Abstract
Iridium(III) hydrido complexes containing N-heterocyclic carbene (NHC)-based pincer ligand 1,3-bis(1-butylimidazolin-2-ylidene)phenyl anion (C1^C^C1) or 1,3-bis(3-butylbenzimidazolin-2-ylidene)phenyl anion (C2^C^C2) and aromatic diimine (2,2′-bipyridine (bpy), 1,10-phenanthroline (phen), 4,4′-dimethyl-2,2′-bipyridine (Me2bpy), or dipyrido-[3,2-f:2′,3′-h]-quinoxaline (dpq)) in the form of [Ir(C^C^C)(N^N)(H)]+ have been prepared. Crystal structures for these complexes show that the Ir–CNHC distances are 2.043(5)–2.056(5) Å. The hydride chemical shifts for complexes bearing C1^C^C1 (−20.6 to −20.3 ppm) are more upfield than those with C2^C^C2 (−19.5 and −19.2 ppm), revealing that C1^C^C1 is a better electron donor than C2^C^C2. Spectroscopic comparisons and time-dependent density functional theory (TD-DFT) calculations suggest that the lowest-energy electronic transition associated with these complexes (λ = 340–530 nm (ε ≤ 103 dm3 mol−1 cm−1)) originate from a dπ(IrIII) → π*(N^N) metal-to-ligand charge transfer transition, where the dπ(IrIII) level contain significant contribution from the C^C^C ligands. All these complexes are emissive in the yellow-spectral region (553–604 nm in CH3CN and CH2Cl2) upon photo-excitation with quantum yields of 10−3–10−1.
Similar content being viewed by others
Introduction
Polypyridyl ruthenium(II) or other d6-transition metal complexes represent an important class of emissive molecular material1,2,3,4,5,6,7,8,9,10,11,12,13,14. Their triplet [dπ(M) → π*(polypyridyl)] metal-to-ligand charge transfer (3MLCT) excited-states are known to derive rich photophysical and photochemical properties and their applications in solar energy conversion11,15, organic light emitting devices (OLEDs)16, photochemistry11,12 and bio-labelling reagents17 have received considerable interest. Regarding the design of transition metal-containing luminophores, iridium(III) center has received great attention on the basis that it is a heavier analogue of ruthenium(II) center18,19.
After the isolation of stable NHCs by Arduengo and co-workers in 199120, intensive investigations on NHCs and the derived metal complexes have been centralized on the development of catalytic reagents for organic transformations21,22,23,24,25. On the other hand, employment of N-heterocyclic carbenes (NHCs)-derived ligands as an alternative of polypyridines in the design of transition metal-based luminophores is growing to be an important research topic recently. For example, emissive Ru(II)26, Ir(III)18,19 and Pt(II)27 complexes supported by NHCs have been reported. Several emissive NHC-containing multinuclear Cu, Ag and Au complexes have also been prepared, in which the NHCs facilitate the metal-metal interaction-induced emissions28,29.
We have initiated a program to develop organometallic Ru(II)/Os(II)–diimine and related luminophores30,31,32,33 and very recently we have reported emissive osmium(II) carbonyl complexes bearing 1,3-bis(1-methylimidazolin-2-ylidene)phenyl anion (MeC1^C^C1Me) or 1,3-bis(3-methylbenzimidazolin-2-ylidene)phenyl anion (MeC2^C^C2Me) and aromatic diimine in the form of [Os(C^C^C)(N^N)(CO)]+30. Spectroscopic and theoretical investigations on [Os(C^C^C)(N^N)(CO)]+ have revealed that the emissive state for [Os(C^C^C)(N^N)(CO)]+ originates from a dπ(OsII) → π*(N^N) MLCT transition, where the C^C^C ligands contribute significantly to both the dπ(OsII) and π*(N^N) levels. This suggests that the NHC-derived ligands would not only act as point charge/spectator ligands, but can also be involved in the emissive excited-state to modify the photophysical properties of a metal–diimine luminophore. As an extension to scrutinize the effect of C^C^C pincer ligands on the photophysical properties of a [M(N^N)] moiety, we now present the preparation, spectroelectrochemical, photophysical and theoretical investigations of a class of emissive hydrido iridium(III) complexes bearing C^C^C pincer ligands and aromatic diimines, [Ir(C^C^C)(N^N)(H)]+.
Results
Synthesis
Emissive Ir(III) complexes [Ir(C^C^C)(N^N)(H)]+ (1–2) were prepared by refluxing [Ir(C^C^C)(CH3CN)(Br)(H)] with N^N in ethylene glycol (Figure 1). [Ir(C^C^C)(CH3CN)(Br)(H)] were synthesized analogously to the corresponding known complexes [Ir(ArC^C^CAr)(CH3CN)(Cl)(H)] and [Ir(MeC1^C^C1Me)(CH3CN)(I)(H)] (ArC^C^CAr = 1,3-bis(1-arylimidazolin-2-ylidene)phenyl anion)23,24. The presence of the hydride ligands in 1–2 was confirmed by the 1H NMR signals at -20.6 to -19.2 ppm and νIr–H at 2126 to 2189 cm−1. Both the 1H and 13C NMR spectra signify that 1–2 possess a pseudo-plane of symmetry in solution on the NMR time scale at room temperature. For instances, there are 17 and 19 sets of aromatic 13C signal for 1a and 1b, respectively. The 13C NMR signals at 167.8–180.6 ppm for 1–2 are typical for metalated NHC. It is noted that the hydride chemical shifts for 1a–1d (-20.55 to -20.27 ppm) are nearly 1 ppm more upfield than those for 2a–2d (-19.50 to -19.21 ppm). Since the hydride chemical shifts indicate that the electronic shielding effect of the hydrido group results from the metal core’s electron cloud, they can be used as probes for the donating ability of the C^C^C ligands34. Therefore, the more upfield hydride chemical shifts for 1a–1d when compared with 2a–2d reveals that C1^C^C1 is a stronger electron donor than C2^C^C2. The same conclusion has recently been made in the comparison of the νCO between [Os(MeC1^C^C1Me)(N^N)(CO)]+ and [Os(MeC2^C^C2Me)(N^N)(CO)]+ 31. These findings are also consistent with the NHC donor strengths determined by Huynh et al., where benzimidazolin-2-ylidene is suggested to have a weaker donor strength compared with imidazolin-2-ylidene35. Complex [Ir(C1^C^C1)2]+ has also been synthesized according to the method reported in literature23 for spectroscopic comparisons.
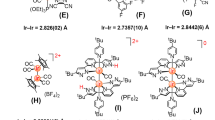
The molecular structures of 1a(ClO4), 2a(ClO4) and [2b(ClO4)]3·CH3CN have been determined by X-ray crystallography. Perspective views of the cations 1a and 2b are depicted in Figure 2; selected bond distances and angles are summarized in Table 1. In each case, the Ir atom adopts a distorted octahedral geometry, with the C^C^C-pincer coordinating in a meridional mode. The ring systems on C^C^C are not perfectly co-planar: the NHC moieties (i.e. imidazolin-2-ylidene or benzimidazolin-2-ylidene units) are tilted towards the hydride ligands and the angles between the NHC planes are 12.36–22.54°. These angles are larger than those found in [Ir(MeC1^C^C1Me)(CH3CN)(I)(H)], [Ir(C^MeCMe^C)(CH3CN)(I)2] (C^MeCMe^C = 1,3-bis(1-butylimidazolium)-4,6-dimethylbenzene) and [Ir(ArC^C^CAr)(CH3CN)(Cl)(H)] in which the angles between the NHC planes are 2.73°, 3.63° and 5.60–15.58° respectively23,24. As a comparison, the ring systems on C^C^C for [Os(MeC1^C^C1Me)(N^N)(CO)]+ and [Os(MeC2^C^C2Me)(N^N)(CO)]+ (N^N = bpy or phen) are more close to a co-planar configuration (angles between the NHC planes are 2.30–13.00°)31. The CNHC–Ir–CPh angles for these complexes are 77.22(11)–78.74(15)°, which are only slightly larger than the CNHC–Os–CPh angles in [Os(MeC1^C^C1Me)(N^N)(CO)]+ and [Os(MeC2^C^C2Me)(N^N)(CO)]+ (75.6(3)–76.8(3)°)31. The Ir–CNHC distances (2.043(5)–2.056(5) Å) are notably longer than the Ir–CPh distances (1.959(4)–1.986(5) Å). Similar findings have been observed in [Ir(MeC1^C^C1Me)(CH3CN)(I)2]23, [Ir(MeC1^C^C1Me)(CH3CN)(I)(H)]24, [Ir(C^MeCMe^C)(CH3CN)(I)2] (C^MeCMe^C = 1,3-bis(1-butylimidazolium)-4,6-dimethylbenzene)23, [Ir(ArC^C^CAr)(CH3CN)(Cl)(H)]24 and Zr, Rh and Os complexes bearing similar C^C^C-pincer ligands31,36. Since the Ir–CPh distances in fac-[Ir(C^C)3] and mer-[Ir(C^C)3] (C^C = 1-phenyl-3-methylbenzimidazolin-2-ylidene-C,C2′) are in the range of 2.071(7)−2.099(4) Å18, the significantly shorter Ir–CPh distances in this work most likely arise from the strain intrinsic to the metal–C^C^C moieties.
Electrochemistry
Cyclic voltammetry has been used to examine the electrochemistry of the complexes (Table 2; all values vs Cp2Fe+/0). 1–2 show irreversible first oxidation waves at Epa = 0.69 to 0.77 V (scan rate = 100 mV s−1) and reversible first reduction couples at E1/2 = -2.00 to -1.73 V. It is noted that both the first oxidation waves and the first reduction couples are sensitive to the change of C^C^C and N^N. For example, the first reduction potentials for 1a–1d (-2.00 to -1.83 V) are slightly more negative than those for 2a–2d (-1.95 to -1.73 V) and the ease of reduction follows the order: d > b ≈ a > c. These findings suggest that both the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecule orbitals (LUMOs) for 1–2 contain contributions from the C^C^C and N^N, in agreement with our DFT calculations (see discussion below). Moreover, the contribution of N^N to the LUMOs of 1–2 is apparent as [Ir(C1^C^C1)2]+ does not feature any reduction wave within the solvent window.
UV–Visible Absorption and Spectroelectrochemistry
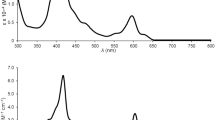
The UV–visible spectral data for 1, 2 and [Ir(C1^C^C1)2]+ are summarized in Table 3 and their absorption spectra are depicted in Figure 3. 1–2 exhibit intense, high-energy absorptions at λ ≤ 340 nm (ε ≥ 104 dm3 mol−1 cm−1) and moderately intense bands at λ > 340 nm (ε ≈ 103 dm3 mol−1 cm−1) with tailing up to 530 nm. In the literature, Ir(III) complexes bearing aromatic diimine ligands such as [Ir(bpy)3]3+ and [Ir(phen)3]3+ feature highly intense absorptions at λ ≤ 320 nm (ε ≥ 104 dm3 mol−1 cm−1) and these are ascribed to π → π*(N^N) intraligand (IL) transitions37,38,39. In addition, [Ir(C1^C^C1)2]+ exhibits intense absorptions at λ ≤ 330 nm (ε ≥ 104 dm3 mol−1 cm−1), which are expected to be a mixture of dπ(IrIII) → π*(C^C^C) metal-to-ligand charge transfer (MLCT) and π → π*(C^C^C) IL transition. With the origin of absorptions for [Ir(N^N)3]3+ and [Ir(C1^C^C1)2]+ as references, the high-energy absorptions at λ ≤ 340 nm for complexes 1–2 are assigned to be a mixing of π → π*(C^C^C) IL, π → π*(N^N) IL and dπ(IrIII) → π*(C^C^C) MLCT transitions.
On the other hand, the electronic transitions at λ = 340–530 nm (ε ≤ 103 dm3 mol−1 cm−1) for 1–2 should contain some dπ(IrIII) → π*(N^N) MLCT character, reasons are as follows: (1) [Ir(bpy)3]3+, [Ir(phen)3]3+ and [Ir(ppy)2(bpy)]+ (ppy = 2-phenylpyridine) feature dπ(IrIII) → π*(N^N) MLCT transitions in similar energy region (λmax = 370–520 nm, ε ≤ 103 dm3 mol−1 cm−1);38,39,40,41,42,43 (2) a red-shift in absorption energy is observed when N^N is changed from Me2bpy to bpy and from phen to dpq; (3) 1–2 display solvatochromic effect in the spectral region concerned. For example, the λmax for 1a within this spectral region is 374 nm in CH3CN and is 384 nm in CH2Cl2; (4) there are no corresponding absorption bands for [Ir(C1^C^C1)2]+. This assignment is consistent with the TD-DFT calculations on complexes 1a and 2a, which suggest that the nature of electronic transitions in the spectral region concerned to be mainly attributed to the HOMO–1 → LUMO and HOMO–2 → LUMO transitions, where the HOMO–1 and HOMO–2 have higher Ir contribution (27–59%) than that in LUMO (3–4%) and LUMO has higher N^N contribution (93%) than those in HOMO–1 and HOMO–2 (3–15%) (see discussion below). The contribution of N^N to the LUMOs for 1 and 2 is further confirmed by spectroelectrochemistry. Thin-layer UV–visible spectroelectrochemistry has been employed to acquire the absorption spectra for 1a− and 2a−, the reduced forms of 1a and 2a respectively (Figure 4). The isosbestic spectral changes suggest that the electrochemical reductions of 1a and 2a are clean conversions. Notably, reductions of 1a and 2a result in enhancement of absorption at ~380 nm and new absorption doublet band near 500 nm. These absorption features were observed in the reduction of [Ir(bpy)3]3+ and are characteristic absorptions for anionic bpy radical (bpy•−)44.
Emission Spectroscopy
The emission properties of the complexes in fluid solution (CH3CN and CH2Cl2) at 298 K have been investigated (Table 4). Figure 5 depicts the emission spectra for 2a, 2c, 2d and [Ir(C1^C^C1)2]+ in CH3CN at 298 K. Emission maxima of 1–2 range from 553 to 604 nm in CH3CN and CH2Cl2 which are significantly blue shifted when compared with [Os(C^C^C)(N^N)(CO)]+ (λem = 676–731 nm, solvents = CH3CN and CH2Cl2)30. Quantum yields (Φ) and emission lifetimes (τ) of 1–2 are around 10−3–10−1 and 102–101 ns respectively, while those parameters for [Os(C^C^C)(N^N)(CO)]+ are around 10−4–10−2 and 1–6 μs respectively30. Similar to Os(C^C^C)(N^N)(CO)]+, these photophysical parameters for 1–2 are sensitive to the change of C^C^C and N^N, revealing that the emissive state involve both the C^C^C and N^N moieties. For example, in both 1–2 and [Os(C^C^C)(N^N)(CO)]+, blue-shift on emission maxima, higher emission quantum yield and longer excited state lifetime are observed when changing the N^N from 2,2-bipyridine to 1,10-phenanthroline30. The resemblance of the excitation profiles to the absorption spectra signifies that the emissions originate from the energy dissipation of the dπ(IrIII) → π*(N^N) MLCT transitions. Interestingly, similar conclusion has been made on the nature of the emissive excited states in [Os(C^C^C)(N^N)(CO)]+ 30. The emission profile for [Ir(C1^C^C1)2]+ is highly structured and the emission maxima (378 and 398 nm) are not sensitive to the change of solvent, therefore these emissions are assigned as π → π* (C^C^C) 3IL emissions.
Theoretical Calculations
Time-dependent density functional theory (TD-DFT) calculations have been performed on modeling complexes [Ir(MeC1^C^C1Me)(bpy)(H)]+ (1a’) and [Ir(MeC2^C^C2Me)(bpy)(H)]+ (2a’), in which their metal cores are the same as 1a and 2a but the butyl chains on the C^C^C are replaced by methyl groups to reduce computational cost. The ground-state structures of 1a’ and 2a’ have been optimized at the DFT level (functional = PBE0)45,46 without symmetry constrain. The conductor-like screening model (COSMO)47 has been applied to account for solvent effects upon the electronic transition. All the optimized geometries are in satisfactory agreement with their crystal structures. For example, the Ir–CNHC and Ir–CPh bond distances calculated for 1a’ (2.05–2.06 and 1.97 Å respectively) are similar to those for 1a determined by X-ray crystallography (Ir–CNHC: 2.049(3) and 2.055(3) Å; Ir–CPh: 1.975(3) Å).
The excitation energies and oscillator strengths for the calculated vertical transitions with λ > 360 nm are summarized in Table 5. Table 6 summarized the compositions of the molecular orbitals (MOs) which are involved in the lowest-energy electronic transitions in these complexes. Figure 6 depicts the simulated absorption spectra. It is noted that the calculated lowest-energy dipole allowed transitions (λ > 360 nm) mainly originate from the HOMO–1 → LUMO and HOMO–2 → LUMO transitions. The HOMOs–1 and HOMOs–2 have higher Ir contribution (27–59%) than that in LUMOs (3–4%), whereas the LUMOs have higher N^N contribution (93%) than those in HOMOs–1 and HOMOs–2 (3–15%), therefore the transitions contain some Ir → π*(N^N) MLCT character. This finding is consistent with the spectroscopic observation that a red-shift in absorption energy is observed when N^N is changed from Me2bpy to bpy and from phen to dpq. Besides, the contribution of C^C^C to both the HOMOs–1 and HOMOs–2 are not low (27–69%), suggesting that the C^C^C ligands contribute significantly to the dπ(IrIII) levels. The electronic difference density plots for 1a’ and 2a’ in their lowest-energy excited state (Figure 6, generated by taking the difference in the excited-state electron density and ground-state electron density) clearly show that electronic charge is depleted from the Ir center and accumulated at the N^N moiety. The emissions from complexes 1–2 are thus believed to be originated from the triplet dπ(IrIII) → π*(N^N) MLCT states.
TD-DFT calculated absorption spectrum for model complexes 1a’ and 2a’ in CH3CN.
Excitation energies and oscillator strengths are shown by the blue vertical lines; spectrum (in black) is convoluted with a Gaussian function having a full width at half-maximum of 3000 cm−1. Inserts show the electronic difference density plots for 1a’ and 2a’ at the vertical transitions marked with * (isodensity value = 0.002 au; charge accumulation and depletion are represented in red and blue respectively).
Conclusion
In this work a series of emissive Ir(III) hydrido complexes bearing the NHC-derived tridentate C^C^C pincer ligands and aromatic diimines have been prepared. This joint experimental and theoretical study reveals that the lowest-energy absorptions associated with these complexes arise from a dπ(IrIII) → π*(N^N) MLCT transition, where the C^C^C ligands contribute significantly to the dπ(IrIII) level. It is therefore evident that the C^C^C ligands can modulate the photophysical properties via the formation of the hybrid [Ir + C^C^C] molecular orbitals and this work highlights the opportunities of using NHC-derived ligands to modulate the photophysics of a [M(N^N)] core.
Methods
General Procedure
All reactions were performed under an argon atmosphere using standard Schlenk techniques unless otherwise stated. All reagents and solvents were used as received. The C^C^C ligand precursors, i.e. benzene-bridged bisimidazolium bromide48 and [Ir(1,5-cod)Cl]2 (cod = 1,5-cyclooctadiene)49, were prepared according to literature methods. [Ir(C^C^C)(CH3CN)(Br)(H)] were synthesized analogously to the corresponding known complexes [Ir(ArC^C^CAr)(CH3CN)(Cl)(H)] and [Ir(MeC1^C^C1Me)(CH3CN)(I)(H)]23,24. 1H, 13C{1H}, DEPT-135, 1H–1H COSY and 1H–13C HSQC NMR spectra were recorded on Bruker 400 DRX FT-NMR spectrometer. Figure 7 depicts the labeling scheme for the H and C atoms. Peak positions were calibrated with solvent residue peaks as internal standard. Electrospray mass spectrometry was performed on a PE-SCIEX API 3000 triple quadrupole mass spectrometer. Infrared spectra were recorded as KBr plates on an Avatar 360 FTIR spectrometer. UV–visible spectra were recorded on a Shimadzu UV-1700 spectrophotometer. Elemental analyses were done on an Elementar Vario Micro Cube carbon–hydrogen–nitrogen elemental micro-analyzer. Cyclic voltammetry was performed with a CH Instrument model 600C series electrochemical analyzer/workstation. All the electrochemical measurements were performed in CH3CN solution with [n-Bu4N]PF6 (0.1 M) as supporting electrolyte at room temperature. The glassy-carbon working electrode was polished with 0.05 μm alumina on a microcloth, sonicated for 5 min in deionized water and rinsed with CH3CN before use. An Ag/AgNO3 (0.1 M in CH3CN) electrode was used as reference electrode, with a platinum wire as the counter electrode. All solutions were degassed with nitrogen before experiments. The E1/2 value of the ferrocenium/ferrocene couple (Cp2Fe+/0) measured in the same solution was used as an internal reference. Steady-state emission spectra were obtained on a Jobin Yvon Fluorolog-3-TCSPC spectrophotometer. Sample and standard solutions were degassed with at least three freeze-pump-thaw cycles. The emission quantum yields for complexes 1–2 were measured by the method of Demas and Crosby50 with [Ru(bpy)3](PF6)2 in degassed CH3CN as standard (Φr = 0.062), whereas that for [Ir(C1^C^C1)2]+ was measured with quinine sulphate in 0.1M H2SO4 as standard (Φr = 0.58)51. The emission quantum yields were calculated by Φs = Φr(Br/Bs)(ns/nr)2(Ds/Dr), where the subscripts s and r refer to sample and reference standard solution, respectively, n is the refractive index of the solvents, D is the integrated intensity and Φ is the luminescence quantum yield. The quantity B is calculated by B = 1 - 10−AL, where A is the absorbance at the excitation wavelength and L is the optical path length52.
[Ir(C^C^C)(N^N)H](ClO4), 1–2(ClO4)
A mixture of [Ir(1,5-cod)Cl]2 (0.10 mmol), benzene bridged bisimidazolium or bisbenzimidazolium bromide (0.20 mmol) and Cs2CO3 (0.43 mmol) was refluxed in CH3CN (30 ml) for 16 h. Upon cooling to room temperature, the solvent was removed by reduced pressure and the residue was extracted with CH2Cl2. The [Ir(C^C^C)(CH3CN)(Br)(H)] obtained from this extract was used for the synthesis of 1–2 without further purification. A mixture of [Ir(C^C^C)(CH3CN)(Br)(H)] (0.15 mmol) and diimine (0.5 mmol) was refluxed in ethylene glycol for 3 h. Upon cooling to room temperature, the resultant solution was added to a saturated NaClO4 solution to give brown solids. The crude product was eluted by column chromatography (neutral alumina, 9:1 (v/v) CH2Cl2/CH3CN as eluent) as a yellow band. After removal of solvent, the yellow solid was recrystallized by slow diffusion of Et2O into CH3CN solution to give yellow crystals.
Complex 1a
Yield: 0.06 g, 50%. Anal. Calcd for C30H34N6Ir(ClO4): C, 46.78; H, 4.45; N, 10.91. Found: C, 46.70; H, 4.51; N, 10.88. 1H NMR (400 MHz, CD3CN): δ –20.46 (s, 1H, Ir−H), 0.58–0.99, 1.18–1.32 (m, 14H, C3H7 of n-Bu); 3.17–3.22 (m, 4H, CH2 of n-Bu); 7.05 (d, 2H, J = 2.1 Hz, Hl); 7.16–7.16 (m, 1H, Hg); 7.22–7.29 (m, 3H, Hi + Hj); 7.41 (d, 1H, J = 5.2 Hz, Hh); 7.65–7.69 (m, 1H, Hb); 7.74 (d, 2H, J = 2.1 Hz, Hk); 7.88 (td, 1H, J = 8.0, 1.6 Hz, Hf); 8.21 (td, 1H, J = 8.0, 1.6 Hz, Hc); 8.38 (d, 1H, J = 8.0 Hz, He); 8.55 (d, 1H, J = 8.0 Hz, Hd); 9.67 (d, 1H, J = 5.2 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.7, 20.2, 34.5, 50.1 (n-Bu); 108.3 (Cj); 117.0 (Ck); 121.6 (Cl); 122.9 (Ci); 124.6 (Ce); 125.3 (Cd); 127.7 (Cg); 129.0 (Cb); 138.2 (Cc); 138.3 (Cf); 142.9 (Ir−CPh); 146.1 (Quaternary C in C1^C^C1); 151.7 (Ch); 156.9, 157.0 (Quaternary C in bpy); 157.2 (Ca); 167.9 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2189, νCl–O = 1086. ESI-MS: m/z 670 [M+].
Complex 1b
Yield: 0.06 g, 50%. Anal. Calcd for C32H34N6Ir(ClO4): C, 48.39; H, 4.31; N, 10.58. Found: C, 48.08; H, 4.36; N, 10.40. 1H NMR (400 MHz, CD3CN): δ –20.27 (s, 1H, Ir−H), 0.14–0.51, 0.89–1.06 (m, 14H, C3H7 of n-Bu); 2.92–3.15 (m, 4H, CH2 of n-Bu); 6.98 (d, 2H, J = 2.0 Hz, Hl); 7.21–7.37 (m, 3H, Hi + Hj); 7.51 (dd, 1H, J = 8.0, 5.0 Hz, Hg); 7.74 (d, 2H, J = 2.0 Hz, Hk); 7.77 (d, 1H, J = 5.0 Hz, Hh); 8.04 (dd, 1H, J = 8.0, 5.0 Hz, Hb); 8.12 (d, 1H, J = 8.8 Hz, He); 8.22 (d, 1H, J = 8.8 Hz, Hd); 8.44 (d, 1H, J = 8.0 Hz, Hf); 8.78 (d, 1H, J = 8.0 Hz, Hc); 9.97 (d, 1H, J = 5.0 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.6, 20.0, 34.4, 50.1 (n-Bu); 108.4 (Cj); 117.1 (Ck); 121.6 (Cl); 123.2 (Ci); 126.4 (Cg); 127.8 (Cb); 128.8 (Cd); 128.9 (Ce); 132.1, 132.6 (Quaternary C in phen); 137.4 (Cc); 137.6 (Cf); 142.6 (Ir−CPh); 146.4 (Quaternary C in C1^C^C1); 148.4, 148.8 (Quaternary C in phen); 152.6 (Ch); 157.3 (Ca); 168.1 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2179, νCl–O = 1094. ESI-MS: m/z 695 [M+].
Complex 1c
Yield: 0.05 g, 40%. Anal. Calcd for C32H38N6Ir(ClO4): C, 48.14; H, 4.80; N, 10.53. Found: C, 48.44; H, 5.08; N, 10.45. 1H NMR (400 MHz, CD3CN): δ –20.55 (s, 1H, Ir−H), 0.58–0.98, 1.19–1.38 (m, 14H, C3H7 of n-Bu); 2.39 (s, 3H, CH3 of Me2bpy); 2.61 (s, 3H, CH3 of Me2bpy); 3.26 (t, 4H, J = 7.8 Hz, CH2 of n-Bu); 6.96 (d, 1H, J = 5.6 Hz, Hg); 7.05 (d, 2H, J = 2.1 Hz, Hl); 7.20 (d, 1H, J = 5.6 Hz, Hh); 7.21–7.29 (m, 3H, Hi + Hj); 7.50 (d, 1H, J = 5.6 Hz, Hb); 7.73 (d, 2H, J = 2.1 Hz, Hk); 8.23 (s, 1H, He); 8.39 (s, 1H, Hd); 9.46 (d, 1H, J = 5.6 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.7, 20.3, 34.5, 50.1 (n-Bu); 21.1, 21.3 (CH3 of Me2bpy); 108.2 (Cj); 117.0 (Ck); 121.5 (Cl); 122.7 (Ci); 125.2 (Ce); 125.8 (Cd); 128.4 (Cg); 129.6 (Cb); 143.4 (Ir−CPh); 146.1 (Quaternary C in C1^C^C1); 150.5, 150.7 (Quaternary C in Me2bpy); 150.9 (Ch); 156.6 (Ca); 156.8, 156.9 (Quaternary C in Me2bpy); 168.4 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2159, νCl–O = 1107. ESI-MS: m/z 699 [M+].
Complex 1d
Yield: 0.06 g, 45%. Anal. Calcd for C34H34N8Ir(ClO4): C, 48.25; H, 4.05; N, 13.24. Found: C, 48.47; H, 4.28; N, 13.06. 1H NMR (400 MHz, CD3CN): δ –20.29 (s, 1H, Ir−H), 0.27–0.75, 1.08–1.21 (m, 14H, C3H7 of n-Bu); 2.91–3.22 (m, 4H, CH2 of n-Bu); 7.00 (d, 2H, J = 2.1 Hz, Hl); 7.24–7.37 (m, 3H, Hi + Hj); 7.65 (dd, 1H, J = 8.2, 5.2 Hz, Hg); 7.76 (d, 2H, J = 2.0 Hz, Hk); 7.87–7.88 (m, 1H, Hh); 8.18 (dd, 1H, J = 8.2, 5.2 Hz, Hb); 9.18 (d, 1H, J = 2.1 Hz, He); 9.22 (d, 1H, J = 2.1 Hz, Hd); 9.41 (dd, 1H, J = 8.2, 1.2 Hz, Hf); 9.75 (dd, 1H, J = 8.2, 1.2 Hz, Hc); 10.09 (dd, 1H, J = 5.2, 1.2 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.5, 20.1, 34.3, 50.2 (n-Bu); 108.5 (Cj); 117.2 (Ck); 121.7 (Cl); 123.3 (Ci); 127.6 (Cg); 128.9 (Cb); 130.8, 131.5 (Quaternary C in dpq); 134.0 (Cc); 134.2 (Cf); 140.5, 140.5 (Quaternary C in dpq); 142.2 (Ir−CPh); 146.3 (Quaternary C in C1^C^C1); 147.7, 147.8 (Cd + Ce); 149.7, 150.0 (Quaternary C in dpq); 153.9 (Ch); 158.5 (Ca); 167.8 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H =2131, νCl–O = 1108. ESI-MS: m/z 747 [M+].
Complex 2a
Yield: 0.07 g, 50%. Anal. Calcd for C38H38N6Ir(ClO4): C, 52.44; H, 4.40; N, 9.66. Found: C, 52.45; H, 4.38; N, 9.46. 1H NMR (400 MHz, CD3CN): δ –19.50 (s, 1H, Ir−H), 0.40–1.07, 1.23–1.47 (m, 14H, C3H7 of n-Bu); 3.30–3.59 (m, 4H, CH2 of n-Bu); 7.07 (t, 1H, J = 6.4 Hz, Hg); 7.28–7.35 (m, 2H, Hm); 7.37–7.50 (m, 6H, Hh + Hi + Hl + Hn); 7.71–7.74 (m, 1H, Hb); 7.77–7.87 (m, 3H, Hj + Hf); 8.21 (d, 2H, J = 8.0 Hz, Hk); 8.27–8.31 (m, 1H, Hc); 8.38 (d, 1H, J = 8.2 Hz, He); 8.60 (d, 1H, J = 8.2 Hz, Hd); 9.51 (d, 1H, J = 5.3 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.8, 20.4, 33.3, 47.5 (n-Bu); 109.7 (Cj); 111.9 (Cl/Cn); 112.2 (Ck); 123.6 (Ci); 123.9 (Cm); 124.6 (Ce); 124.7 (Cl/Cn); 125.5 (Cd); 127.9 (Cg); 129.3 (Cb); 133.0, 135.6 (Quaternary C in C2^C^C2); 138.7 (Cf); 138.8 (Cc); 142.9 (Ir−CPh); 146.6 (Quaternary C in C2^C^C2); 152.2 (Ch); 156.6 (Quaternary C in bpy); 157.2 (Ca); 157.3 (Quaternary C in bpy); 180.1 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2126, νCl–O = 1075 ESI-MS: m/z 771 [M+].
Complex 2b
Yield: 0.05 g, 40%. Anal. Calcd for C40H38N6Ir(ClO4): C, 53.71; H, 4.28; N, 9.40. Found: C, 53.75; H, 4.30; N, 9.45. 1H NMR (400 MHz, CD3CN): δ –19.21 (s, 1H, Ir−H), 0.19–0.35, 0.38–0.45, 0.52–0.67 (m, 14H, C3H7 of n-Bu); 3.26–3.46 (m, 4H, CH2 of n-Bu); 7.26–7.39 (m, 4H, Hl/Hm + Hn); 7.39–7.48 (m, 3H, Hg + Hm/Hl); 7.52 (t, 1H, J = 8.0 Hz, Hi); 7.81 (d, 2H, J = 5.1 Hz, Hf); 7.87 (d, 2H, J = 8.0 Hz, Hj); 8.08–8.18 (m, 2H, Hb + He); 8.21–8.29 (m, 3H, Hd + Hk); 8.41 (d, 1H, J = 8.2 Hz, Hh); 8.88 (d, 1H, J = 8.2 Hz, Hc); 9.96 (d, 1H, J = 8.2 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.8, 20.3, 33.3, 47.6 (n-Bu); 109.8 (Cj); 111.9 (Cl/Cm/Cn); 112.3 (Ck); 123.9 (Ci); 124.0 (Cl/Cm/Cn); 124.8 (Cl/Cm/Cn); 126.7 (Cg); 128.1 (Cb); 128.8 (Cd); 129.1 (Ce); 132.2, 132.7 (Quaternary C in phen); 133.1, 135.7 (Quaternary C in C2^C^C2); 137.9 (Ch); 138.1 (Cc); 142.6 (Ir−CPh); 146.9 (Quaternary C in C2^C^C2); 148.3, 148.9 (Quaternary C in phen); 153.2 (Cf); 157.4 (Ca); 180.4 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2129, νCl–O = 1090. ESI-MS: m/z 795 [M+].
Complex 2c
Yield: 0.05 g, 40%. Anal. Calcd for C40H42N6Ir(ClO4): C, 53.47; H, 4.71; N, 9.35. Found: C, 53.53; H, 4.77; N, 9.57. 1H NMR (400 MHz, CD3CN): δ –19.48 (s, 1H, Ir−H), 0.60–0.97, 1.34–1.47 (m, 14H, C3H7 of n-Bu); 2.33 (s, 3H, CH3 of Me2bpy); 2.67 (s, 3H, CH3 of Me2bpy); 3.39–3.67 (m, 4H, CH2 of n-Bu); 6.90 (d, 1H, J = 5.7 Hz, Hg); 7.25 (d, 1H, J = 5.7 Hz, Hh); 7.28–7.38 (m, 2H, Hm); 7.40–7.50 (m, 5H, Hi + Hl + Hn); 7.54–7.65 (m, 1H, Hb); 7.81 (d, 2H, J = 8.0 Hz, Hj); 8.17–8.35 (m, 3H, He + Hk); 8.46 (s, 1H, Hd); 9.42 (d, 1H, J = 5.6 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.8, 20.5, 33.4, 47.6 (n-Bu); 21.1, 21.3 (CH3 of Me2bpy); 109.6 (Cj); 111.9 (Cl/Cn); 112.2 (Ck); 123.5 (Ci); 123.9 (Cm); 124.7 (Cl/Cn); 125.3 (Ce); 126.0 (Cd); 128.6 (Cg); 130.0 (Cb); 133.0, 135.7 (Quaternary C in C2^C^C2); 143.4 (Ir−CPh); 146.7 (Quaternary C in C2^C^C2); 151.1, 151.3 (Quaternary C in Me2bpy); 151.3 (Ch); 156.6 (Quaternary C in Me2bpy); 156.7 (Ca); 157.1 (Quaternary C in Me2bpy); 180.6 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2133, νCl–O = 1094. ESI-MS: m/z 799 [M+].
Complex 2d
Yield: 0.06 g, 40%. Anal. Calcd for C42H38N8Ir(ClO4): C, 53.30; H, 4.05; N, 11.84. Found: C, 53.41; H, 4.25; N, 11.94. 1H NMR (400 MHz, CD3CN): δ –19.22 (s, 1H, Ir−H), 0.23–0.79, 1.18–1.48 (m, 14H, C3H7 of n-Bu); 3.17–3.61 (m, 4H, CH2 of n-Bu); 7.28–7.46 (m, 6H, Hl + Hm + Hn); 7.54 (t, 1H, J = 8.0 Hz, Hi); 7.59 (dd, 1H, J = 8.2, 5.2 Hz, Hg); 7.89 (d, 2H, J = 8.0 Hz, Hj); 7.91–7.93 (m, 1H, Hh); 8.16–8.35 (m, 3H, Hb + Hk); 9.18 (d, 1H, J = 2.1 Hz, He); 9.24 (d, 1H, J = 2.1 Hz, Hd); 9.37 (dd, 1H, J = 8.2, 1.4 Hz, Hf); 9.85 (dd, 1H, J = 8.2, 1.3 Hz, Hc); 10.11 (dd, 1H, J = 5.2, 1.3 Hz, Ha). 13C NMR (100 MHz, CD3CN): δ 13.7, 20.4, 33.3, 47.7 (n-Bu); 109.9 (Cj), 112.0 (Cl/Cm/Cn); 112.3 (Ck); 124.1 (Ci); 124.1, 124.9 (Cl/Cm/Cn); 127.8 (Cg); 129.2 (Cb); 130.9, 131.6 (Quaternary C in dpq); 133.1 (Quaternary C in C2^C^C2); 134.5 (Cc), 134.6 (Cf), 135.7 (Quaternary C in C2^C^C2); 140.4, 140.5 (Quaternary C in dpq); 142.3 (Ir−CPh), 146.9 (Quaternary C in C2^C^C2), 147.8 (Ce); 147.9 (Cd); 149.5, 150.2 (Quaternary C in dpq); 154.5 (Ch); 158.6 (Ca); 180.1 (Ir−Ccarbene). IR (KBr, cm−1): νIr−H = 2130, νCl–O = 1097. ESI-MS: m/z 847 [M+].
X-ray Crystallography
X-ray diffraction data for 1a(ClO4), 2a(ClO4) and [2b(ClO4)]3·CH3CN were collected on an Oxford Diffraction Gemini S Ultra X-ray single crystal diffractometer with Cu Kα radiation (λ = 1.54178 Å) at 133 K. The data were processed using CrysAlis53. The structures were solved by Patterson and Fourier methods and refined by full-matrix least-squares based on F2 with program SHELXS-97 and SHELXL-9754 within WinGX55. All non-hydrogen atoms were refined anisotropically in the final stage of least-squares refinement. The positions of H atoms were calculated based on riding mode with thermal parameters equal to 1.2 times that of the associated C atoms. CCDC 1416088–1416090 contain the supplementary crystallographic data for this paper, which can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Computational Methodology
DFT calculations were performed on model complexes [Ir(MeC1^C^C1Me)(bpy)(H)]+ (1a’) and [Ir(MeC2^C^C2Me)(bpy)(H)]+ (2a’). Their electronic ground states were optimized without symmetry constrain using the density functional PBE045,46. The def2-SVP basis sets were used for the H, C and N atoms, while the def2-TZVP(-f) basis sets were used for the Ir atoms56. Zero-order regular approximation (ZORA) was employed to account for relativistic effects. Tight SCF convergence (10−8au) was used for all calculations. The vertical transition energies for these model complexes in CH3CN were computed at their respective gas-phase optimized ground-state geometries using time-dependent-DFT (TD-DFT) method with the same density functional and basis sets in the geometry optimizations. The combination of the resolution of the identity and the “chain of spheres exchange” algorithms (RIJCOSX)57 was used to accelerate all DFT and TD-DFT calculations with the use of appropriate auxiliary basis sets. The conductor-like screening model (COSMO)47 was used to account for solvent effects upon the electronic transition. All the calculations were performed using the ORCA software package (version 3.0.2)58.
Additional Information
How to cite this article: Chung, L.-H. et al. Luminescent Iridium(III) Complexes Supported by N-Heterocyclic Carbene-based C^C^C-Pincer Ligands and Aromatic Diimines. Sci. Rep. 5, 15394; doi: 10.1038/srep15394 (2015).
References
Kalyanasundaram, K. Photophysics, Photochemistry and Solar Energy Conversion with Tris(bipyridyl)ruthenium(II) and its Analogues. Coord. Chem. Rev. 46, 159–244 (1982).
Balzani, V., Sabbatini, N. & Scandola, F. “Second-sphere” Photochemistry and Photophysics of Coordination Compounds. Chem. Rev. 86, 319–337 (1986).
Juris, A. et al. Ru(II) Polypyridine Complexes: Photophysics, Photochemistry, Electrochemistry and Chemiluminescence. Coord. Chem. Rev. 84, 85–277 (1988).
Meyer, T. J. Chemical Approaches to Artificial Photosynthesis. Acc. Chem. Res. 22, 163–170 (1989).
Balzani, V., Barigelletti, F. & De Cola, L. Metal Complexes as Light Absorption and Light Emission Sensitizers. Top. Curr. Chem. 158, 31–71 (1990).
Sauvage, J.-P. et al. Ru(II) and Osmium(II) Bis(terpyridine) Complexes in Covalently-Linked Multicomponent Systems: Synthesis, Electrochemical Behavior, Absorption Spectra and Photochemical and Photophysical Properties. Chem. Rev. 94, 993–1019 (1994).
Balzani, V. et al. Luminescent and Redox-Active Polynuclear Transition Metal Complexes. Chem. Rev. 96, 759–833 (1996).
De Silva, A. P. et al. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 97, 1515–1566 (1997).
Dixon, I. M. et al. A Family of Luminescent Coordination Compounds: Iridium(III) Polyimine Complexes. Chem. Soc. Rev. 29, 385–391 (2000).
De Cola, L. et al. Design, Synthesis and Photophysics of Ruthenium and Osmium Complexes through 20 Years of Collaboration. Inorg. Chim. Acta. 360, 775–784 (2007).
Campagna, S. et al. Photochemistry and Photophysics of Coordination Compounds: Ruthenium. Top. Curr. Chem. 280, 117–214 (2007).
Flamigni, L. et al. Photochemistry and Photophysics of Coordination Compounds: Iridium. Top. Curr. Chem. 281, 143–203 (2007).
Flamigni, L., Collin, J.-P. & Sauvage, J.-P. Iridium Terpyridine Complexes as Functional Assembling Units in Arrays for the Conversion of Light Energy. Acc. Chem. Res. 41, 857–871 (2008).
Lainé, P. P., Campagna, S. & Loiseau, F. Conformationally Gated Photoinduced Processes within Photosensitizer-Acceptor Dyads Based on Ruthenium(II) and Osmium(II) Polypyridyl Complexes with an Appended Pyridinium Group. Coord. Chem. Rev. 252, 2552–2571 (2008).
Balzani. V. & Juris, A. Photochemistry and Photophysics of Ru(II)-Polypyridine Complexes in the Bologna Group. From Early Studies to Recent Developments. Coord. Chem. Rev. 211, 97–115 (2001).
Chou, P.-T. & Chi, Y. Phosphorescent Dyes for Organic Light-Emitting Diodes. Chem. Eur. J. 13, 380–395 (2007).
Erkkila, K. E., Odom, D. T. & Barton, J. K. Recognition and Reaction of Metallointercalators with DNA. Chem. Rev. 99, 2777–2795 (1999).
Sajoto, T. et al. Blue and Near-UV Phosphorescence from Iridium Complexes with Cyclometalated Pyrazolyl or N-Heterocyclic Carbene Ligands. Inorg. Chem. 44, 7992–8003 (2005).
Chang, C.-F. et al. Highly Efficient Blue-Emitting Iridium(III) Carbene Complexes and Phosphorescent OLEDs. Angew. Chem. Int. Ed. 47, 4542–4545 (2008).
Arduengo, A. J., Harlow, R. L. & Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 113, 361–363 (1991).
Herrmann, W. A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 41, 1290–1309 (2002).
Clavier, H. & Nolan, S. P. Percent Buried Volume For Phosphine and N-Heterocyclic Carbene Ligands: Steric Properties in Organometallic Chemistry. Chem. Commun. 46, 841–861 (2010).
Raynal, M. et al. An Unprecedented, Figure-Of-Eight, Dinuclear Iridium(I) Dicarbene and New Iridium(III) ‘Pincer’ Complexes. Chem. Commun. 3983–3985 (2008).
Raynal, M. et al. Reaction Intermediates in the Synthesis of New Hydrido, N-Heterocyclic Dicarbene Iridium(III) Pincer Complexes. Organometallics 28, 4028–4047 (2009).
Chianese, A. R. et al. Iridium Complexes of CCC-Pincer N-Heterocyclic Carbene Ligands: Synthesis and Catalytic C-H Functionalization. Organometallics, 29, 3019–3026 (2010).
Son, S. U. et al. Synthesis of Ru(II) Complexes of N-Heterocyclic Carbenes and Their Promising Photoluminescence Properties in Water. Inorg. Chem. 43, 6896–6898 (2004).
Unger, Y. et al. Green–Blue Emitters: NHC-Based Cyclometalated [Pt(C^C*)(acac)] Complexes. Angew. Chem. Int. Ed. 49, 10214–10216 (2010).
Barnard, P. J. et al. Luminescence Studies of The Intracellular Distribution of a Dinuclear Gold(I) N-Heterocyclic Carbene Complex. Angew. Chem. Int. Ed. 45, 5966–5970 (2006).
Strasser, C. E. & Catalano, V. J. “On-off” Au(I)···Cu(I) Interactions in a Au(NHC)2 Luminescent Vapochromic Sensor. J. Am. Chem. Soc. 132, 10009–10011 (2010).
Chung, L.-H. et al. Emissive Osmium(II) Complexes Supported by N-Heterocyclic Carbene-based C^C^C-Pincer Ligands and Aromatic Diimines. Inorg. Chem. 51, 8693–8703 (2012).
Chung, L.-H. et al. Ruthenium(II) and Osmium(II) Complexes Bearing Bipyridine and the N-Heterocyclic Carbene-Based C^N^C Pincer Ligand: An Experimental and Density Functional Theory Study. Inorg. Chem. 52, 9885–9896 (2013).
Chung, L.-H. et al. Metal–Indolizine Zwitterion Complexes as a New Class of Organometallic Material: A Spectroscopic and Theoretical Investigation. Organometallics 33, 3443–3452 (2014).
Tsui, W.-K. et al. Luminescent Ruthenium(II) Complex Bearing Bipyridine and N-Heterocyclic Carbene-based C^N^C Pincer Ligand for Live-Cell Imaging of Endocytosis. Sci. Rep. 5, 9070 (2014).
Darensbourg, M. Y., Ludwig, M. & Riordan, C. G. Spectroscopic and Chemical Studies of Nickel(II) Hydrides. Inorg. Chem. 28, 1630–1634 (1989).
Huynh, H. V. et al. 13C NMR Spectroscopic Determination of Ligand Donor Strengths Using N-Heterocyclic Carbene Complexes of Palladium(II). Organometallics 28, 5395–5404 (2009).
Cho, J. et al. An Improved Method for The Synthesis of Zirconium (CCC-N-Heterocyclic Carbene) Pincer Complexes and Applications in Hydroamination. Chem. Commun. 5001–5003 (2008).
Flynn, C. M. & Demas, J. N. Synthesis and Luminescence of the Tris(2,2’-Bipyridine)Iridium(III) Ion. J. Am. Chem. Soc. 96, 1959–1960 (1974).
Ayala, N. P. et al. Synthesis, Luminescence and Excited-State Complexes of the Tris(1,10-Phenanthroline)- and Bis(Terpyridine)-Iridium(III) Cations. J. Am. Chem. Soc. 112, 3837–3844 (1990).
Wunschel, K. R. & Ohnesorge, W. E. Luminescence of iridium(III) chelates with 2,2’-bipyridine and with 1,10-phenanthroline. J. Am. Chem. Soc. 89, 2777–2778 (1967).
Garces, F. O., King, K. A. & Watts, R. J. Synthesis, Structure, Electrochemistry and Photophysics of Methyl-Substituted Phenylpyridine Ortho-Metalated Iridium(III) Complexes. Inorg. Chem. 27, 3464–3471 (1988).
King, K. A. & Watts, R. J. Dual Emission from an Ortho-Metalated Ir(III) Complex. J. Am. Chem. Soc. 1987, 109, 1589–1590 (1987).
Ohsawa, Y. et al. Electrochemistry and Spectroscopy of Ortho-Metalated Complexes of Ir(III) and Rh(III). J. Phys. Chem. 91, 1047–1054 (1987).
Ichimura, K. et al. Excited-state Absorption Spectroscopy of Ortho-Metalated Ir(III) Complexes. J. Phys. Chem. 91, 6104–6106 (1987).
Coombe, V. T. et al. Spectroelectrochemical Studies on Tris(Bipyridyl)Iridium Complexes: Ultraviolet, Visible and Near-Infrared Spectra of the Series [Ir(bpy)3]3+/2+/+/0. Inorg. Chem. 23, 3423–3425 (1984).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Klamt, A. & Schüürmann, G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc., Perkin Trans. 2, 799–805 (1993).
Vargas, V. C. et al. Efficient Route to 1,3-Di-N-imidazolylbenzene. A Comparison of Monodentate vs. Bidentate Carbenes in Pd-Catalyzed Cross Coupling. Org. Lett. 5, 4847–4849 (2003).
Komiya, S. Synthesis of Organometallic Compounds: A Practical Guide (ed. Komiya, S. ) (John Wiley and Sons Ltd, 1997).
Demas, J. N. & Crosby, G. A. The Measurement of Photoluminescence Quantum Yields. A Review. J. Phys. Chem. 75, 991–1024 (1971).
Brouwer, A. M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Reports). Pure Appl. Chem. 83, 2213–2228 (2011).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd edn. (Kluwer Academic/Plenum Publishers, 1999).
CrysAlis, version 1.171.31.8; Oxford Diffraction Ltd.: Oxford, UK (2007).
Sheldrick, G. M. SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinements; University of Göttingen: Göttingen, Germany (1997).
Farrugia, L. J. WinGX. J. Appl. Crystallogr. 32, 837–838 (1999).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 24, 1740–1747 (2003).
Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2012).
Acknowledgements
The work described in this paper was supported by the Hong Kong Research Grants Council (Project No. CityU 103911) and the Special Equipment Grant from the Hong Kong University Grants Committee (SEG_CityU02). We are grateful to Dr. Shek-Man Yiu for X-ray diffraction data collection and Mr. Pak-Kei Pat for technical support.
Author information
Authors and Affiliations
Contributions
L.H.C, H.S.L. and S.W.N. carried out all the experiments and performed the data analysis; D.L.M., C.H.L. and C.Y.W. designed the experiments, analyzed the results and wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chung, LH., Lo, HS., Ng, SW. et al. Luminescent Iridium(III) Complexes Supported by N-Heterocyclic Carbene-based C^C^C-Pincer Ligands and Aromatic Diimines. Sci Rep 5, 15394 (2015). https://doi.org/10.1038/srep15394
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15394
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.