Abstract
We study the roughening process and morphology transition of organic semiconductor thin film induced by molecular orientation in the model of molecular semiconductor copper hexadecafluorophthalocyanine (F16CuPc) using both experiment and simulation. The growth behaviour of F16CuPc thin film with the thickness, D, on SiO2 substrate takes on two processes divided by a critical thickness: (1) D ≤ 40 nm, F16CuPc thin films are composed of uniform caterpillar-like crystals. The kinetic roughening is confirmed during this growth, which is successfully analyzed by Kardar-Parisi-Zhang (KPZ) model with scaling exponents α = 0.71 ± 0.12, β = 0.36 ± 0.03 and 1/z = 0.39 ± 0.12; (2) D > 40 nm, nanobelt crystals are formed gradually on the caterpillar-like crystal surface and the film growth shows anomalous growth behaviour. These new growth behaviours with two processes result from the gradual change of molecular orientation and the formation of grain boundaries, which conversely induce new molecular orientation, rapid roughening process and the formation of nanobelt crystals.
Similar content being viewed by others
Introduction
Organic electronics is an increasing attractive research field, especially with greatly potential commercialization of organic electronic devices such as organic light-emitting diodes (OLED), organic field-effect transistors (OFETs) and organic photovoltaics (OPVs)1,2,3,4. One of the key components in these devices is the active layer which is composed of organic semiconductor thin film. Controlling their morphology and structure can dramatically optimize the physical properties and accordingly improve the device performance5,6,7. The prerequisite for their controllability is to penetratingly understand the growth mechanism and evolution process of organic semiconductor thin film. Because of the inherent anisotropy of organic molecules, organic thin film growth shows more complex growth scenarios than conventional inorganic film. It normally exhibits its own growth features although sometimes the similar growth behaviour to inorganic thin film could be observed8,9,10,11,12,13,14,15,16,17. Furthermore, organic molecules with specific shapes, for example, rod-like molecule para-sexiphenyl (p-6P) and disk-like molecule metal phthalocyanine (MPc), often display different growth behaviours and mechanisms11,18,19.
In this study, we are dedicated to study the film roughening and morphology transition of organic molecular semiconductor in the model of copper hexadecafluorophthalocyanine (F16CuPc, Fig. 1a), which is an air-stable n-type material and widely used in OFETs and OPVs20,21. The experiments show that the growth of F16CuPc semiconductor thin film has two roughening processes with the thickness, i.e., traditional kinetic roughening and anomalously rapid roughening, accompanying morphology transition. The new growth mechanism supported by both experiments and simulation is unveiled that the gradual change of molecular orientation and grain boundaries results in the rapid roughening and morphology transition and accordingly accelerate the formation of new molecular orientation and nanobelt crystals.
Results and Discussion
The theoretical framework related to the mechanisms of film growth has been established in inorganic systems22 and was also developed to analyze the growth of organic thin films9,14,17,19. Height difference correlation function (HDCF) has been successfully analyzed the film morphological evolution and the scaling exponents reflecting the film growth process and mechanism can be derived. The mean square height difference g(R) = <[h(x1, y1) − h(x2, y2)]> can be obtained based on all pairs of points (x1, y1) and (x2, y2) separated laterally by the distance R = [(x1 − x2)2 + (y2 − y2)2]1/2. The relative magnitudes of R and the correlation length, ξ, can divide the HDCF into two distinct behaviours: (1) R ≪ ξ, g(R) ∝ R2α, where α is the roughness scaling exponent; (2) R ≫ ξ, g(R) = 2σ2, where σ is the surface root-mean-square (RMS) roughness. The parameters ξ and σ are dependent on the film thickness, D and fit the power laws σ ∝ Dβ and ξ ∝ D1/z, where β and z are growth and dynamic scaling exponents, respectively. The ξ at each thickness can be determined by fitting the HDCF to the function g(R) = 2 σ2{1 − exp [−(R/ξ)2α]}. Based on the HDCF analysis, the scaling exponents α, β and z can be obtained. Normally, they obey the scaling equation β ≈ α/z.
Fig. 1 shows the morphology of F16CuPc films with different thicknesses grown on SiO2 substrate. The film morphology takes on an obvious transition from small caterpillar-like crystals to nanobelt crystals with increasing the thickness. As D < 30 nm, the film is composed of uniform caterpillar-like crystals. At D = 30 nm, some light spots can be found on the surface, as indicated by the circle in Fig. 1b. Increasing D, more and more light spots are formed (Fig. 1c). Furthermore, longer nanobelt crystals are also formed out of surface and leave a long gap. It suggests that the longer nanobelt crystals are lying-down on the surface at the beginning and then they stand up when reaching a certain size for relaxing the strains (Fig. 1c and d). More and more nanobelt crystals can form accordingly as D continuously increases and finally form the fibre-like crystal surface (Fig. 1f). At D ≥ 70 nm, it is difficult to image the morphology of F16CuPc films with AFM because of longer standing-up nanobelt crystals and SEM is used to image the films. It obviously indicates that thick films include two layers: caterpillar-like crystal layer and nanobelt crystal layer. The similar growth behaviour could also be found on the ITO substrate23.
Based on the images by AFM (D ≤ 70 nm), the growth and roughening process can be analyzed. The surface RMS roughness, σ, as a function of film thickness, D, is shown in Fig. 2a. The data can be fitted using scaling equation σ ∝ Dβ. The growth-induced surface roughening normally includes kinetic roughening and mound growth. The absolute upper bound on the roughness fits the equation σ = d(D/d)1/2, where d is the molecular size and β is usually 0.5. Analysis of the data for F16CuPc grown on SiO2 shows that β = 0.36 for D < 40 nm, while β = 2.43 for 40 nm ≤ D ≤ 70 nm (Fig. 2 also includes F16CuPc grown on the ITO substrate23, β = 0.12 for D ≤ 30 nm, while β = 3.09 for 30 nm ≤ D ≤ 48 nm. The growth of organic semiconductor thin film is related to the substrate, so the β and critical transition thickness show a little difference). These results suggest that F16CuPc film growth undertakes two different roughening mechanisms for D ≤ 40 nm and 40 nm ≤ D ≤ 70 nm on SiO2 substrate respectively. The dramatic increase in roughness for thick films can not be explained by conventional mound growth.
(a) Logarithmic plots of surface RMS roughness, σ, as a function of film thickness, D, for F16CuPc thin films. The data were obtained from AFM images. The σ values are the average values for scanning areas of 1, 2 and 5 μm2 on the ITO substrate23 and 1.5, 2.5 and 5 μm2 on the SiO2 substrate. (b) Logarithmic plots of averaged g(R) as a function of R with the film thickness D for F16CuPc thin films. The upper inset (b1) is the static roughness scaling exponent, α, as a function of D by fitting the linear part of g(R), where the average α is 0.71. The inset below (b2) is logarithmic plots of the correlation length, ξ, as a function of D, obtained by fitting HDCF to the analytical function g(R) = 2 σ{1 − exp[−(R/ξ)2α]}, where dynamic scaling exponents, z, can be obtained.
Fig. 2b shows the plot of the average HDCF, g(R), as a function of film thickness and an average value of α = 0.71 can be derived. On the other hand, the inverse dynamic scaling exponent 1/z is determined by logarithmic plots of the correlation length, ξ, as a function of D, with fitting HDCF to the analytical function g(R) = 2 σ{1 − exp[−(R/ξ)2α]}. It is obvious that two 1/z values, i.e., 1/z1 = 0.39 and 1/z2 = 7.45, are obtained divided at the critical thickness of 40 nm.
For D ≤ 40 nm, the growth behaviour can be analyzed with normal kinetic roughening and the scaling exponents are consistent with those based on Kardar-Parisi-Zhang (KPZ) model. In KPZ model, α ≈ 2/3, 1/z ≈ 0.3 and β ≈ 0.2; while in our case, α ≈ 0.71, 1/z ≈ 0.39 and β ≈ 0.36. The scaling exponents α, β and z obey scaling equation 1/z ≈ β/α, so 1/zcal ≈ 0.51, which is a little bigger than that obtained by experiment. This discrepancy results from the existence of different potential energy barrier at the step edges of existing islands19.
As the thickness increases and 40 nm ≤ D ≤ 70 nm (for D ≥ 70 nm, we cannot image the morphology of F16CuPc films with AFM because of longer nanobelt crystals and rough surface), the scaling exponents increase dramatically, in which 1/z ≈ 7.45 and β ≈ 2.43 although α is almost the same. The changes of scaling exponents are consistent with the transition of film morphology (Fig. 1b–d). This film growth behaviour can be divided into two roughening process: traditional kinetic roughening process and anomalous rapid roughening process accompanying morphology transition. In the later stage, the special large scaling exponents and growth behaviour cannot be analyzed with the existing mechanisms, i.e., kinetic roughening and mound growth, in the literatures. Here a new growth mechanism dominates the growth process.
XRD experiments were carried out for these films. Fig. 3 shows the representative XRD curves of films with three different thicknesses. The main diffraction peaks show a gradual change in peak positions where new peaks at higher 2θ values emerge with increasing film thickness. The 30 nm film show only one diffraction peak at 2θ = 5.95° with d = 14.84 Å, indexed as (002). However, the 70 nm and 120 nm films obviously show one strong (002) diffraction peak with shoulders. For 120 nm films, the peak and shoulders can be fitted to three diffraction peaks, in which 2θ values are 5.95°, 6.02° and 6.20° respectively, with the d values can be obtained as 14.84 Å, 14.67 Å and 14.24 Å accordingly. It suggests that molecular layer distance decrease slightly as increasing the thickness. Furthermore, a new peak emerges at 2θ = 31.4° with d = 2.84 Å for the 120 nm film, which can be indexed as (108) diffraction plane, as shown in the left inset in Figure 3a obtained between 30° and 32°. (This peak was not observed when F16CuPc films grown on ITO substrate23. It is because that F16CuPc film has a weaker crystallinity on ITO substrate than that on SiO2 substrate and it is difficult to get the weak signal of (108) diffraction peak by normal XRD experiment.) It means, with increasing the thickness, not only the distance of molecular layer decreases, but also another molecular orientation emerges when the film thickness is over a critical value. The right inset in Fig. 3a shows the schematic of molecular arrangement of (00l) and (108) orientations.
(a) XRD curves of F16CuPc films grown on SiO2 substrate with the thickness D = 30 nm, 70 nm and 120 nm, respectively. The main diffraction peaks show a gradual change in peak positions where new peaks at higher 2θ values emerge with increasing the film thickness. The fitted curves for the (002) diffraction peaks are shown as well. The left inset is the XRD data obtained between 30° and 32°. Increasing the film thickness not only changes the positions of (002) diffraction peak, but also a new diffraction peak emerges at 31.4°, indexed as (108). The right inset is the schematic of molecular arrangement of (00l) and (108) orientations. The diffraction peaks were indexed using single crystal data: a = 4.7960 Å, b = 10.228 Å, c = 28.002 Å, α = 86.41°, β = 87.89°, γ = 81.39° 24. (b) Schematic of F16CuPc molecular orientations in films as increasing the thickness D. The gaps between the grain boundaries or molecules become bigger as increasing the thickness D, in which it provides more probabilities that new molecules move into the gaps and arrange with new orientations. They aggregate, nucleate, grow up and accelerate the formation of nanobelt crystals.
Meanwhile, Fig. 3b shows the schematic of the change of molecular orientation with the film thickness D. The stacking angle δ decreases as the film thickness increases. For example, δ1 < δ2 < δ3 and it results in 14.24 Å ≤ d ≤ 14.84 Å when D ≤ 120 nm. The stacking angle δ decreases, the gaps between the grain boundaries or molecules increase. Thus it provides more probabilities that new molecules move into the gaps and arrange with new orientations. As indicated with the black circle in Fig. 3b, the (108) orientation forms in the gaps of (002) orientated crystals. After the nucleation happens with new molecular orientation, it can grow up and accelerate the formation of nanobelt crystals with new molecules coming under the continuous deposition. It is anomalous growth behaviour with rapid roughening process compared with the traditional KPZ film growth. Based on the experiment results, the critical thickness of the transition from KPZ to anomalous growth behaviour is about 40 nm for F16CuPc grown on SiO2. It suggests that no molecules or few molecules fill in the gaps of grain boundaries as D ≤ 40 nm and it is advantageous to form smooth film with KPZ growth model. While D > 40 nm, the gaps become bigger and bigger with increasing D. Then more and more molecules move into the gaps with different orientations, in which they aggregate, nucleate and grow up.
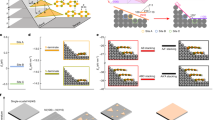
In order to rationalize the scaling behaviour and roughening processes, the potential energy variation of impinging and then laterally diffusing F16CuPc molecules was simulated. Molecular structural parameters were obtained from density functional theory (DFT) methods and the reported unit cell lattice parameters24 with a = 4.7960 Å, b = 10.228 Å, c = 28.002 Å, α = 86.41°, β = 87.89° and γ = 81.39° were used (The details are shown in the Supplementary Information). The intermolecular interaction energy calculation method and molecular mechanics force field parameters used were described elsewhere25,26. The calculations were performed for the two different crystal models consisting of three molecular layers as shown in Fig. 4. One model, denoted as Case A, with relatively narrow terrace (Fig. 4a) can represent the typical small clusters at the initial stage of the molecular deposition, i.e. D ≤ 40 nm. The other model, denoted as Case B, with relatively long terrace, can be a model for bigger clusters such as nanobelts of the thick films, i.e. D ≥ 70 nm. The intermolecular interaction energies of impinging F16CuPc molecule were then calculated as it diffuses laterally along a axis. In Fig. 4, the impinging molecules are drawn palely. The positions at b and c axes and molecular tilting values of the impinging molecule were varied to minimize the energy at each a-axis position.
The intermolecular interaction energies are plotted in Fig. 5. For both the models, the calculations yielded similar minimum energies of 3.01 eV at the energetically most stable position. However, it is interesting to note that significantly different energy barriers were observed at the step edges. The step edge barrier, ΔEb, at the largest value of 0.11 eV was observed for the Case A (Fig. 5a), while the ΔEb significantly increased to 0.44 eV for the Case B (Fig. 5b). The small step edge barrier for the Case A probably stemmed from nearly perpendicular molecular arrays. The impinging molecule moving at the step edge could easily slide down to the lower layer without breaking the intermolecular interactions a lot with existing molecules, as shown in Fig. 4a. This small edge barrier can explain the experimental results that the growth behaviour of F16CuPc thin films when D ≤ 40 nm was consistent with conventional scaling behaviour based on the KPZ model. In contrast, for the Case B, the impinging molecule on the large terrace was stabilized by adopting a configuration with its molecular plane almost parallel to the surface in order to maximize the intermolecular interactions, as shown in Fig. 4b. This configuration breaks at the step edge when the diffusing molecule moves down to the lower layer, leading to a significant decrease in the molecular interactions and large energy barriers. This kind of large step edge barrier is similar to the growth of PTCDA thin films with surface-parallel orientation27. The simulation supports the gradual change of molecular orientations and the formation of nanobelt crystals with increasing the film thickness.
Conclusion
In conclusion, we use both experiment and simulation to study organic semiconductor thin-film growth in the model of molecular semiconductor F16CuPc. It is revealed that molecular semiconductor thin-film growth can take on more than one processes, i.e., conventional KPZ growth and anomalous growth behaviour with rapid roughening process, accompanying morphology transition as the film thickness increases. This new growth behaviour results from the gradual change of molecular orientation and the formation of grain boundaries, which induce new molecular orientations, rapid roughening process and the formation of nanobelt crystals.
Methods
Materials and Thin Film Growth
F16CuPc material (Sigma-Aldrich) was purified twice by thermal gradient sublimation prior to use. Films with different thicknesses were grown by high vacuum organic molecular beam deposition (base pressure of about 4 × 10−8 mbar) with the substrate held at room temperature. All films were grown on either commercially available pre-cleaned SiO2 (IDB Technologies Ltd) or indium tin oxide (ITO)-coated glass (CRL Opto) substrates with root-mean square (RMS) roughness of ~0.4 nm and ~3.5 nm respectively. A deposition rate of 0.15–0.20 Å s−1 was used for all F16CuPc thin films.
Characterization
The morphology of fresh films was characterized by AFM (Asylum Research MFP-3D, Santa Barbara, USA) with taping mode and field emission SEM (FE-SEM, Zeiss Supra 55VP, Germany) with operating voltage at 5 kV. X-ray diffraction (XRD) was used to characterise the structure of films using an X′ Pert PRO (PANalytical, Netherlands) instrument with Cu Kα radiation (λ = 1.540 56 Å), where the selected voltage and current were 45 kV and 40 mA, respectively. The scanning rate was set at 0.4°/min from 2° to 32° (2θ).
References
Sokolov, A. N., Tee, B. C. K., Bettinger, C. J., Tok, J. B. H. & Bao, Z. N. Chemical and engineering approaches to enable organic field-effect transistors for electronic skin applications. Acc. Chem. Res. 45, 361–371 (2012).
Irimia-Vladu, M., Głowacki, E. D., Voss, G., Bauer, S. & Sariciftci, N. S. Green and biodegradable electronics. Mater. Today 15, 340–346 (2012).
Torsi, L., Magliulo, M., Manoli, K. & Palazzo, G. Organic field-effect transistor sensors: a tutorial review. Chem. Soc. Rev. 42, 8612–8628 (2013).
Krebs, F. C., Espinosa, N., Hosel, M., Sondergaard, R. R. & Jorgensen, M. 25th anniversary article: rise to power - OPV-based solar parks. Adv. Mater. 26, 29–39 (2014).
Diao, Y., Shaw, L., Bao, Z. A. & Mannsfeld, S. C. B. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ. Sci. 7, 2145–2159 (2014).
Yang, J. L. & Yan, D. H. Weak epitaxy growth of organic semiconductor thin film. Chem. Soc. Rev. 38, 2634–2645 (2009).
Wen, Y. G., Liu, Y. Q., Guo, Y. L., Yu, G. & Hu, W. P. Experimental techniques for the fabrication and characterization of organic thin films for field-effect transistors. Chem. Rev. 11, 3358–3604 (2011).
Mayer, A. C., Kazimirov, A. & Malliaras, G. G. Dynamics of bimodal growth in pentacene thin films. Phys. Rev. Lett. 97, 105503 (2006).
Dürr, A. C. et al. Rapid roughening in thin film growth of an organic semiconductor (diindenoperylene). Phys. Rev. Lett. 90, 016104 (2003).
de Oteyza, D. G., Barrena, E., Ossó, J. O., Sellner, S. & Dosch, H. Thickness-dependent structural transitions in fluorinated copper-phthalocyanine (F16CuPc) films. J. Am. Chem. Soc. 128, 15052–15053 (2006).
Hlawacek, G. et al. Characterization of step-edge barriers in organic thin-film growth. Science 321, 108–111 (2008).
Ruiz, R. et al. Dynamic scaling, island size distribution and morphology in the aggregation regime of submonolayer pentacene films. Phys. Rev. Lett. 91, 136102 (2003).
Meyer zu Heringdorf, F. J., Reuter, M. C. & Tromp, R. M. Growth dynamics of pentacene thin flms. Nature 412, 517–520 (2001).
Biscarini, F., Samori, P., Greco, O. & Zamboni, R. Scaling behavior of anisotropic organic thin films grown in high vacuum. Phys. Rev. Lett. 78, 2389–2392 (1997).
Zhang, X. N. et al. Evidence for a layer-dependent Ehrlich-Schwöbel barrier in organic thin film growth. Phys. Rev. Lett. 103, 136101 (2009).
Kowarik, S. et al. Real-time observation of structural and orientational transitions during growth of organic thin films. Phys. Rev. Lett. 96, 125504 (2006).
Yim, S. & Jones, T. S. Growth dynamics of C60 thin films: effect of molecular structure. Appl. Phys. Lett. 94, 021911 (2009).
Yang, J. L. et al. Ultrathin-film growth of para-sexiphenyl (I): submonolayer thin-film growth as a function of the substrate temperature. J. Phys. Chem. B 112, 7816–7820 (2008).
Yim, S. & Jones, T. S. Anomalous scaling behavior and surface roughening in molecular thin-film deposition. Phys. Rev. B 73, 161305 (2006).
Sekitani1, T., Zschieschang, U., Klauk, H. & Someya, T. Flexible organic transistors and circuits with extreme bending stability. Nature Mater. 9, 1015–1022 (2010).
Yang, J. L., Sullivan, P., Schumann, S., Hancox, I. & Jones, T. S. Organic photovoltaic cells based on unconventional electron donor fullerene and electron acceptor copper hexadecafluorophthalocyanine. Appl. Phys. Lett. 100, 023307 (2012).
Pelliccione, M. & Lu, T. M. Evolution of Thin Film Morphology: Modeling and Simulations (Springer-Verlag, Berlin, Heidelberg, 2008).
Yang, J. L., Schumann, S. & Jones, T. S. Morphology and structure transitions of copper hexadecafluorophthalocyanine (F16CuPc) thin films. J. Phys. Chem. C 114, 1057–1063 (2010).
Yoon, S. M., Song, H. H., Hwang, I. C., Kim, K. S. & Choi, H. C. Single crystal structure of copper hexadecafluorophthalocyanine (F16CuPc) ribbon. Chem. Commun. 46, 231–233 (2010).
Yim, S., Heutz, S. & Jones, T. S. Influence of intermolecular interactions on the structure of phthalocyanine layers in molecular thin film heterostructures. Phys. Rev. B, 67, 165308 (2003).
Halgren, T. A. The representation of van der Waals (vdW) interactions in molecular mechanics force fields: potential form, combination rules and vdW parameters. J. Am. Chem. Soc. 114, 7827–7843 (1992).
Yim, S., Kim, K. & Jones, T. S. Growth morphology of perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) thin films: influence of intermolecular interactions and step-edge barriers. J. Phys. Chem. C 111, 10993–10997 (2007).
Acknowledgements
This work was funded by Engineering and Physical Sciences Research Council (EPSRC) through the Basic Technology Project “Molecular Spintronics”. J.L. Yang also acknowledges the support of the National Natural Science Foundation of China (51203192) and the Program for New Century Excellent Talents in University (NCET-13-0598).
Author information
Authors and Affiliations
Contributions
J.L.Y. and T.S.J. conceived the idea. J.L.Y. carried out all the experiments as well as the data collection and analysis. S.Y. contributed to the simulation calculation and analysis. J.L.Y. and S.Y. prepared the first draft. All authors discussed the results and provided comments on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, J., Yim, S. & Jones, T. Molecular-Orientation-Induced Rapid Roughening and Morphology Transition in Organic Semiconductor Thin-Film Growth. Sci Rep 5, 9441 (2015). https://doi.org/10.1038/srep09441
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09441
This article is cited by
-
Enhanced photoelectrical response of thermodynamically epitaxial organic crystals at the two-dimensional limit
Nature Communications (2019)
-
Post-Deposition Wetting and Instabilities in Organic Thin Films by Supersonic Molecular Beam Deposition
Scientific Reports (2018)
-
Interface modification for organic and perovskite solar cells
Science China Materials (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.