Abstract
We investigated the lethal effects of visible light on insects by using light-emitting diodes (LEDs). The toxic effects of ultraviolet (UV) light, particularly shortwave (i.e., UVB and UVC) light, on organisms are well known. However, the effects of irradiation with visible light remain unclear, although shorter wavelengths are known to be more lethal. Irradiation with visible light is not thought to cause mortality in complex animals including insects. Here, however, we found that irradiation with short-wavelength visible (blue) light killed eggs, larvae, pupae and adults of Drosophila melanogaster. Blue light was also lethal to mosquitoes and flour beetles, but the effective wavelength at which mortality occurred differed among the insect species. Our findings suggest that highly toxic wavelengths of visible light are species-specific in insects and that shorter wavelengths are not always more toxic. For some animals, such as insects, blue light is more harmful than UV light.
Similar content being viewed by others
Introduction
Understanding the influence of visible light (400–780 nm) on organisms is important for identifying novel uses and examining hazards of exposure to visible light. However, little is known about the biological toxicity of visible light. Although recent studies have described damage by short-wavelength visible light (blue light, 400–500 nm) to the mammalian retina, called the ‘blue light hazard’1,2,3,4,5, there have been no reports on the lethal effects of irradiation with visible light on complex animals, including insects. On the other hand, the toxicity of shortwave UV light to organisms is well known. UVC (100–280 nm) and UVB (280–315 nm) induce mutagenic and cytotoxic DNA lesions6,7 and UVC irradiation has lethal effects on insects8 and microorganisms9. The use of UVC irradiation for control of pests such as Tribolium castaneum, T. confusum, Cadra cautella and Trogoderma granarium, which infest stored grains, has been studied10,11. Lethal effects of UVC against larvae of the silkworm Bombyx mori are also well known12,13. Lethal effects of UVB have been reported for spider mites14, in which UVB irradiation strongly decreases survivorship and egg production. However, there are no reports that describe lethal effects of UVB or UVA (315–400 nm) on insects, although UVA irradiation slightly decreases adult longevity in the lepidopteran Helicoverpa armigera15. It is well known that shorter wavelengths of light are more lethal9,16,17. In addition, positive effects of wavelengths ranging from UVA to green (500–560 nm) have been reported for spider mites; irradiation with UVA, blue and green light caused photoreactivation of mites damaged by UVB irradiation18. Therefore, irradiation with visible light is not considered lethal to complex animals, including insects. Here, in contrast, we show a strong lethal effect of blue light on insects. In this study, we found that blue-light irradiation by a common LED can kill insect pests of various orders and that highly lethal blue-light wavelengths are species-specific in insects.
Results
Lethal effects of irradiation with various wavelengths of light on D. melanogaster pupae
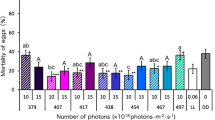
First, we investigated the lethal effect of light (wavelengths from 378 to 732 nm) on D. melanogaster pupae using LEDs. Irradiation with wavelengths of 378, 417, 440, 456 and 467 nm at 3.0 × 1018 photons·m−2·s−1 throughout the pupal stage significantly increased the mortality of D. melanogaster pupae compared with their mortality under DD (24-h dark) conditions (Fig. 1a, Supplementary Table 1). In particular, we identified two peak wavelengths (440 and 467 nm; Fig. 1a) that had strong lethal effects. More than 90% and 70% of pupae died before adult emergence after irradiation with wavelengths of 467 and 440 nm, respectively; the lethal effects of these wavelengths were stronger than those of UVA (378 nm). Wavelengths of 404 nm and ≥496 nm did not have a lethal effect on D. melanogaster pupae (Fig. 1a, Supplementary Table 1). In wavelengths ranging from 378 to 508 nm, mortality increased with increasing numbers of photons (Fig. 1b). Wavelengths of 440, 456 and 467 nm led to 100% mortality at 4.0 × 1018 photons·m−2·s−1; this number of photons did not have a lethal effect at wavelengths of 508, 657 and 732 nm. These results reveal, for the first time, that complex animals such as insects can be killed by irradiation with certain wavelengths of visible light and that visible light is more harmful than UV light to some animals.
Comparison of the lethal effects of light irradiation on Drosophila melanogaster pupae using various wavelengths of LED light.
(a) Mortality of pupae irradiated with 3.0 × 1018 photons·m−2·s−1. Data are means ± standard error (SE). Different lowercase letters next to bars indicate significant differences (Steel–Dwass test, P < 0.05). LL, DD and LD indicate 24-h light, 24-h dark and 16L:8D photoperiod conditions, respectively. (b) Dose–response relationships for lethal effects of irradiation on pupae for each wavelength. Data are mean values.
Lethal effects of irradiation with blue light on eggs, larvae and adults of D. melanogaster
Irradiation with a wavelength of 467 nm had the strongest lethal effect on Drosophila pupae, although this wavelength was also lethal to other developmental stages. The mortality rate of eggs increased with increasing numbers of photons (Fig. 2a); the majority of eggs died after 48-h irradiation at ≥5.0 × 1018 photons·m−2·s−1, whereas most eggs hatched under dark conditions. Irradiation with a wavelength of 467 nm for 24 h was lethal to final-instar larvae (L1–L2)19 and showed a dose–response relationship (Fig. 2b). Most flies died before adult emergence after irradiation at 7.0 × 1018 photons·m−2·s−1. Flies died during earlier developmental stages as the number of photons increased. Forty percent and 27% of flies died during the larval stage following irradiation at 12.0 × 1018 and 10.0 × 1018 photons·m−2·s−1, respectively. Using these same irradiation levels, more than 90% of flies died during the larval or prepupal stages (L1–P4). With irradiation at 7.0 × 1018 photons·m−2·s−1, the flies that died before adult emergence were almost evenly divided among the flies that died during the larval or prepupal stages (L1–P4) and those that died during the pupal stage (P5–P15). Interestingly, none of the irradiated flies died during the developmental stages of P5–P9. Adult longevity decreased significantly as the number of photons increased (Fig. 2c, Supplementary Table 2). In contrast, the longevity of adult flies maintained under dark conditions was approximately 60 d. Irradiation with a wavelength of 467 nm affected fly fecundity (Fig. 2d); the mean number of eggs deposited by surviving females decreased with increasing numbers of photons. These results show that irradiation with blue light has a lethal effect on the pupal stage of Drosophila and also on other developmental stages of this insect—including the adult stage, which is typically considered tolerant of light irradiation.
Effects of irradiation with 467-nm blue light on eggs, larvae and adults of Drosophila melanogaster.
(a) Dose–response relationships for lethal effects of irradiation with LED light on eggs. “0” photons represents the 24-h dark condition. Data are means ± standard error (SE). (b) Relationship between light dose and developmental stage at which mortality occurred. Developmental stages of larvae and pupae were classified according to Bainbridge and Bownes (1981)19. L1 and L2 are third-instar larvae, P1–P4 are prepupae and P5–P15 are phanerocephalic pupae. No irradiated flies died during the P5–P9 developmental stages. Data are mean values. Mortality (mean ± SE) of flies that could not emerge is indicated by the black line. (c) Dose–response relationships for effects of irradiation with LED light on adult longevity. “0” photons represents the 24-h dark condition. Data are means ± SE. Different lowercase letters above bars indicate significant differences (Steel–Dwass test, P < 0.05). (d) Dose–response relationships for the effects of irradiation with LED light on fecundity. DD indicates the 24-h dark condition. Inset numbers (1.0, 5.0 and 10.0 × 1018) indicate light dose in photons·m−2·s−1. Data are mean values.
Lethal effects of blue-light irradiation on C. pipiens molestus and T. confusum
We also investigated the lethal effects of various blue-light wavelengths (404–508 nm) on pupae of the mosquito Culex pipiensmolestus. Blue light irradiation was lethal to mosquito pupae, although their tolerance was higher than that of D. melanogaster pupae (Fig. 3a, b). Compared with DD conditions, irradiation with wavelengths of 404, 417 and 456 nm at 10.0 × 1018 photons·m−2·s−1 throughout the pupal stage significantly increased the mortality of C. pipiens molestus (Supplementary Table 3); the peak wavelength of 417 nm was highly lethal (Fig. 3a). Wavelengths of 404 and 417 nm killed substantial proportions of pupae before adult emergence, whereas wavelengths ≥ 440 nm were non- or negligibly lethal (Fig. 3a). The lethal effect of 417 nm increased with increasing numbers of photons; in contrast, the lethal effect of 404 nm was nominal and the lethal effects of 440-, 456- and 467-nm wavelengths increased only slightly with increasing numbers of photons (Fig. 3b). Irradiation with a wavelength of 417 nm was lethal to mosquito eggs and the mortality increased over time (Fig. 3c, Supplementary Table 4). Whereas only 34% of mosquitos died before hatching following 48 h of irradiation at 10.0 × 1018 photons·m−2·s−1, approximately 90% of hatchlings from the irradiated eggs died within 72 h after irradiation; this is compared with a 2% mortality rate of hatchlings from the eggs maintained under dark conditions. Accordingly, even if irradiated eggs hatched, most hatchlings died soon thereafter. These results show that the lethal effect of blue light is not confined to flies; however, the effective wavelength at which mortality occurs is species-specific and tolerance to blue-light irradiation differs among insect species.
Lethal effects of blue-light irradiation on the mosquito Culex pipiensmolestus and the confused flour beetle Tribolium confusum.
(a) Mortality of C. pipiensmolestus pupae irradiated with various wavelengths of blue light at 10.0 × 1018 photons·m−2·s−1 during the pupal stage. Data are means ± standard error (SE). Different lowercase letters next to bars indicate significant differences (Steel–Dwass test, P < 0.05). DD indicates the 24-h dark condition. (b) Dose–response relationships for lethal effects of irradiation with each wavelength of light on pupae. Data are mean values. (c) Mortality of C. pipiensmolestus that were irradiated with 417-nm light for 48 h at 10.0 × 1018 photons·m−2·s−1 during the egg stage. Data are means ± SE. Hours in parentheses show the elapsed time after discontinuation of irradiation. Different lowercase or capital letters next to bars indicate significant differences among the three treatments for each time period (Steel–Dwass test, P < 0.05). LL and DD indicate 24-h light and 24-h dark conditions, respectively. (d) Mortality of T. confusum pupae irradiated with various wavelengths of light at 2.0 × 1018 photons·m−2·s−1 during the pupal stage. Data are means ± SE. Different lowercase letters next to bars indicate significant differences (Steel–Dwass test, P < 0.05). LD indicates 16L:8D photoperiod condition.
Blue-light irradiation was lethal to pupae of the confused flour beetle T. confusum (Fig. 3d). All beetles irradiated with wavelengths ranging from 404 to 467 nm throughout the pupal stage at 2.0 × 1018 photons·m−2·s−1 died before adult emergence. However, irradiation with the 532-nm wavelength did not have a lethal effect. These findings show that blue-light irradiation can kill insects of various orders.
Discussion
In this study, we revealed for the first time that blue-light irradiation can kill insect pests and that effective wavelengths of visible light are species-specific. Our findings show that visible light is more harmful than UV light to some animals. The insides of the containers and media in which insects were housed did not register temperatures that would have affected the survival of any of the developmental stages in any of the irradiation treatments (Supplementary Tables 5 and 6). In addition, increases in lethal effects did not always correspond to increases in temperature. In the irradiation treatments in which increasing temperature corresponded to lethal effects, the temperatures were not high enough to affect insect survival20. Therefore, we concluded that temperature increases caused by LED light did not cause the mortality. UVB and UVC directly damage DNA by inducing the formation of DNA lesions, notably cis-syn cyclobutane pyrimidine dimers and pyrimidine (6-4) pyrimidone photoproducts21. The maximum absorption spectrum of DNA ranges from 260 to 265 nm and absorption rapidly declines at longer wavelengths22. DNA damage induced by UVA is minimal because UVA is not absorbed by native DNA6,7. However, UVA indirectly damages lipids, proteins and DNA by enhancing the production of reactive oxygen species (ROS)23,24,25. Increases in oxidative stress caused by UVA irradiation have also been shown in insects such as the cotton bollworm Helicoverpa armigera26. In addition, molecular-level responses to stress and damage by UVA irradiation have been confirmed in insects27,28. However, lethal effects of UVA irradiation on insects have not been shown15,27. Blue-light irradiation injures organisms by stimulating the production of ROS. Many microbial cells are highly sensitive to blue light as a result of the accumulation of photosensitizers such as porphyrins and flavins29. Mammalian retinas can also be severely damaged by ROS produced by blue-light irradiation4,5. It is probable that the lethal effect of blue light on insects is caused by the production of ROS, because the effective wavelength is species-specific and not always associated with the amount of photon energy delivered. In addition, light transmission of D. melanogaster puparia was not wavelength-specific (Supplementary Figure 1). These findings suggest that light absorption by certain inner tissues of the fly is wavelength-specific. That is, species-specific chromophores or photosensitizers in insect tissues absorb specific wavelengths of light, thereby generating free radicals. Insects subsequently die from tissue damage caused by free-radical formation.
We selected three insect species for the experiments presented here. D. melanogaster is a major model animal species with a short life cycle. Investigation of the lethal effects of blue-light irradiation on D. melanogaster can be conducted with ease and can be useful for studying damage caused by blue light or free radicals in animals. C. pipiensmolestus is a major mosquito species that is easily reared and thus is an appropriate model species for mosquito experiments. Mosquitoes are one of the most medically important insect pests and they transmit serious diseases, including malaria, dengue fever, yellow fever, West Nile fever and Japanese encephalitis. This study showed lethal effects of blue light on mosquito pupae and eggs. Reproduction of mosquitoes might be prevented by blue-light irradiation of water containing eggs, larvae and pupae and might consequently prevent outbreaks of mosquito-borne diseases. T. confusum is a globally important insect pest of stored grain. Our findings showed the potential of blue-light irradiation for pest control in stored products. That is, blue-light irradiation may be useful for pest control in various situations including agriculture, sanitation and food storage. D. melanogaster and C. pipiensmolestus belong to the order Diptera, whereas T. confusum belongs to Coleoptera. This implies that blue-light irradiation has lethal effects against multiple insect orders. Current techniques in pest management utilize light to influence insect behaviours, including attraction, repulsion and light adaptation in nocturnal species30. The present study suggests the potential for a novel, clean and safe pest-control technique that can easily kill insect pests simply by radiating blue light (e.g., LED).
However, tolerance to blue light varied widely among the insect species studied here. The order of tolerance was C. pipiensmolestus ≫ D. melanogaster ≥ T. confusum. The tolerance of C. pipiensmolestus to blue light was much higher than that of D. melanogaster, although both species belong to the order Diptera. The habitats of these three species differ. T. confusum inhabits stored foods in indoor environments. D. melanogaster lives in both outdoor and indoor habitats, but it occupies dark environments until adult emergence. C. pipiensmolestus usually lives in water in areas with low light until adult emergence. Therefore, the quantity of light to which these species are exposed is highest for C. pipiensmolestus, followed (in decreasing order) by D. melanogaster and T. confusum. Tolerance of insects to blue-light irradiation is thought to be closely related to the light exposure experienced in their natural habitats. The numbers of photons of 470 nm and blue-light wavelengths (400–500 nm) in direct sunlight in the field associated with our laboratory (Sendai, Japan; 38°N, 140°E) were approximately 1.0–2.5 × 1018 and 7.5–9.0 × 1018 photons·m−2·s−1, respectively (1:00–2:00 PM in early summer). Therefore, we assume that D. melanogaster and T. confusum cannot survive under direct sunlight because of the lethal effect of blue light. Accordingly, eggs, larva and pupae of D. melanogaster and T. confusum require dark habitats. The relationships between insect species (or habitats) and tolerance to blue light require further investigation in order to utilize blue-light irradiation for pest control.
Blue-light irradiation may be useful for controlling various insect pests. However, because the effective wavelengths of blue light are species-specific, several wavelengths (or broad-spectrum blue light) are needed for the simultaneous control of multiple species. In addition, genetic variation in resistance to UVC or ionizing irradiation has been confirmed in D. melanogaster31,32. It is probable that there is genetic variation in insect resistance to blue-light irradiation; this variation should be investigated so that the use of blue-light irradiation for pest control can be realized in the near future.
The purpose of this study was to reveal the lethal effects of light; the effects of low doses of blue-light irradiation on insects have not yet been clarified. In mammals, low doses of UV exposure provide health benefits including energy improvement, mood elevation and vitamin D production, although high rates of exposure can present health risks such as increased susceptibility to cancer33. It is possible that low doses of blue light can also have beneficial effects on insects.
Our findings facilitate the development of clean and safe pest-control techniques and provide important information on the hazards of exposure to visible light.
Methods
Insects
Eggs, final instar larvae, pupae and adults of Drosophila melanogaster; eggs and pupae of Culex pipiensmolestus; and pupae of Tribolium confusum were maintained in our laboratory and used for the experiments. D. melanogaster was purchased from Sumika Technoservice Co. (Takarazuka, Japan). The flies were reared on culture medium consisting of glucose (2.5 g), dry brewer's yeast (2.5 g), agar (0.5 g), propionic acid (0.25 mL), 20% butyl p-hydroxybenzoate in 70% ethyl alcohol (0.25 mL) and water (total medium volume = 50 mL) in a plastic box (72 × 72 × 100 mm). C. pipiensmolestus were supplied by Earth Chemical Co., Ltd. (Tokyo, Japan). The eggs, larvae and pupae were maintained in a plastic container (150 mm dia × 91 mm tall) containing 250 mL of water, with a constant supply of fishery feed (trout juveniles). Adults were maintained in a plastic cage (340 × 250 × 340 mm) containing two plastic cups (30 mm dia × 35 mm tall). Absorbent cotton impregnated with 3% honey solution was placed in one of the cups as a food source and absorbent cotton soaked with water was placed in the other cup as an oviposition substrate. T. confusum were provided by Fuji Flavor Co., Ltd. (Tokyo, Japan) and were reared in a plastic container (130 mm dia × 77 mm tall) on wheat flour containing 5% dry brewer's yeast. All insects were wild type and were maintained at 25 ± 1°C under a photoperiod of 16L:8D.
LED light radiation
LED lighting units (IS-mini®, ISL-150 × 150 Series; CCS Inc., Kyoto, Japan; light emission surface: 150 × 150 mm; 360 LEDs were equally arranged on a panel; LED type: φ 3-mm plastic mould) with power supply units (ISC-201-2; CCS Inc.) were used for UV and visible light radiation. Insects were irradiated with LED light in a multi-room incubator (LH-30CCFL-8CT; Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The emission spectrum was measured using a high-resolution spectrometer (HSU-100S; Asahi Spectra Co., Ltd., Tokyo, Japan; numerical aperture of the fibre: 0.2) Comparison of the emission spectra used in the experiments is shown in Fig. 4. The number of photons (photons·m−2·s−1) was measured using the spectrometer in a dark room and was adjusted using the power-supply unit. The distance between the light source and the spectrometer sensor during measurements was approximately the same as that between the insects and light source in the incubator. Because the insects were irradiated through a glass lid, polystyrene lid, or glass plate, the same lid or plate was placed between the light source and sensor during measurement. The distances between the lid or plate and the light source during measurements were approximately the same as those in the incubator. Insect containers were placed directly under the light source during irradiation. We confirmed that the upper surfaces of the containers were irradiated homogeneously by measuring the numbers of photons. In addition, we assumed that temperature changes caused by the light source would not affect survival of the insects because LED light emits little heat. To check this assumption, we measured the temperature inside the containers using a button-type temperature logger (3650, Hioki E. E. Co., Ueda, Japan), of the insects and in the media except for water (filter paper, culture medium, bottom of dish) using a radiation thermometer (IR-302, Custom Co., Tokyo, Japan). We measured water temperature using a digital thermometer (TP-100MR, Thermo-port Co., Iruma, Japan). Temperatures that showed lethal effects in several light treatments were measured in each experiment and under DD and LD (16L:8D photoperiod) conditions. The temperature data are summarized in Supplementary Tables 5 and 6.
Lethal effects of irradiation with various wavelengths of light on D. melanogaster pupae
Thirty pupae were collected from the rearing boxes within 24 h of pupation and placed on a sheet of filter paper (Advantec, No. 1, 70 mm dia) impregnated with 700 μL of water in a glass petri dish (60 mm dia × 20 mm tall). The petri dish was sealed with parafilm, placed in the incubator and irradiated with LED light for 7 d at 25 ± 1°C. The numbers of emerging adults were counted 7 d after the start of irradiation. Eight replications (petri dishes) were performed for each light dose and wavelength. Initially, lethal effects at 3.0 × 1018 photons·m−2·s−1 were compared among 12 wavelengths (378, 404, 417, 440, 456, 467, 496, 508, 532, 592, 657 and 732 nm). We investigated mortality of pupae under 24 h light (LL), 24 h dark (DD) and 16L:8D photoperiod (LD) conditions using white cold cathode fluorescent lamps (CCFLs) in the light periods. The relationships between lethal effects and numbers of photons were compared among the 12 wavelengths.
Lethal effects of irradiation with blue light on eggs, larvae and adults of D. melanogaster
1) Eggs
Five pairs of mated adults were released onto 10 mL of culture medium (same as rearing stock culture) in a glass petri dish (60 mm dia × 90 mm tall) and allowed to lay 10 eggs on the medium within 6 h. The petri dish with eggs was immediately sealed with parafilm and placed in the incubator. The eggs were then irradiated with 467-nm LED light for 48 h at 25 ± 1°C and the numbers of newly hatched larvae were counted under a stereomicroscope. The lethal effects of irradiation at 3.0 × 1018, 4.0 × 1018, 5.0 × 1018 and 10.0 × 1018 photons·m−2·s−1 were investigated. We also investigated egg mortality under DD conditions. Ten replications (petri dishes) were performed for each light dose.
2) Larvae
Ten final-instar larvae (wandering third-instar stage, L119) were collected from the rearing boxes within 24 h of wandering out of the culture medium and placed in a polystyrene petri dish (55 mm dia × 15 mm tall). The petri dish was sealed with parafilm, placed in the incubator and irradiated with 467-nm LED light for 24 h at 25 ± 1°C. After irradiation, the petri dish was transferred to the thermostatic chamber (LP-1PH; Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan) and maintained under 16L:8D (white fluorescent lamps were used during the light period) at 25 ± 1°C. The number of adults that emerged was counted after 10 d. Pupae that died before emergence were dissected under a stereomicroscope and their developmental stages were determined19. We investigated the lethal effects of irradiation at 5.0 × 1018, 7.0 × 1018, 10.0 × 1018 and 12.0 × 1018 photons·m−2·s−1. Ten replications (petri dishes) were performed for each light dose.
3) Adults
One pair of adults was collected from rearing boxes within 12 h of emergence and released onto 10 mL of culture medium (same composition as for rearing stock cultures) in a glass petri dish (60 mm dia × 90 mm tall). The petri dish was irradiated with 467-nm LED light in the incubator at 25 ± 1°C. Flies were irradiated for 24 h d−1 until both the male and female died. Every 24 h, we counted the number of surviving adults and eggs deposited and replaced the petri dish containing culture medium with a fresh one. Ten replications (petri dishes) were performed for each light dose.
Lethal effects of blue-light irradiation on C. pipiens molestus and T. confusum
1) C. pipiens molestus pupae
Ten pupae were collected from the stock cultures within 1 h of pupation and released into water (100 mL) in a polyethylene terephthalate (PET) ice-cream cup (101 mm dia × 49 mm tall), the opening of which was covered with a glass plate. The cup was placed in the incubator and irradiated with LED light for 5 d at 25 ± 1°C. The numbers of emerging adults were counted 5 d after the start of irradiation. Ten replications (cups) were performed for each light dose and wavelength. Initially, lethal effects at 10.0 × 1018 photons·m−2·s−1 were compared among five wavelengths (404, 417, 440, 456 and 467 nm). We also investigated pupal mortality rates under DD conditions. The relationships between lethality and number of photons were then compared among seven wavelengths (404, 417, 440, 456, 467, 496 and 508 nm).
2) C. pipiens molestus eggs
Thirty eggs were collected from the stock cultures within 1 h of deposition and placed in water (50 mL) in a PET ice-cream cup (60 mm dia × 38 mm tall), the opening of which was covered with a glass plate. The cup was placed in the incubator (25 ± 1°C) and irradiated with 417-nm LED light at 10.0 × 1018 photons·m−2·s−1 for 48 h. The number of newly hatched larvae was counted 48 h after the start of irradiation. After checking hatchability, the cup with mosquitoes was maintained under DD conditions for 72 h (25 ± 1°C) and the mortality of newly hatched larvae was then investigated. For comparison, hatchability and mortality rates were investigated under LL (white CCFLs provided light for 48 h, after which darkness was provided for 72 h) and DD (no irradiation, darkness for 120 h) conditions. Ten replications (ice-cream cups) were performed for each light dose.
3) T. confusum pupae
Ten pupae were collected from the stock cultures within 24 h of pupation and placed in a glass petri dish (30 mm dia × 15 mm tall). The petri dish was placed in the incubator (25 ± 1°C) and irradiated with LED light at 2.0 × 1018 photons·m−2·s−1 for 14 d, after which we counted the number of adults that emerged. The lethal effects of irradiation were compared among five wavelengths (404, 417, 456, 467 and 532 nm). Ten replications (petri dishes) were performed for each wavelength. We also investigated mortality of pupae under LD conditions (white CCFLs were used).
Statistical analyses
Mortality and adult longevity were analysed using a generalized linear model (GLM) followed by the Steel–Dwass test. Mortality of T. confusum pupae was analysed by Steel–Dwass test without GLM, because 100% mortality occurred under blue-light irradiation (404–467 nm) and 0% mortality occurred under LD conditions. The lethal effects on C. pipiens molestus eggs were analyzed by using GLM followed by the Steel–Dwass test among 417 nm irradiation, LL and DD in each of 0 and 72 h after discontinuing irradiation.
References
Bynoe, L. A., Del Priore, L. V. & Hornbeck, R. Photosensitization of retinal pigment epithelium by protoporphyrin IX. Graefe's Arch. Clin. Exp. Ophthalmol. 236, 230–233 (1998).
Algvere, P. V., Marshall, J. & Seregard, S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 84, 4–15 (2006).
Różanowska, M. & Sarna, T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem. Photobiol. 81, 1305–1330 (2005).
Wu, J., Seregard, S. & Algvere, P. V. Photochemical damage of the retina. Surv. Ophthalmol. 51, 461–481 (2006).
Kuse, Y., Ogawa, K., Tsuruma, K., Shimazawa, M. & Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 4, 10.1038/srep05223 (2014).
Sinha, R. P. & Häder, D-P. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 2002, 225–236 (2002).
Rastogi, R. P., Richa, Kumar, A., Tyagi, M. B. & Sinha, R. P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 10.4061/2010/592980 (2010).
Beard, R. L. Lethal action of UV irradiation on insect. J. Econ. Entomol. 65, 650–654 (1971).
Reed, N. G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 125, 15–27 (2010).
Faruki, S. I., Das, D. R., Khan, A. R. & Khatun, M. Effects of ultraviolet (254nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth, Cadra cautella. J. Insect Sci. 7, insectscience.org/7.36 (2007).
Ghanem, I. & Shamma, M. Effect of non-ionizing radiation (UVC) on the development of Trogoderma granarium Everts. J. Stored Prod. Res. 43, 362–366 (2007).
Nakajima, M. & Yoshida, H. Studies on ultraviolet sensitivity in silkworm, with special reference to the effect of UV-irradiation on melanin formation in the cuticle of the striped silkworm. Jpn. J. Appl. Entomol. Zool. 15, 51–55 (1971).
Nakajima, M. & Yoshida, H. Studies on ultraviolet sensitivity in the silkworm, with special reference to variations in its killing effect during the larval instar stage. Jpn. J. Appl. Entomol. Zool. 15, 17–22 (1971).
Ohtsuka, K. & Osakabe, M. Deleterious effects of UV-B radiation on herbivorous spider mites: They can avoid it by remaining on lower leaf surfaces. Environ. Entomol. 38, 920–929 (2009).
Zhang, C. Y., Meng, J. Y., Wang, X. P., Zhu, F. & Lei, C. L. Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera and on the development of its F1 generation. Insect Sci. 18, 697–702 (2011).
Clark, J. H. The physiological action of light. Physiol. Rev. 2, 277–309 (1922).
McMillan, T. J. et al. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 60, 969–976 (2008).
Murata, Y. & Osakabe, M. Factors affecting photoreactivation in UVB-irradiated herbivorous spider mite (Tetranychus urticae). Exp. Appl. Acarol. 63, 253–265 (2014).
Bainbridge, S. P. & Bownes, M. Staging the metamorphosis of Drosophila melanogaster. J. Embyrol. Exp. Morph. 66, 57–80 (1981).
Hoffmann, A. A., Sørensen, J. G. & Loeschcke, V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216 (2003).
Pfeifer, G. P. Formation and processing of UV photoproducts: Effects of DNA sequence and chromatin environment. Photochem. Photobiol. 65, 270–283 (1997).
Beggs, C. B. A quantitative method for evaluating the photoreactivation of ultraviolet damaged microorganisms. Photochem. Photobiol. Sci. 2002, 431–437 (2002).
Chamberlain, J. & Moss, S. H. Lipid peroxidation and other membrane damage produced in Escherichia coli K1060 by near-UV radiation and deuterium oxide. Photochem. Photobiol. 45, 625–630 (1987).
Moan, J. & Peak, M. J. Effects of UV radiation on cells. J. Photochem. Photobiol. B: Biology 4, 21–34 (1989).
Santos, A. L. et al. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch. Microbiol. 195, 63–74 (2013).
Meng, J. Y., Zhang, C. Y., Zhu, F., Wang, X. P. & Lei, C. L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect. Physiol. 55, 588–592 (2009).
Sang, W., Ma, W. H., Qiu, L., Zhu, Z. H. & Lei, C. L. The involvement of heat shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J. Insect. Physiol. 58, 830–836 (2012).
Zhou, L. J. et al. Identification and transcriptional profiling of differentially expressed genes associated with response to UVA radiation in Drosophila melanogaster (Diptera: Drosophilidae). Environ. Entomol. 42, 1110–1117 (2013).
Yin, R. et al. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light and beyond. Curr. Opin. Pharmacol. 13, 731–762 (2013).
Shimoda, M. & Honda, K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 48, 413–421 (2013).
Gomez, F. H., Loeschcke, V. & Norry, F. M. QTL for survival to UV-C radiation in Drosophila melanogaster. Int. J. Radiat. Biol. 89, 583–589 (2013).
Vaisnav, M. et al. Genome-wide association analysis of radiation resistance in Drosophila melanogaster. PLoS One 9, e104858 (2014).
Sivamani, R. K., Crane, L. A. & Dellavalle, R. P. The benefits and risks of ultraviolet (UV) tanning and its alternatives: the role of prudent sun exposure. Dermatol. Clin. 27, 149–154 (2009).
Acknowledgements
This study was supported by a grant entitled “Elucidation of biological mechanisms of photoresponse and development of advanced technologies utilizing light” from the Ministry of Agriculture, Forestry and Fisheries (MAFF), Japan and by JSPS KAKENHI Grant Number 25660261. We wish to thank Earth Chemical Co., Ltd. and Fuji Flavor Co., Ltd. for kindly supplying insects for use in our study. We wish to thank for Dr. Yoshihara (Graduate School of Agricultural Science, Tohoku University) for kindly advice on statistical analyses.
Author information
Authors and Affiliations
Contributions
M.H. designed the experiments. K.S., M.S. and Y.S. performed the experiments. M.H. and K.S. analysed the data. M.H. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Lethal effects of short-wavelength visible light on insects
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Hori, M., Shibuya, K., Sato, M. et al. Lethal effects of short-wavelength visible light on insects. Sci Rep 4, 7383 (2014). https://doi.org/10.1038/srep07383
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07383
This article is cited by
-
Negative phototaxis of jumping cocooned parasitoid wasp larvae against short wavelengths and physicochemical properties of the cocoon shell
Scientific Reports (2023)
-
Lethal effect of blue light on Liposcelis bostrychophila (Psocoptera: Liposcelididae)
Applied Entomology and Zoology (2023)
-
Age-dependent effects of blue light exposure on lifespan, neurodegeneration, and mitochondria physiology in Drosophila melanogaster
npj Aging (2022)
-
Effects of insemination and blood-feeding on locomotor activity of wild-derived females of the malaria mosquito Anopheles coluzzii
Parasites & Vectors (2021)
-
Effects of artificial light on bird movement and distribution: a systematic map
Environmental Evidence (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.