Abstract
Osteoarthritis (OA) and dementia are prevalent causes of disability in geriatric patients. To date, information on the temporal correlation between these progressive diseases and the risk of dementia in patients with OA is limited. This retrospective population-based 4-year cohort study investigated the risk of dementia in patients with OA. We performed a case-control matched analysis by using the Taiwan Longitudinal Health Insurance Database 2005. Patients were selected on the basis of International Classification of Diseases, Ninth Revision, Clinical Modification codes for OA between January 1, 2004 and December 31, 2007. The prevalence and the adjusted hazard ratio (HR) of dementia in patients with and without OA were estimated. The OA cohort comprised 35,149 patients and the non-OA cohort (comparison cohort) comprised 70,298 patients (1:2). The incidence of dementia was 21.7 per 10,000 person-years in the OA cohort and 14.7 per 10,000 person-years in the non-OA cohort. The HR for dementia during the follow-up period was 1.33 (95% confidence interval [CI], 1.17−1.50, P < 0.001) for patients with OA. The adjusted HR for dementia was 1.25 (95% CI, 1.10−1.43, P < 0.001) for patients with OA. The results of this study indicated that OA is an independent risk factor for dementia.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) of the knee is one of the leading causes of disability among noninstitutionalized elderly adults1. Consequences of severe knee OA, including loss of mobility and limited daily activities, affect individuals and society economically. The World Health Organization (WHO) Global Burden of Disease Study, conducted in 21 epidemiological regions worldwide, reported a 26.6% increase in the burden of knee OA from 1990 to 20102. Although nonsteroidal antiinflammatory therapy, exercise and control of body weight remain the primary treatment options, there is no effective treatment for severe knee and hip OA and total joint replacement is typically the final and only effective solution for relieving pain and disability.
Dementia, an age-related disease characterised by a gradual decline in mental abilities that affects reasoning, memory and other cognitive functions, is one of the major causes of mortality in patients with knee and hip OA3,4. The incidence of dementia is 10% in people aged older than 65 years and approximately 20% in people aged older than 80 years. The prevalence of dementia in people older than 65 years was estimated to be 7.29% in Western Europe, 5.65% in South Asia, 8.50% in Latin America and 4.98% in East Asia5. The prevalence of neurodegenerative disorders is increasing because of changes in the population demographics. As of 2010, 35.6 million people worldwide were diagnosed with dementia and the numbers were estimated to nearly double every 20 years, to 65.7 million in 2030 and 115.4 million in 20505. Health care costs can impose a substantial economic burden on patients with dementia6. Moreover, dementia causes a decline in cognitive and social functioning that interferes with independent living, affecting the individual and increasing the burden on family members and caregivers7. Therefore, preventing dementia in geriatric patients is crucial.
The correlation between OA and dementia has not been investigated thoroughly. An animal study reported that OA can accelerate the progression of and exacerbate Alzheimer’s disease (AD) by causing peripheral inflammation, triggering neuroinflammation and inducing AD pathogenesis8. A previous study reported that primary total knee replacement can improve not only the knee function but also the mental health of patients with OA and mental disability. We hypothesized that OA can be an accelerating factor in the development of dementia. However, information regarding the occurrence of dementia after the onset of OA is limited. No large-scale nationwide population study has investigated OA and the risk of dementia. Therefore, we conducted a retrospective population-based case-controlled cohort study to investigate the risk of dementia among patients with OA.
Methods
Study design and study population
Data source
This retrospective population-based cohort study was conducted using case-control matched analysis. The patient data were obtained from the Taiwan Longitudinal Health Insurance Database 2005 (LHID2005). In March 1995, the National Health Insurance programme was established in Taiwan and currently has over 25 million enrolees, covering more than 99% of the population of Taiwan. The LHID2005 comprises claims data for 1,000,000 beneficiaries randomly sampled from the Registry for Beneficiaries of the National Health Insurance Research Database (NHIRD), which contains 25.68 million claims files. The claims files contain information on ambulatory care; inpatient care; pharmacy use; date of service; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes; and claimed medical expenses. The National Health Research Institutes (NHRI) manages the claims data and provides scrambled random identification numbers for insured patients to protect patient privacy. Because the NHIRD comprises deidentified secondary data that were analysed anonymously, the need for informed consent in this study was waived.
Study patients
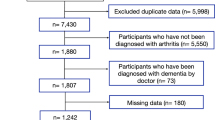
Between January 1, 2004 and December 31, 2007, patients whose ambulatory care claims contained ICD-9-CM code 715 (OA) were identified. For an accurate diagnosis of OA, patients who received at least 5 consistent diagnoses according to the ICD-9-CM in outpatient clinics or a primary diagnosis of OA during hospitalisation within 1 year were selected. Initially, 35,901 OA patients were enrolled in this study. However, 450 patients were excluded because of missing data and 302 OA patients with a previous sustained diagnosis of dementia (ICD-9-CM code 290) were also excluded. The OA cohort comprised 35,149 patients with OA and the non-OA cohort comprised 70,298 patients without OA who were matched with the patients with OA in a 1:2 ratio according to age and sex. Each patient was monitored for as many as 4 years, from the patient’s entry date until the patient received 2 consecutive diagnoses of dementia (ICD-9-CM codes 290, 294 and 331) in outpatient clinics or 1 primary diagnosis during hospitalisation, or until the end of 2011. The database consists of deidentified secondary data and the data were analyzed anonymously; therefore, the need for informed consent was waived.
Baseline comorbidities
Baseline variables, namely age, diabetes mellitus (DM; ICD-9-CM codes 250 and 251), hypertension (ICD-9-CM codes 401−405), hyperlipidaemia (ICD-9-CM codes 272.0−272.4), autoimmune disease (rheumatoid arthritis [RA], ICD-9-CM code 714.0; systemic lupus erythematosus [SLE], ICD-9-CM code 710.0), cerebral vascular accident (CVA; ICD-9-CM codes 430−438), chronic obstructive pulmonary disease (COPD; ICD-9-CM code 496) and Parkinson’s disease (ICD-9-CM code 332), were obtained for all patients.
Statistical analysis
First, we calculated the dementia hazard function for as many as 4 years by using the Cox model to examine the differences in the risk of dementia between the 2 OA and non-OA cohorts, after adjusting for patient physical characteristics, namely age and sex; comorbidities, namely DM, hyperlipidaemia, RA, CVA, COPD and Parkinson’s disease; and the external factor of urbanisation level. To adjust for the hospital cluster random effect, we proposed a frailty model for modelling corrections among the dementia diagnoses of hospital clusters, incorporating a random component for the hazard function.
Let the hazard function for the ith patient in the kth hospital cluster be
 , where h0(t) is the baseline hazard function, x is the vector of the fixed-effect covariance (including OA, age, sex, autoimmune disease, DM, hypertension, hyperlipidaemia, CVA, COPD and Parkinson’s disease), β is the regression coefficient, andτ is the random effect for the hospital cluster. Let τ ~ iidN(0,Δ). The penalised partial log likelihood is
, where h0(t) is the baseline hazard function, x is the vector of the fixed-effect covariance (including OA, age, sex, autoimmune disease, DM, hypertension, hyperlipidaemia, CVA, COPD and Parkinson’s disease), β is the regression coefficient, andτ is the random effect for the hospital cluster. Let τ ~ iidN(0,Δ). The penalised partial log likelihood is
 , where lpartial(β) is the partial likelihood function for the Cox model. Thus, the marginal log likelihood of this shared frailty model is
, where lpartial(β) is the partial likelihood function for the Cox model. Thus, the marginal log likelihood of this shared frailty model is

Using a Laplace approximation, we computed the optimal linear unbiased predictors of β,τ and Δ by maximising the penalised partial log likelihood9,10,11. All data analyses were performed using the SAS statistical package (Version 9.1.3; SAS Institute, Cary, NC, USA). A P value of <0.05 was considered statistically significant.
Results
The OA cohort comprised 35,149 patients with OA and the non-OA cohort comprised 70,298 patients without OA. Women constituted 63.2% in both cohorts and the prevalence of comorbidities was higher in the OA cohort than in the non-OA cohort (Table 1). The incidence of dementia was 21.7 per 10,000 person-years in the OA cohort, whereas it was 14.7 per 10,000 person-years in the non-OA cohort. In patients with OA, the crude hazard ratio (HR) for developing dementia was 1.33 (P < 0.001) and the adjusted HR was 1.25 (P < 0.001; Table 2). Figure 1 shows the Kaplan–Meier hazard curves for dementia in the OA and non-OA cohorts over a 4-year follow-up period. The log-rank test analysis in Fig 1 revealed that patients in the OA cohort had higher hazard rates (HR = 1.24, P < 0.001) than did patients in the non-OA cohort. According to the Cox regression analysis, the HR for dementia during the follow-up period was 1.32 (95% confidence interval [CI], 1.17−1.49) for the OA cohort compared with the non-OA cohort. After adjustment for patient age and sex, DM, hyperlipidaemia, hypertension, coronary heart disease, COPD, stroke and Parkinson’s disease in hospital or clinics, the HR for dementia during the 4-year follow-up period was 1.22 (95% CI, 1.07−1.38) for patients in the OA cohort (Table 3).
Discussion
Our study revealed that OA patients are at a high risk for dementia. This longitudinal cohort study is the first large-scale population-based study to investigate the risk of dementia in patients with OA. A community cohort study of geriatric patients with chronic, painful hip or knee OA reported that uncontrolled symptoms can cause functional disability and fatigue, which in turn lead to a depressed mood and ADL dependence12. In addition, patients with OA exhibit a high coprevalence of other chronic diseases. Among patients with OA, 90% have at least one additional chronic disease, of which cardiovascular diseases are the most common. Weight-bearing activities such as walking can exacerbate the pain and reduce the activity level of patients with OA13. A lack of physical activity may result in poor fitness and a higher risk of cardiovascular disease14,15. Nuesch et al. studied the causes of mortality in patients with OA and reported high mortality rates for all disease-specific causes of death, particularly for deaths associated with cardiovascular diseases and dementia3.
Previous studies have reported the preventive effects of physical activity and exercise on dementia later in later16,17,18,19. We suggest that improving physical activity after joint replacement is crucial in preventing dementia, particularly in geriatric patients with OA. Pain relief and restoration of ambulation function can enable patients to achieve higher levels of physical activity. A prospective cohort study revealed a strong correlation between total daily activity and a lower risk of dementia20. In addition, obesity is a key risk factor for OA, as indicated by the high prevalence of hip and knee OA in overweight patients21. Furthermore, a recent population-based study indicated that high midlife leisure and physical activity levels protect against dementia and AD, particularly in overweight people22. Studies have suggested that physical activity reduces cardiovascular risk by controlling blood pressure and exerting positive effects on brain structures21,23,24,25. Conversely, discontinuing daily activities because of OA can expose patients with OA to a higher risk of dementia.
OA is a slow degenerative disease and the pathogenesis of OA includes loss of articular cartilage with subchondral bone remodelling. Proinflammatory cytokines induce inflammation, which is one of the pathological mechanisms of OA development26. Interleukin-1 (IL-1) and tumour necrosis factor (TNF alpha) are the key proinflammatory cytokines that induce cartilage catabolism in patients with OA26,27,28. Similarly, peripheral inflammation may be associated with an increased risk of dementia. In addition, previous studies have revealed associations between the serum levels of proinflammatory cytokines such as IL-1b, IL-6 and TNF alpha, which all increase the risk of dementia and AD.9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. An animal model study revealed that OA with peripheral inflammation triggers neuroinflammation and subsequently induces AD pathogenesis8. Therefore, we hypothesised that inflammation caused by OA is one of the possible mechanisms of dementia development.
Another possible mechanism through which patients with OA develop dementia is related to the influence of the depression symptoms of OA patients. A meta-analysis reported that patients with a history of depression were highly likely to be diagnosed with dementia later in life32. The prevalence of depression is 18% in patients with arthritis aged older than 45 years in the United States33. Another article stated that approximately 19% of patients with OA experience moderate-to-severe depression, reporting that chronic pain and impairment of daily activities caused by OA play a crucial role in depression34. A previous study revealed that knee joint replacement can improve both mental health and knee function, particularly in patients with OA and a poor preoperative mental health status35. Therefore, we infer that a poor mental status caused by OA is among the risk factors contributing to dementia development.
This study had several limitations that must be addressed. First, the diagnosis of dementia and medical comorbidities was determined using ICD-9-CM codes from the NHRID; however, no information on the accuracy of these codes is available. To reduce the bias caused by the incorrect use of codes, we included only patients who received 5 consecutive diagnoses of OA in outpatient clinics or a primary diagnosis of OA during hospitalisation. Second, information on daily physical activity, individual behaviour and psychological status is not recorded in the NHIRD. These factors are crucial in the development of dementia; however, quantifying these parameters in such a large database is challenging. Finally, the NHIRD does not include data on body weight or the severity and duration of OA. We controlled for age as one of the confounding factors in both cohorts, potentially eliminating this bias because OA is a degenerative disease.
Conclusion
Patients with OA are at a higher risk for dementia than are those without OA. Impaired daily physical activity, OA-induced inflammatory processes and depression are possible mechanisms of dementia development. Multidisciplinary interventions that involve controlling pain and symptoms of depression, preventing the decline of physical function and encouraging participation in leisure activities can be used for OA patients to prevent the development dementia. In the future, studies can focus on the prevention of dementia by controlling the progression of OA symptoms.
Additional Information
How to cite this article: Huang, S.-W. et al. Osteoarthritis Increases the Risk of Dementia: A Nationwide Cohort Study in Taiwan. Sci. Rep. 5, 10145; doi: 10.1038/srep10145 (2015).
References
Guccione, A. A. et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. American journal of public health. 84, 351–358 (1994).
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380, 2163–2196, doi:10.1016/S0140-6736(12)61729-2 (2012).
Nuesch, E. et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. Bmj. 342, d1165, 10.1136/bmj.d1165 (2011).
Rowe, J. W. Health care of the elderly. The New England journal of medicine. 312, 827–835, 10.1056/NEJM198503283121305 (1985).
Europe, A. World Alzheimer Report. London: Alzheimer’s Disease International (2009).
Schaller, S., Mauskopf, J., Kriza, C., Wahlster, P. & Kolominsky-Rabas, P. L. The main cost drivers in dementia: a systematic review. International journal of geriatric psychiatry, 10.1002/gps.4198 (2014).
Feldman, H. et al. The disability assessment for dementia scale: a 12-month study of functional ability in mild to moderate severity Alzheimer disease. Alzheimer disease and associated disorders. 15, 89–95 (2001).
Kyrkanides, S. et al. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. Journal of neuroinflammation. 8, 112, 10.1186/1742-2094-8-112 (2011).
Breslow, N. E. & Clayton, D. G. Approximate inference in generalized linear mixed models. Journal of the American Statistical Association. 88, 9–25 (1993).
Ripatti, S. & Palmgren, J. Estimation of Multivariate Frailty Models Using Penalized Partial Likelihood. Biometrics. 56, 1016–1022 (2000).
Therneau, T. M. & Grambsch, P. M. Modeling Survival Data : Extending the Cox Model New York: Springer-Verlag. (2000).
Hawker, G. A. et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis care & research. 63, 1382–1390, 10.1002/acr.20298 (2011).
Sale, J. E., Gignac, M. & Hawker, G. How “bad” does the pain have to be ? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis and rheumatism. 55, 272–278, 10.1002/art.21853 (2006).
Kodama, S. et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. Jama. 301, 2024–2035, 10.1001/jama.2009.681 (2009).
Gulati, M. et al. The prognostic value of a nomogram for exercise capacity in women. The New England journal of medicine. 353, 468–475, 10.1056/NEJMoa044154 (2005).
Bowen, M. E. A prospective examination of the relationship between physical activity and dementia risk in later life. American journal of health promotion : AJHP. 26, 333–340, 10.4278/ajhp.110311-QUAN-115 (2012).
Sofi, F. et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. Journal of internal medicine. 269, 107–117, 10.1111/j.1365-2796.2010.02281.x (2011).
Ahlskog, J. E., Geda, Y. E., Graff-Radford, N. R. & Petersen, R. C. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic proceedings. 86, 876–884, 10.4065/mcp.2011.0252 (2011).
Scarmeas, N. et al. Physical activity, diet and risk of Alzheimer disease. Jama. 302, 627–637, 10.1001/jama.2009.1144 (2009).
Buchman, A. S. et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 78, 1323–1329, 10.1212/WNL.0b013e3182535d35 (2012).
Lau, E. C. et al. Factors associated with osteoarthritis of the hip and knee in Hong Kong Chinese: obesity, joint injury and occupational activities. American journal of epidemiology. 152, 855–862 (2000).
Tolppanen, A. M. et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. Journal of Alzheimer’s disease : JAD. 38, 201–209, 10.3233/JAD-130698 (2014).
Aarsland, D., Sardahaee, F. S., Anderssen, S., Ballard, C. & Alzheimer’s Society Systematic Review, g. Is physical activity a potential preventive factor for vascular dementia ? A systematic review. Aging & mental health. 14, 386–395, 10.1080/13607860903586136 (2010).
Rovio, S. et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiology of aging. 31, 1927–1936, 10.1016/j.neurobiolaging.2008.10.007 (2010).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 108, 3017–3022, 10.1073/pnas.1015950108 (2011).
Martel-Pelletier, J., Alaaeddine, N. & Pelletier, J. P. Cytokines and their role in the pathophysiology of osteoarthritis. Frontiers in bioscience : a journal and virtual library. 4, D694–703 (1999).
Goldring, M. B., Suen, L. F., Yamin, R. & Lai, W. F. Regulation of Collagen Gene Expression by Prostaglandins and Interleukin-1beta in Cultured Chondrocytes and Fibroblasts. American journal of therapeutics. 3, 9–16 (1996).
Westacott, C. I. & Sharif, M. Cytokines in osteoarthritis: mediators or markers of joint destruction ? . Seminars in arthritis and rheumatism. 25, 254–272 (1996).
Engelhart, M. J. et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of neurology. 61, 668–672, 10.1001/archneur.61.5.668 (2004).
Tan, Z. S. et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 68, 1902–1908, 10.1212/01.wnl.0000263217.36439.da (2007).
Holmes, C. et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 73, 768–774, 10.1212/WNL.0b013e3181b6bb95 (2009).
Ownby, R. L., Crocco, E., Acevedo, A., John, V. & Loewenstein, D. Depression and risk for Alzheimer disease: systematic review, meta-analysis and metaregression analysis. Archives of general psychiatry. 63, 530–538, 10.1001/archpsyc.63.5.530 (2006).
Murphy, L. B., Sacks, J. J., Brady, T. J., Hootman, J. M. & Chapman, D. P. Anxiety and depression among US adults with arthritis: prevalence and correlates. Arthritis care & research. 64, 968–976, 10.1002/acr.21685 (2012).
Rosemann, T. et al. Predictors of depression in a sample of 1,021 primary care patients with osteoarthritis. Arthritis and rheumatism. 57, 415–422, 10.1002/art.22624 (2007).
Clement, N. D., MacDonald, D. & Burnett, R. Primary total knee replacement in patients with mental disability improves their mental health and knee function: a prospective study. The bone & joint journal. 95-B, 360–366, 10.1302/0301-620X.95B3.29563 (2013).
Acknowledgements
This study was based partly on data from the NHIRD provided by the Bureau of National Health Insurance, Department of Health and managed by the NHRI in Taiwan and was supported by the National Science Council of Taiwan under grant number NSC 101-2118-M-031 -001 -MY2.
Author information
Authors and Affiliations
Contributions
S.W.H. conceptualised and designed the study and drafted the article; S.W.H. and H.W.L. analysed and interpreted the data; S.W.H. and C.D.L. critically revised the article for important intellectual content; T.H.L. and H.W.L. provided final approval of the article; L.C.C. provided study materials and patients; H.W.L. offered statistical expertise; and W.T.W. provided administrative, technical and logistical support.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, SW., Wang, WT., Chou, LC. et al. Osteoarthritis Increases the Risk of Dementia: A Nationwide Cohort Study in Taiwan. Sci Rep 5, 10145 (2015). https://doi.org/10.1038/srep10145
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10145
This article is cited by
-
Impact of frailty severity and severe pain on cognitive function for community-dwelling older adults with arthritis: a cross-sectional study in Korea
Scientific Reports (2024)
-
Knee osteoarthritis accelerates amyloid beta deposition and neurodegeneration in a mouse model of Alzheimer’s disease
Molecular Brain (2023)
-
Minimal amount of exercise prevents incident dementia in cognitively normal older adults with osteoarthritis: a retrospective longitudinal follow-up study
Scientific Reports (2023)
-
Nonpharmacological approaches for pain and symptoms of depression in people with osteoarthritis: systematic review and meta-analyses
Scientific Reports (2023)
-
Patterns of change and factors associated with IADL function decline in community-dwelling older adults with arthritis
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.