Abstract
Despite the increasing number of studies conducted recently to evaluate the association between HPV infections and the risk of prostate cancer, the results remain inconclusive. Furthermore, the prevalence and distribution of overall and individual HPV types worldwide in prostate cancer has not been reported until now. Therefore, we estimated the prevalence of HPV in prostate cancer by pooling data of 46 studies with 4919 prostate cancer cases, taking into account the heterogeneity of major related parameters, including study region, specimen type, HPV DNA source, detection method, publication calendar period and Gleason score. Moreover, we tested the association of HPV infections with prostate cancer risks by a meta-analysis of 26 tissue-based case-control studies. We found that the prevalence of HPV infection was 18.93% (95% CI = 17.84–20.05%) in prostate cancer cases and most of which were high-risk HPV types (17.73%, 95% CI = 16.52–18.99%). The prevalence varied by region, PCR primers used, publication calendar period and Gleason score. Our study also showed a significantly increased risk of prostate cancer with the positivity of overall HPV detected in prostate tissues (OR = 1.79, 95% CI = 1.29–2.49) and revealed the geographic variation of association strength (P < 0.001). In conclusion, HPV infections may contribute to the risk of prostate cancer.
Similar content being viewed by others
Introduction
Human papillomavirus (HPV) is most commonly transmitted through sexual activity. Carcinogenetic types of HPV, such as HPV 16 and 18, have been proved to be a necessary cause of invasive cervical cancer1. Studies have also suggested possible links between HPV infections and other female cancers, such as vulva2, vagina2 and breast3. In addition to cancers specific to females, HPV has also been shown to be associated with the risk of cancers in male anogenital and urinary sites, such as penis2, anus2 and bladder4.
Studies have found positive associations of prostate cancer with sexual activities and sexually transmitted diseases5. It has been hypothesized that the prostate gland can also be infected by HPV for its anatomic proximity of the anogenital and urinary sites. In 1992, Rabkin et al. reported men with anal cancer, a disease that has been associated with HPV, had an increased risk for developing subsequent prostate cancer6. Then, a series of epidemiological and laboratory studies detected HPV in malignant, benign or normal prostate tissues. However, results on the association between HPV infections and prostate cancer risks remain controversial.
We retrieved published data on HPV prevalence and combined individual studies on the association between HPV infections and prostate cancer risks through a meta-analysis. We further evaluated how influential parameters (such as study region, sample source, detection method and publication calendar period) affected the results from the meta-analysis.
Results
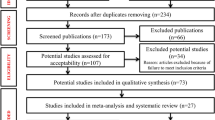
Overall, 465,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 publications were included in the present study, among which 328,9,10,11,12,15,16,17,20,22,23,24,25,26,27,28,29,30,31,33,34,35,36,37,38,44,46,47,48,49,50,51 and 115,7,14,18,19,21,32,39,41,42,43 respectively reported HPV prevalence in tissues and sera of prostate cancer cases, as well as 313,40,45 presented data on both tissues and sera. In addition, one study40 was conducted in both African-American and Italian men and therefore, data in this study were consequently divided into two subgroups when stratified analyses were done by study region. Therefore, 25 countries and/or regions presented data on HPV prevalence in 6365 prostate cancer cases (Supplementary Table 1). Since 5 studies5,7,13,14,31 only presented data of individual HPV type, a total of 4919 and 5547 (Table 1) men from the 6365 prostate cancer cases were used to estimate the prevalence of overall HPV and HPV 16, respectively.
The prevalence of HPV ranged from 0% to 76.92% (Supplementary Table 1) and yielded an average of 18.93% (95% CI = 17.84–20.05%) (Table 1) and 17.18% (95% CI = 15.66–18.81%) after adjusted for study region, specimen type and publication calendar period. Thirteen HPV types (HPV6, 11, 16, 18, 31, 33, 35, 39, 45, 52, 58, 59 and 68) were detected in prostate cancer cases across studies. The prevalence of high-risk HPV types (17.73%, 95% CI = 16.52–18.99%) was much higher than that of low-risk HPV types (4.31%, 95% CI = 2.84–6.25%) (OR = 4.78, 95% CI = 3.20–7.45) (Table 1). Furthermore, the prevalence of HPV clade A9 (17.20%, 95% CI = 16.08–18.37%) was higher than that of HPV clade A7 (6.60%, 95% CI = 5.92–7.34%) and clade A10 (3.44%, 95% CI = 2.17–5.17%) (Table 1) (ORA9/A7 = 2.94, 95% CI = 2.55–3.38 and OR A9/A10 = 5.83, 95% CI = 3.78–9.43, respectively). The 5 most common high-risk HPV types identified, in order of decreasing prevalence, were HPV16 (13.68%, 95% CI = 12.89–14.62%), HPV31 (11.82%, 95% CI = 9.73–14.17%), HPV33 (8.39%, 95% CI = 7.26–9.63%), HPV18 (6.60%, 95% CI = 5.92–7.34%) and HPV58 (3.55%, 95% CI = 1.16–8.08%). HPV11 and HPV6 were also common in prostate cancer with the prevalence of 2.34% (95% CI = 1.29–3.90%) and 1.02% (95% CI = 0.28–2.60%) (Table 1). Further analysis showed the prevalence of all the individual HPV types based on sera was consistently higher than that on tissues but HPV 16. HPV 16 was the most common type in tissues but ranked the third in sera. Meanwhile, HPV 18 was the second common type when detected in tissues but ranked the sixth in sera (Fig. 1).
After stratified by study region, we found that overall HPV prevalence in prostate cancer cases was highest in Africa (68.29%, 95% CI = 61.45–74.60%) with only one study, followed by Asia (20.25%, 95% CI = 16.74–24.13%) and Latin America (18.63%, 95% CI = 12.94–25.52%) (Table 1). The HPV prevalence in prostate cancer cases was significantly higher (OR = 1.29, 95% CI = 1.03–1.63) from studies published in 2000–2015 (19.43%, 95% CI = 18.25–20.65%) than those in 1990–1999 (15.74%, 95% CI = 13.06–18.73%) (Table 1). In addition, the cancer cases with high Gleason score (≥7) had higher (OR = 1.40, 95% CI = 1.12–1.75) HPV prevalence (18.70%, 95% CI = 16.28–21.32%) than those with low Gleason score (<7, 14.10%, 95% CI = 12.42–15.91%) (Table 1).
For studies with HPV DNA detection in prostate cancer tissues, there was no significant difference of HPV prevalence between using fresh (17.13%, 95% CI = 14.04–20.59%) and fixed tissues (16.71%, 95% CI = 14.18–19.47%) (OR = 1.03, 95% CI = 0.76–1.39) (Table 2). With respect to HPV detection methods in prostate cancer tissues, the broad spectrum PCR primers, type–specific PCR primers and both combined, as well as non–PCR methods, namely in situ hybridization (ISH), immunohistochemistry and HC2, were used. HPV prevalence was 17.72% (95% CI = 15.84–19.72%) when PCR-based method was used but only 5.36% (95% CI = 1.12–14.87%) when non-PCR-based method was used. Further analysis showed that the type-specific PCR primers were generally demonstrated to be more efficient with a detection rate of 27.34% (95% CI = 22.28–32.86%) (Table 2). Meanwhile, the HPV prevalence was again significantly higher (OR = 1.70, 95% CI = 1.12–2.58) in Gleason score ≥7 (22.68%, 95% CI = 18.16–27.73%) than those in Gleason score <7 (14.73%, 95% CI = 11.20–18.86%), when HPV DNA was detected in tissues (Table 1).
Thirty eight case-control studies8,9,10,12,13,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 with 4741 prostate cancer cases and 7074 controls were retrieved to estimate overall HPV and prostate cancer risks, among which 818,19,21,32,39,41,42,43 and 288,9,10,12,13,16,17,20,22,23,24,25,26,27,28,29,30,35,36,37,38,44,46,47,48,49,50,51 case-control studies respectively used HPV serology and DNA detection in prostate tissues, as well as 240,45 studies used both of them. We additionally excluded 416,25,40,50 studies because of the absence of HPV in both cases and controls. This resulted in 34 case-control8,9,10,12,13,17,18,19,20,21,22,23,24,26,27,28,29,30,32,35,36,37,38,39,41,42,43,44,45,46,47,48,49,51 studies with 4540 prostate cancer cases and 6892 controls in our analysis for the association between overall HPV infections and prostate cancer risks, among which 818,19,21,32,39,41,42,43 and 258,9,10,12,13,17,20,22,23,24,26,27,28,29,30,35,36,37,38,44,49,51 case-control studies respectively used HPV serology and DNA detection in prostate tissues, as well as 1 study45 used both of them (Table 3).
Overall, infection of any type of HPV was significantly associated with an increased risk of prostate cancer (OR = 1.32, 95% CI = 1.12–1.55) with significant between-studies heterogeneity (Q = 73.87, P<0.001). However, the statistically significant association was absent for studies based on sera (OR = 1.03, 95% CI = 0.95–1.12) while persisted in studies based on tissues (OR = 1.79, 95% CI = 1.29–2.49). Hence, the following analyses focused on 26 studies8,9,10,12,13,17,20,22,23,24,26,27,28,29,30,35,36,37,38,44,49,51 with 1218 prostate cancer cases and 1346 controls with HPV DNA detection in tissues. According to the result of the heterogeneity test (Q = 56.29, P < 0.001), the random-effect model was chosen to evaluate the pooled OR (Fig. 2 and Table 3). Generally, there was a significantly increased prostate cancer risk in relation to infection of any type of HPV (OR = 1.79, 95% CI = 1.29–2.49) (Fig. 2 and Table 3). Egger’s and Begg’s tests, which were used to indicate publication bias, proved to be insignificant (P = 0.133 and 0.261, respectively). In subgroup analysis, we found the geographic heterogeneity of the magnitude of association estimates between overall HPV infections and prostate cancer risks (P for between-strata heterogeneity < 0.001) and revealed that the increased risk of prostate cancer with HPV infections was evident in Europe (OR = 1.95, 95% CI = 1.35–2.81), Asia (OR = 2.82, 95% CI = 1.89–4.21) and Latin America (OR = 5.76, 95% CI = 2.25–14.76). However, there was no significant heterogeneity for OR estimates across the other subgroups.
Discussion
Epidemiological and biological studies have now conclusively proved that a variety of infectious agents are the major causes of cancers worldwide52. In the last two decades, at least six different viruses have been linked to the development of specific types of human cancers. HPV, one of the most important infectious agents, has been shown to be linked to penile cancer2, arousing research interests in male genital and urinary systems. The prostate inflammation resulting from sexually transmitted infections in the course of carcinogenesis has been speculated53.
Three meta-analyses have been published on the correlation between HPV infections and the risk of prostate cancer54,55,56, but the results were not consistent. The first analysis in 200555 with 10 studies found a significantly increased risk of prostate cancer in relation to HPV infections (OR = 1.39, 95% CI = 1.12–2.06) by combining studies in tissues and sera together. The latter two studies in 201154 and 201556, which mainly focused on the infection of the most common oncogenic types (HPV 16 and/or HPV 18) in relation to prostate cancer, found that the overall risk of prostate cancer was not significantly increased by either HPV 16 (OR = 1.09, 95% CI = 0.97–1.23) or HPV 18 (OR = 1.05, 95% CI = 0.89–1.24) infection when HPV was detected in sera and tissues combined54, but significantly increased when HPV DNA detected in prostate tissues54,56.
Whether HPV infections are associated with increased risk of prostate cancer and if so, whether the bio-samples used for detection would affect the results still need to be answered. In addition, although positive associations have been reported in previous meta-analyses, no study, until today, has comprehensively accessed the type-specific prevalence of HPV infections in prostate cancer by region, detection method and publication year, which might provide supportive information for the wider use of HPV vaccine in addition to its use in cervical cancer prevention. Hence, in our present study, with the updated data and more detailed analyses, we analyzed the relationship between HPV infections and the risk of prostate cancer, particularly by detection method and provided evidence for the link between HPV infections and increased risk of prostate cancer. The link was prominent in studies with HPV detected in tissues. Furthermore, we revealed the geographic variations in the association strengths and emphasized other methodological parameters (e.g., detection method) in further analyses that have never been shown in the previous studies.
In general, the prevalence of overall and individual HPV types was higher when detected in sera than in tissues except HPV 16. The distribution of individual types also varied despite bio-samples used in HPV detection (Fig. 1). This suggested that the HPV prevalence in sera was not well corresponding to that in tissues. The higher prevalence in sera might be due to HPV infections from anatomical sites other than prostate. The results also suggested the caveat of sero-epidemiological studies on HPV detection, such as the indistinguishable origins of the antibody from other mucosal sites of the body and the complex link between seropositivity and historical infections and current disease status. Hence, further analyses in prostate cancer tissues merit more attention.
Further analysis focused on cancer tissues showed that PCR-based method with the type-specific primer was more suitable in HPV DNA detection in prostate cancer cases. Comparing with the low detection rate of HPV DNA when PCR amplification of long DNA fragments in the L1 gene is adopted, HPV type-specific primers are usually designed to amplify shorter sequences of HPV DNA and might be more sensitive to detect HPV DNA sequences4. Therefore, it is possible that the detection rate for HPV using HPV type-specific PCR primers may be higher than other PCR methods. On the other hand, type-specific PCR based method for HPV detection in prostate tissues might be more useful, partly due to the low copy numbers of HPV DNA in prostate cancer tissues.
Our study also suggested a moderate geographical variation in HPV prevalence and association strengths with prostate cancer. Meta-analyses on the prevalence of cervical HPV DNA worldwide showed a higher HPV detection rate in Africa and a lower prevalence in North America and Europe57. So higher HPV prevalence in prostate cancers in Africa (68.29%, 95% CI = 61.45–74.60%) is expected. Meanwhile, the risk of prostate cancer by HPV infection was highest in Latin America (OR = 5.76, 95% CI = 2.25–14.76) and Asia (OR = 2.82, 95% CI = 1.89–4.21) (no cases-control comparison for mem with Africa origin). Since Asia has the lowest prostate cancer incidence and mortality, this may due to the moderate risk magnitude and the complex risk profiles (e.g., hygienic habits, sexual and smoking behaviors) for prostate cancer.
The prevalence of HPV was higher (OR = 1.29, 95% CI = 1.03–1.63) in studies published in 2000–2015 (19.43%, 95% CI = 18.25–20.65%) than those in 1990–1999 (15.74%, 95% CI = 13.06–18.73%). The increased prevalence is expected and it is primarily due to improved HPV detection protocols. Since the majority of histological type of prostate cancer is adenocarcinoma, it has been hypothesized that HPV 18 to be more predominant than HPV 16 as that in cervical adenocarcinoma58, which was similar to the HPV type distribution in breast carcinoma4. However, the present study showed that HPV 16 was the most common type among all types included. It also should be noted that the prevalence of clade 10 (3.94%, 95% CI = 1.82–7.36%) was lower either than HPV 6 (16.35%, 95% CI = 9.82–24.88%) or HPV 11 (8.13%, 95% CI = 5.43–11.61%) in sera. The reason was that the data on prevalence of clade 10 could not be extracted from 2 publications13,59, which only presented data of individual HPV types in case of the occurrence of multiple infections. However, these 2 studies13,59 presented relatively higher prevalence of HPV 6 (24.1% and 18.9%, respectively) and 11 (12.5% and 18.9%, respectively) in sera. Thus, the prevalence of HPV clade 10 in sera was under-estimated in the present study.
In addition to the prostate, HPV are also discussed in the pathogenesis of anogenital and urinary cancers, including penis, anus, bladder, testis and renal cancer. HPV appears not to play a major causative role in renal60,61 and testicular carcinogenesis62 for the failure detection of HPV DNA in cancer cases. On the contrary, there is sufficient evidence in humans for the carcinogenicity of certain HPV types, (i.e. HPV 16 and 18) associated anal and penile cancers2. About 90% of anal squamous cell cancers occur in individuals with detectable HPV infections63. Of those, HPV 16 and/or HPV 18 are detectable in more than 90% of cases63. Although the etiology of penile cancer is still unclear, approximately 40% of all penile tumors are thought to be attributable to HPV infections2. A quantitative review of studies that used PCR methods for HPV DNA detection found HPV presented in 45.4% of invasive penile tumors after adjusting for PCR primer, histology subtype and year and geographical location of the study64. However, HPV infections should be kept in mind regarding cases of bladder cancer and prostate cancer. The prevalence of HPV in prostate cancer cases (17.18%) was lower than that in the anal and penile cancers, while it is similar to the HPV prevalence (16.88%) in bladder cancer cases (most occurred in males) reported in our previous meta-analysis4.
Very limited studies exploring the association between HPV infections and main clinical features of prostate cancer, e.g., cancer types and prostate-specific antigen (PSA) levels, made it difficult to do further sub-analysis by these features in the present meta-analysis. Most of the cancer cases were adenocarcinoma. Only one study14 showed that sero-positivity of HPV 18 was associated with 92% increased risk of adenocarcinoma (OR = 2.92, 95% CI = 1.15–7.38) and the association was slightly attenuated (OR = 2.59, 95% CI = 1.17–5.75) when all histologic tumor types were included (127 adenocarcinoma, 14 unspecified carcinoma and 1 transitiocellular carcinoma). Since 2 case-control studies24,38 reported the null association of HPV infections to PSA levels, the prevalence of HPV might be irrelevant to PSA levels based on the present evidence. However, this present meta-analysis indicated a higher prevalence of HPV infections in high-grade prostate cancer (Gleason score ≥7). Due to the retrospective temporality in case-control studies, whether HPV infections precede prostate cancer carcinogenesis or tumor environment is amiable for HPV invasion needs to be confirmed in prospective studies. Moreover, no study has explored the association between HPV infections and hormone response in prostate cancer till today.
The mechanism of HPV infections and prostate cancer development is far from clear. It has been proposed that exposure to environmental factors such as infectious agents and dietary carcinogens and hormonal imbalances lead to injury of the prostate gland and to the development of chronic inflammation and regenerative ‘risk factor’ lesions, referred to as proliferative inflammatory atrophy (PIA)65.Two meta-analyses with the statistically significant evidence of the association between prostatitis and prostate cancer66,67 also suggest that inflammation resulting from infections may be one mechanism for prostate cancer carcinogenesis. Although a case-control study has showed that HPV infections are not related with prostatitis-related symptoms when urethral swab was used for HPV detection68, another study has showed that HPV was identified in the prostatic secretions from patients with type III prostatitis and might be associated with the degree of intraprostatic inflammation69. Whether HPV infections associated with the risk of prostate cancer is mediated by chronic prostatic inflammation which leads to initiation and progression of prostate cancer, needs further functional research.
Until today, prostate cancer is not clearly linked to any preventable risk factors70. Although the causal involvement of HPV in prostate carcinogenesis is still a matter of controversial debate, the association of HPV infections with prostate cancer, if substantiated, would be unexpectedly good news for cancer prevention14.
Conclusions
Generally, the present study suggested the link between HPV infections and prostate cancer, though the risk estimates varied by study region. Furthermore, as we are aware, it is the first study to summarize the HPV prevalence and the distribution of individual oncogenic types worldwide. Our study highlighted the importance of detection methods in studies of HPV infections and prostate cancer. Considering the great variation in HPV prevalence and the risk estimates by the influential parameters, multi-center large-scale prospective studies are needed. In addition, the etiological and biology functional researches on HPV infections and prostate cancer are necessary.
Materials and Methods
Study selection
We used Medline to search for relevant articles published from January 1989 to May 2015 using the MeSH terms “Papillomavirus”, “Human” and “Prostate cancer”. We also evaluated citations in retrieved articles. The work flow is shown in the Supplementary Figure 1. We tried to include all studies on HPV DNA or antibodies detected in biopsy tissues or sera. We included the most recent study when multiple reports were published with substantial overlaps26,45. The exclusion criteria were as follows: (i) studies on immunosuppression patients, for example, patients after renal and cardiac transplantation; (ii) case reports; (iii) publications not in English; (iv) studies without extractable data from the original article.
Data extraction
Two reviewers (Lin Yang and Shuanghua Xie) independently extracted data from selected articles according to a standard form created a priori for this study. Disagreement was resolved by consensus. For each study included, the following information was extracted: first author, year of publication, country of origin, specimens type (tissue or serum), HPV DNA source (fixed or fresh), detection method (PCR or not and types of primers), sample size, HPV prevalence overall by disease status (case or control) and type-specific: HPV 6, 11, 16, 18, 31, 33, 35, 39, 45, 52, 58, 59 and 68) and matching criteria if controls were present. When HPV was assessed in both prostate tissues and sera, only the results obtained from tissues was used13,40,45. Detailed information on all included studies was presented in supplementary Table 1.
Gleason score of prostate cancer cases was also extracted. The majority of studies classified prostate cancers into two subgroups as high (≥7) and low (<7) Gleason scores. However, 2 studies32,34 categorized Gleason score into 2–4, 5–7 and 8–10. We collapsed the two lower categories in to the group of low Gleason score in the present meta-analysis. Additionally, data of HPV infections and Gleason score in one study29 was excluded because the classification of Gleason score was not compatible with the other studies and the data could not be merged.
Statistical Analyses
We described characteristics of included studies and calculated prevalence and its 95% confidence intervals of overall, clade-specific (clade A7: HPV 18 and 39; clade A9: HPV 16, 31, 33, 35, 52, 58; clade A10: HPV 6 and 11) and type–specific HPV prevalence (11 high-risk HPV types: HPV 16, 18, 31, 33, 35, 39, 45, 52, 58, 59 and 68; 2 low-risk HPV types: HPV 6 and 11) in total prostate cancers and by detection method. An unconditional logistic regression model was used to adjust and compare the HPV prevalence by the influential parameters including study region, specimen type and published year.
We also pooled risk estimates for HPV infections and prostate cancer risk using included case-control studies. A fix-effect or random-effect model was used to pool the data, based on the Mantel-Haenszel method and the DerSimonian and Laird method, respectively. These two models provide similar results when between-studies heterogeneity is absent; otherwise, random-effect model is more appropriate. Between-studies heterogeneity test was performed by using the χ2-based Q test and the heterogeneity was considered significant if P < 0.05. Publication bias was evaluated using Egger’s linear regression asymmetry test71 and Begg’s rank correlation test72. All analyses were performed using the Stata statistical software (version 11.0, StataCorp, College Station, TX).
Additional Information
How to cite this article: Yang, L. et al. Worldwide Prevalence of Human Papillomavirus and Relative Risk of Prostate Cancer: A Meta-analysis. Sci. Rep. 5, 14667; doi: 10.1038/srep14667 (2015).
References
Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 90, 1–636 (2007).
Humans, I. W. G. o.t.E.o.C.R.t. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 90, 1–636 (2007).
Li, N. et al. Human papillomavirus infection and sporadic breast carcinoma risk: a meta-analysis. Breast Cancer Res Treat 126, 515–520 (2011).
Li, N. et al. Human papillomavirus infection and bladder cancer risk: a meta-analysis. J Infect Dis 204, 217–223 (2011).
Huang, W. Y. et al. Sexually transmissible infections and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 17, 2374–2381 (2008).
Rabkin, C. S., Biggar, R. J., Melbye, M. & Curtis, R. E. Second primary cancers following anal and cervical carcinoma: evidence of shared etiologic factors. Am J Epidemiol 136, 54–58 (1992).
Adami, H. O., Kuper, H., Andersson, S. O., Bergstrom, R. & Dillner, J. Prostate cancer risk and serologic evidence of human papilloma virus infection: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 12, 872–875 (2003).
Aghakhani, A. et al. The role of human papillomavirus infection in prostate carcinoma. Scand J Infect Dis 43, 64–69 (2011).
Anderson, M. et al. Analysis of prostate tissue DNA for the presence of human papillomavirus by polymerase chain reaction, cloning and automated sequencing. J Med Virol 52, 8–13 (1997).
Anwar, K. et al. Presence of ras oncogene mutations and human papillomavirus DNA in human prostate carcinomas. Cancer Res 52, 5991–5996 (1992).
Balis, V. et al. Prevalence of BK virus and human papillomavirus in human prostate cancer. Int J Biol Markers 22, 245–251 (2007).
Carozzi, F. et al. Association of human papillomavirus with prostate cancer: analysis of a consecutive series of prostate biopsies. Int J Biol Markers 19, 257–261 (2004).
Chen, A. C. et al. Human papillomavirus in benign prostatic hyperplasia and prostatic adenocarcinoma patients. Pathol Oncol Res 17, 613–617 (2011).
Dillner, J. et al. Sero-epidemiological association between human-papillomavirus infection and risk of prostate cancer. Int J Cancer 75, 564–567 (1998).
Effert, P. J., Frye, R. A., Neubauer, A., Liu, E. T. & Walther, P. J. Human papillomavirus types 16 and 18 are not involved in human prostate carcinogenesis: analysis of archival human prostate cancer specimens by differential polymerase chain reaction. J Urol 147, 192–196 (1992).
Gazzaz, F. S. & Mosli, H. A. Lack of detection of human papillomavirus infection by hybridization test in prostatic biopsies. Saudi Med J 30, 633–637 (2009).
Ghasemian, E. et al. Evaluation of human papillomavirus infections in prostatic disease: a cross-sectional study in Iran. Asian Pac J Cancer Prev 14, 3305–3308 (2013).
Hayes, R. B. et al. Sexual behaviour, STDs and risks for prostate cancer. Br J Cancer 82, 718–725 (2000).
Hisada, M. et al. Human papillomavirus antibody and risk of prostate cancer. JAMA 283, 340–341 (2000).
Ibrahim, G. K. et al. Detection of human papillomavirus in the prostate by polymerase chain reaction and in situ hybridization. J Urol 148, 1822–1826 (1992).
Korodi, Z. et al. Human papillomavirus 16, 18 and 33 infections and risk of prostate cancer: a Nordic nested case-control study. Cancer Epidemiol Biomarkers Prev 14, 2952–2955 (2005).
Kuczyk, M., Serth, J., Machtens, S. & Jonas, U. Detection of viral HPV 16 DNA in prostate cancer and benign prostatic hyperplasia by quantitative PCR-directed analysis. Prostate Cancer Prostatic Dis 3, S23 (2000).
Leiros, G. J. et al. Detection of human papillomavirus DNA and p53 codon 72 polymorphism in prostate carcinomas of patients from Argentina. BMC Urol 5, 15 (2005).
Martinez-Fierro, M. L. et al. Identification of viral infections in the prostate and evaluation of their association with cancer. BMC Cancer 10, 326 (2010).
Masood, S., Rhatigan, R. M., Powell, S., Thompson, J. & Rodenroth, N. Human papillomavirus in prostatic cancer: no evidence found by in situ DNA hybridization. South Med J 84, 235–236 (1991).
McNicol, P. J. & Dodd, J. G. High prevalence of human papillomavirus in prostate tissues. J Urol 145, 850–853 (1991).
Michopoulou, V. et al. Detection of human papillomavirus (HPV) DNA prevalence and p53 codon 72 (Arg72Pro) polymorphism in prostate cancer in a Greek group of patients. Tumour Biol 35, 12765–12773 (2014).
Mokhtari, M., Taghizadeh, F. & Hani, M. Is prostatic adenocarcinoma in a relationship with Human Papilloma Virus in Isfahan -Iran. J Res Med Sci 18, 707–710 (2013).
Moyret-Lalle, C. et al. ras, p53 and HPV status in benign and malignant prostate tumors. Int J Cancer 64, 124–129 (1995).
Noda, T. et al. Detection of human papillomavirus (HPV) DNA in archival specimens of benign prostatic hyperplasia and prostatic cancer using a highly sensitive nested PCR method. Urol Res 26, 165–169 (1998).
Rogler, A. et al. P53 codon 72 (Arg72Pro) polymorphism and prostate cancer risk: association between disease onset and proline genotype. Pathobiology 78, 193–200 (2011).
Rosenblatt, K. A., Carter, J. J., Iwasaki, L. M., Galloway, D. A. & Stanford, J. L. Serologic evidence of human papillomavirus 16 and 18 infections and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 12, 763–768 (2003).
Rotola, A. et al. Presence and physical state of HPV DNA in prostate and urinary-tract tissues. Int J Cancer 52, 359–365 (1992).
Saad, F., Gu, K., Jean-Baptiste, J., Gauthier, J. & MesMasson, A. M. Absence of human papillomavirus sequences in early stage prostate cancer. Can J Urol 6, 834–838 (1999).
Salehi, Z. & Hadavi, M. Analysis of the codon 72 polymorphism of TP53 and human papillomavirus infection in Iranian patients with prostate cancer. J Med Virol 84, 1423–1427 (2012).
Serth, J., Panitz, F., Paeslack, U., Kuczyk, M. A. & Jonas, U. Increased levels of human papillomavirus type 16 DNA in a subset of prostate cancers. Cancer Res 59, 823–825 (1999).
Silvestre, R. V. et al. Low frequency of human papillomavirus detection in prostate tissue from individuals from Northern Brazil. Mem Inst Oswaldo Cruz 104, 665–667 (2009).
Singh, N. et al. Implication of high risk human papillomavirus HR-HPV infection in prostate cancer in Indian population–a pioneering case-control analysis. Sci Rep 5, 7822 (2015).
Sitas, F. et al. The relationship between anti-HPV-16 IgG seropositivity and cancer of the cervix, anogenital organs, oral cavity and pharynx, oesophagus and prostate in a black South African population. Infect Agent Cancer 2, 6 (2007).
Strickler, H. D. et al. A multifaceted study of human papillomavirus and prostate carcinoma. Cancer 82, 1118–1125 (1998).
Strickler, H. D. et al. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur J Cancer Prev 7, 305–313 (1998).
Sutcliffe, S. et al. Plasma antibodies against Chlamydia trachomatis, human papillomavirus and human herpesvirus type 8 in relation to prostate cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 16, 1573–1580 (2007).
Sutcliffe, S. et al. Human papillomavirus types 16, 18 and 31 serostatus and prostate cancer risk in the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 19, 614–618 (2010).
Suzuki, H. et al. Detection of human papillomavirus DNA and p53 gene mutations in human prostate cancer. Prostate 28, 318–324 (1996).
Tachezy, R. et al. HPV persistence and its oncogenic role in prostate tumors. J Med Virol 84, 1636–1645 (2012).
Terris, M. K. & Peehl, D. M. Human papillomavirus detection by polymerase chain reaction in benign and malignant prostate tissue is dependent on the primer set utilized. Urology 50, 150–156 (1997).
Tu, H., Jacobs, S. C., Mergner, W. J. & Kyprianou, N. Rare incidence of human papillomavirus types 16 and 18 in primary and metastatic human prostate cancer. Urology 44, 726–731 (1994).
Whitaker, N. J. et al. Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate 73, 236–241 (2012).
Wideroff, L. et al. Human papillomavirus DNA in malignant and hyperplastic prostate tissue of black and white males. Prostate 28, 117–123 (1996).
Yow, M. A. et al. Detection of infectious organisms in archival prostate cancer tissues. BMC Cancer 14, 579 (2014).
Zambrano, A., Kalantari, M., Simoneau, A., Jensen, J. L. & Villarreal, L. P. Detection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infections. Prostate 53, 263–276 (2002).
de Martel, C. et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The Lancet. Oncology 13, 607–615 (2012).
Nakai, Y. & Nonomura, N. Inflammation and prostate carcinogenesis. Int J Urol (2012).
Lin, Y. et al. Human papillomavirus 16 or 18 infection and prostate cancer risk: a meta-analysis. Ir J Med Sci 180, 497–503 (2011).
Taylor, M. L., Mainous, A. G., 3rd & Wells, B. J. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med 37, 506–512 (2005).
Bae, J. M. Human papillomavirus 16 infection as a potential risk factor for prostate cancer: an adaptive meta-analysis. Epidemiology and Health 37 (2015). 10.4178/epih/e2015005.
de Sanjose, S. et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 7, 453–459 (2007).
Li, N., Franceschi, S., Howell-Jones, R., Snijders, P. J. & Clifford, G. M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 128, 927–935 (2011).
Hrbacek, J. et al. Serum antibodies against genitourinary infectious agents in prostate cancer and benign prostate hyperplasia patients: a case-control study. BMC Cancer 11, 53 (2011).
Grce, M. et al. Human papillomaviruses are not associated with renal carcinoma. Anticancer research 17, 2193–2196 (1997).
Khoury, J. D. et al. Landscape of DNA virus associations across human malignant cancers: analysis of 3,775 cases using RNA-Seq. Journal of virology 87, 8916–8926 (2013).
Bertazzoni, G. et al. Lack of evidence for an association between seminoma and human papillomavirus infection using GP5+/GP6+ consensus primers. J Med Virol 85, 105–109 (2013).
Parkin, D. M. & Bray, F. Chapter 2: The burden of HPV-related cancers. Vaccine 24 Suppl 3, S3/11–25 (2006).
Backes, D. M., Kurman, R. J., Pimenta, J. M. & Smith, J. S. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer causes & control: CCC 20, 449–457 (2009).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nature reviews. Cancer 7, 256–269 (2007).
Dennis, L. K., Lynch, C. F. & Torner, J. C. Epidemiologic association between prostatitis and prostate cancer. Urology 60, 78–83 (2002).
Jiang, J. et al. The role of prostatitis in prostate cancer: meta-analysis. PloS one 8, e85179 (2013).
Bartoletti, R. et al. Human papillomavirus infection is not related with prostatitis-related symptoms: results from a case-control study. International braz j urol: official journal of the Brazilian Society of Urology 40, 247–256 (2014).
Xiao, J. et al. Atypical microorganisms in expressed prostatic secretion from patients with chronic prostatitis/chronic pelvic pain syndrome: microbiological results from a case-control study. Urologia internationalis 91, 410–416 (2013).
Cogliano, V. J. et al. Preventable exposures associated with human cancers. Journal of the National Cancer Institute 103, 1827–1839 (2011).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Acknowledgements
This work is supported in part by the scholarship from China Scholarship Council (CSC) and Scientific Research Foundation for Returned Scholars, Ministry of Education of China for the conduct of the research and preparation of the article. This work was supported in part by the National Natural Science Fund from the National Natural Science Foundation of China (grant no. 81172757 and 81373079), Beijing Nova Program (grant no.xx2012067) and Natural Science Foundation of Beijing Municipality (grant no.7123225). Cindy Ke Zhou was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute.
Author information
Authors and Affiliations
Contributions
This manuscript has not been submitted to any other journals for publication. N.L. design of the study, L.Y., S.X., X.F. and Y.C. collected and analyzed data, L.Y. drafted the article, T.Z., M.D., C.K.Z., Z.H., N.L. and D.H. revised it critically for important intellectual content. All of the authors gave final approvals of the version submitted. There are no commercial associations that might pose a conflict of interest with the submitted article.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, L., Xie, S., Feng, X. et al. Worldwide Prevalence of Human Papillomavirus and Relative Risk of Prostate Cancer: A Meta-analysis. Sci Rep 5, 14667 (2015). https://doi.org/10.1038/srep14667
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14667
This article is cited by
-
Human papillomavirus and prostate cancer: systematic review and meta-analysis
Scientific Reports (2023)
-
Urinary microbiota and prostatic diseases: the key for the lock? A systematic review
Prostate Cancer and Prostatic Diseases (2023)
-
Detection of high-risk Human Papillomavirus in prostate cancer from a UK based population
Scientific Reports (2023)
-
A matched case-control study in Taiwan to evaluate potential risk factors for prostate cancer
Scientific Reports (2023)
-
Evidence for a causal role by human papillomaviruses in prostate cancer – a systematic review
Infectious Agents and Cancer (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.