Abstract
The terminal root branch orders composed mainly of primarily-developed tissues are increasingly recognized as an ephemeral module specialized for resource absorption. This root module is crucial in driving a range of ecosystem processes such as belowground productivity, carbon and nutrient cycling. Traditionally, acquisition of the ephemeral root module is achieved by separating these primarily-developed branch orders with forceps. However, obtaining this root segment with forceps approach is labor-intensive which may not be applicable for studies with an appreciable amount of root samples. To address this challenge, we developed a new idea to obtain the ephemeral root module. In this new view, root samples were tenderly kneaded by hand and the detached roots are considered as the ephemeral root module. To test this idea, four species with contrasting growing environment were selected and a range of chemicals were determined including C, N, P, Ca, S, Mg, Ba, Zn, Mn, Pb, Cu, V and Li. We found no or little difference of these chemicals in roots by hand-kneading approach from roots by forceps approach. These results suggested that hand-kneading method could be a convenient way to acquire ephemeral root module.
Similar content being viewed by others
Introduction
Unraveling relationships between plant structures and their functions has been a long-standing focus in ecological studies1,2. In roots with a complex branching structure, mounting evidence has demonstrated a close linkage of root function with its structure, the position of a root or root branch order in a root branch2,3,4. Unlike the higher-order woody roots in the branch used for water and nutrient transport, the lower-order roots with primarily-developed tissues serve as the principal agent for water and nutrient absorption. Recently, a range of evidences have support that lower-order root segments are a relative independent subunit with a shorter lifespan than2,5 and different functions6,7 and responses to environment change8,9 from higher-order counterparts. These lower-order roots, analogous to the short-lived modularity of leaves relative to the long-lived twigs, were then proposed as the ephemeral root module specialized for absorptive function2. The ephemeral root module is crucial for plant growth and nutrient cycling as it is disproportionally higher in absorptive area, metabolic activity and nutrient concentration yet slower in decomposition rate after death1,10,11,12,13,14.
Despite the importance of ephemeral root module in ecosystem processes, collecting enough biomass of this root segment is still difficult, especially for species with very fine root systems7. This is because despite the ephemeral root module in a root branch can be determined by examining root anatomical structures this root segment is still needed to be dissected order by order with forceps2,3. Acquiring ephemeral root module by this way is usually labor-intensive and costly2,4. It has been regarded as a great handicap in a range of studies on root functional traits and root decomposition which require appreciable samples of ephemeral root modules rate. Therefore, it is imperative to develop a convenient approach to facilitate acquisition of ephemeral root modules.
It has been noted earlier that dense branch scars occurs frequently on high-order woody roots which is proposed to arise from frequent death of lower-order root segments or ephemeral root modules1. Furthermore, these primarily-developed roots lack mechanical protection from secondary root tissues such as secondary conduits and fibers which makes them susceptible to detachment from woody roots when suffering from mechanical force2,3,4,15,16. Given the above facts, it is reasonable to speculate that ephemeral root modules may fall off a root branch when exerting external force, for example with properly hand-kneading. We expect that hand-kneading of root branches can be a promising approach to obtain ephemeral root module conveniently.
One possible way to test effectiveness of hand-kneading method as a convenient and alternative way to the traditional forceps method is to compare chemicals of roots from these two approaches. This is because chemical composition has been reported to be different greatly within a root branch1,12,17. Here, a couple of macro- and micro-elements were determined for ephemeral root modules by each of the two methods in four species. If these elements are similar between the two methods, hand-kneading method can thus be a new and convenient approach to obtain ephemeral root modules.
Results
The C concentration of ephemeral root modules showed no significant difference between the two methods for L. gmelinii (P = 0.089), A. mangium (P = 0.191) and F. mandshurica (P = 0.091) (1Table 2). In P. elliotti, C concentration of forceps method was higher than that with hand-kneading method by 3.7% (P = 0.025) (Table 2). There was no difference of N concentration between the two methods in each of the four species. As for P concentration, differences of the two methods were 2.9%, 8.1% and 8.8% for L. gmelinii, A. mangium and F. mandshurica, respectively. There was significant difference of root Ca in A. mangium (11.7%), P. elliottii (4.1%) and F. mandshurica (10.0%). In the microelements, differences between the two methods were frequently larger than 10% (Table 2). This was especially the case for Pb and V in A. mangium (84.2%, 38.3%).
However, in the paired t-test, no difference of the 13 elements between the two methods were found in L. gmelinii (P = 0.06), P. elliotti (P = 0.55), A. mangium (P = 0.38) and F. mandshurica (P = 0.70) (Fig. 2). When the four species were considered together, the chemical ratio by the two methods showed no difference from the 1:1 line (F = 1.25, P = 0.30 in ANCOVA, Fig. 2).
Discussion
Our results showed similar chemical concentrations in ephemeral root modules obtained by forceps and hand-kneading methods (Fig. 2). This supports our hypothesis that there was no difference of root chemicals between these two methods. The success of this new method in acquiring ephemeral root modules may come from different mechanical strength of this root segment from that of the higher-order woody roots. As proposed recently, ephemeral root modules are mainly composed of primarily-developed tissues2,3,4. The lack of secondary tissues (e.g. secondary xylem and continuous cork layer) in this root segment may cause its junction with higher-order woody roots fragile to external mechanical force and hence can easily fall off by hand-kneading.
Along root branch orders, root chemicals are in paralleled with root anatomical structures and root lifespan7,20,21,22. Thus, the overall similar root chemicals by hand-kneading method to that by forceps method may suggest the former method can track this ephemeral root segment with dramatic difference in structure and functions from that of higher-order roots. In turn, effectiveness of hand-kneading method can thus be an indirect evidence of the existence of ephemeral root modules in a root branch. It is increasingly recognized that ephemeral root modules or the most dynamic part in a root branch is closely linked with a range of ecological processes because of its high nutrient content, fast turnover rate but slow decomposition2,4,23. Compared with forceps method, hand-kneading method is easy to operate and economic in labor investment which is promising to represent a novel and convenient way to acquire ephemeral root modules.
As for the three most studied macro-elements, C, N and P, they were almost identical between the two methods except P. Although there was indeed difference of root P in some species (Table 2), they were usually less than 10% between the two methods. As such, measurement of these nutrients in root samples by hand-kneading method may not result in a considerable error in studies on root nutrient cycling. As a convenience and economic way to acquire ephemeral root modules, hand-kneading method can be applicable in root studies. For example, in measuring root functional traits, it is increasingly acknowledged that interspecific comparison should be conducted by comparing root segments with a similar function24,25. These studies can be greatly facilitated by easily collecting enough ephemeral root modules with hand-kneading approach. The new method can also be applied to another important ecological process, litter decomposition in roots. Given the importance of lower-order roots in nutrient cycling12,23 and formation of soil organic matter26 in the process of root decomposition, it arose a great interest in unraveling patterns of decomposition of ephemeral root modules. However, studies of decomposition for this root segment are greatly constrained by limited root samples as preparation of root samples by forceps method is labor-intensive and costly23. Thus, hand-kneading approach is promising in applying to studies on root decomposition.
In contrast with macro-elements, significant difference of micro-elements between the two methods appeared frequently. Among the 10 microelements, most were heavy metals. It has been proposed recently that several distal orders, similar to ephemeral root modules, are an important agent for plants to alleviate heavy metal damage27,28. The reason lies in that this root segment high in metabolic activity can absorb and store many heavy metals. Given the short lifespan of ephemeral root modules, heavy metals in this root segment can be easily released by dying away quickly from higher-order woody roots hence reducing toxic effects on plants27.
One reason for ineffectiveness of hand-kneading method for determining micro-elements may be that these elements are in trace amount which may be more sensitive than macro-elements to difference of root samples collected by these two methods. In forceps methods, ephemeral root modules were dissected to an order with prominent occurrence of secondary tissues. This order is usually determined by examining anatomical structures along root branch orders. However, there was still a great variation of root structures even in the same order of a species29, which with forceps method may result in some bias of the true ephemeral root modules. On the other hand, hand-kneading method may track the portion of ephemeral root modules that most easily falls off when exerting external force. The size of this portion may depend on root toughness and strength and duration of force when exerting hand-kneading. The difference of micro-elements between the two methods may be a result from more sensitive of micro-elements compared with macro-elements to the above difference of root samples collected by these two methods. Difference of root samples by these two methods may also contribute to difference of macro-elements, as presented previously, by these two methods. Thus, hand-kneading method may not apply for estimation of heavy metals in ephemeral root modules.
In conclusion, our study provides a novel approach in acquisition of ephemeral root modules, the most dynamic part of a root branch. The hand-kneading method could be considered as an indirectly evidence of existence of modularity of in plant roots as well as directly evidenced by root morphology1, anatomy2 and lifespan4. Furthermore, by providing lower-order root samples more conveniently and economically than forceps method, hand-kneading method has great potential in advancing studies of root functional traits and root decomposition. Despite the advantage of hand-kneading method, it should be noted that there are still some critical issues should be stressed in future studies. One is how to knead the roots? Given the diverse root properties in toughness and elasticity among species, it is necessary to determine the force and duration in hand-kneading of the roots. This may also be an important source for root chemical difference between the two methods. Also, more species should be included in future studies to test the generality of hand-knead method and draw out general rules to be followed in collecting ephemeral root modules.
Methods
Study site and species selection
Four tree species with different phylogeny and mycorrhizal types (Table 1) were selected in this study. A conifer, Fraxinus mandshurica and a deciduous broad leaf species, Larix gmelinii, were collected at Maoershan Forest Research Station (45°21′–45°23′N, 127°30′–127°30′E) of the Northeast Forestry University, Heilong Jiang province, China (Site 1). They have been in mono-culture plantations since 1986. The long term mean annual, January and July temperatures are 2.8, −19.6 and 20.9°C, respectively. Mean annual precipitation is 723 mm and the soil is loamy Hap-Boric Luvisols18. Another two species, Acacia mangium, an evergreen broad leaf species and Pinus elliottii, a conifer species, were sampled at Heshan Hilly Land Interdisciplinary Experimental Station (22°34′–22°41′N, 112°50′–112°54′E) of South China Botanical Garden, the Chinese Academy of Sciences, Guangdong Province, China (Site 2). The two species were also planted in mono-culture in 1984. The long term mean annual, January and July temperatures is 21.7, 13.1 and 28.7°C, respectively. Mean annual precipitation is 1760 mm. The soil is acrisol19. The soil with broad leaf species was more fertile (higher in soil C and N) than soil with conifer species in each site (Table 1). Details of the species and site information are presented in Table 1. All necessary permits in this study have been obtained from Maoershan Forest Research Station of the Northeast Forestry University and Heshan Hilly Land Interdisciplinary Experimental Station of South China Botany Garden, Chinese Academy of Sciences, respectively.
Root excavation and dissection
Root samples were collected in early May 2012. For each sample, three mature trees were randomly selected. Soil blocks measuring 30 cm × 30 cm × 30 cm were excavated with a distance of 1 m from the tree trunk. The soil was carefully removed to expose the main lateral root branch. The intact root branches with at least the first five branch orders were selected. These samples were put immediately into a cooler and transported to the laboratory for subsequent processing.
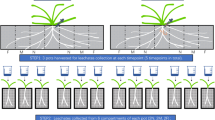
The root branches were gently washed in deionized water to remove soil. The definition of root branch order followed Pregitzer's method with the most distal roots as the first-order roots1. According to previous studies on root anatomic structures2,4, the first three orders of F. mandshurica and the first two orders of L. gmelinii, P. elliottii and A. mangium were composed of primary-developed tissues and regarded as ephemeral root modules. In forceps method, these root modules were collected by dissecting roots to the above orders of these two species (see Fig. 1A, B). Before dissecting, root branches were wetted in a petri dish. Then, roots belonging to different orders were carefully dissected with forceps. For root samples by hand-kneading method, they were gently kneaded and roots falling to a white paper were collected (see Fig. 1C, D). In the course of hand-kneading, some roots and barks from higher-order woody roots might also fell down. These roots were usually larger and in deeper color than other root segments and were easily picked out.
Chemical measurements
Root samples were oven-dried at 50°C and then were ground for chemical measurement. Root total carbon (C) and nitrogen (N) were determined by an elemental analyzer (Vario Microcube; Elementar, Hanau, Germany). The concentration of other elements including P, Ca, S, Mg, Ba, Zn, Mn, Pb, Cu, V and Li were measured by ICP-MS (Elan DRC-e, PerkinElmer, USA).
Data analysis
The difference of each chemical between root-order-based and hand-kneading methods was analyzed by independent t-test. In each species, paired t-test with averaged chemical values was employed to evaluate overall difference of 13 chemicals between the two methods. Finally, for all the four species, analysis of covariance (ANCOVA) was used to assess whether the ratio of root chemicals by hand-kneading method to those by root-order-based method was different from 1. The level of significance was at 0.05. All analyses were conducted in R 3.00 statistical platform (R Development Core Team).
References
Pregitzer, K. S. et al. Fine root architecture of nine North American trees. Ecological Monographs 72, 293–309 (2002).
Xia, M. X., Guo, D. L. & Pregitzer, K. S. Ephemeral root modules in Fraxinus mandshurica. New Phytologist 188, 1065–1074 (2010).
Guo, D. L. et al. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist 180, 673–683 (2008).
Long, Y. Q., Kong, D. L., Chen, Z. X. & Zeng, H. Variation of the Linkage of Root Function with Root Branch Order. PloS one 8, e57153 (2013).
Espeleta, J. F., West, J. B. & Donovan, L. A. Tree species fine-root demography parallels habitat specialization across a sandhill soil resource gradient. Ecology 90, 1773–1787 (2009).
Hishi, T. Heterogeneity of individual roots within the fine root architecture: causal links between physiological and ecosystem functions. Journal of Forest Research 12, 126–133 (2007).
Valenzuela-Estrada, L. R., Vera-Caraballo, V., Ruth, L. E. & Eissenstat, D. M. Root anatomy, morphology and longevity among root orders in Vaccinium corymbosum (Ericaceae). American Journal of Botany 95, 1506–1514 (2008).
Salguero-Gómez, R. & Casper, B. B. Keeping plant shrinkage in the demographic loop. Journal of Ecology 98, 312–323 (2010).
Wang, G. L., Fahey, T. J. & Xue, S. Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 171, 583–590 (2013).
Jackson, R. B., Mooney, H. A. & Schulze, E. D. A global budget for fine root biomass, surface area and nutrient contents. Proceedings of the National Academy of Sciences 94, 7362–7366 (1997).
Wang, W. et al. Effects of litter types, microsite and root diameters on litter decomposition in Pinus sylvestris plantations of northern China. Plant and Soil 374, 677–688 (2014).
Goebel, M. et al. Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecological Monographs 81, 89–102 (2011).
Matamala, R., Gonzalez-Meler, M. A., Jastrow, J. D., Norby, R. J. & Schlesinger, W. H. Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 302, 1385–1387 (2003).
Strand, A. E., Pritchard, S. G., McCormack, M. L., Davis, M. A. & Oren, R. Irreconcilable differences: fine-root life spans and soil carbon persistence. Science 319, 456–458 (2008).
Sun, Y., Gu, J. C., Zhuang, H. F., Guo, D. L. & Wang, Z. Q. Lower order roots more palatable to herbivores: a case study with two temperate tree species. Plant and soil 347, 351–361 (2011).
Hunter, M. D. in Root Feeders: An Ecosystem Perspective. (eds. Johnson, S. N. & Murray, P. J.) 68–95 (CABI Publishing, Cambridge; 2008)
Li, A., Guo, D. L., Wang, Z. Q. & Liu, H. Y. Nitrogen and phosphorus allocation in leaves, twigs and fine roots across 49 temperate, subtropical and tropical tree species: a hierarchical pattern. Functional Ecology 24, 224–232 (2010).
Wang, Z. Q., Guo, D. L., Wang, X. R., Gu, J. C. & Mei, L. Fine root architecture, morphology and biomass of different branch orders of two Chinese temperate tree species. Plant and Soil 288, 155–171 (2006).
Li, Z. A., Peng, S. L., Rae, D. J. & Zhou, G. Y. Litter decomposition and nitrogen mineralization of soils in subtropical plantation forests of southern China, with special attention to comparisons between legumes and non-legumes. Plant and Soil 229, 105–116 (2001).
Tjoelker, M. G., Craine, J. M., Wedin, D., Reich, P. B. & Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist 167, 493–508 (2005).
Withington, J. M., Reich, P. B., Oleksyn, J. & Eissenstat, D. M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecological Monographs 76, 381–397 (2006).
Rewald, B., Ephrath, J. E. & Rachmilevitch, S. A root is a root is a root? Water uptake rates of Citrus root orders. Plant, cell & environment 34, 33–42 (2011).
Fan, P. P. & Guo, D. L. Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163, 509–515 (2010).
Comas, L. H. & Eissenstat, D. M. Patterns in root trait variation among 25 co-existing North American forest species. New Phytologist 182, 919–928 (2009).
Freschet, G. T., Cornelissen, J. H. C., Van Logtestijn, R. S. P. & Aerts, R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology 98, 362–373 (2010).
Clemmensen, K. E. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618 (2013).
Wang, J. J. et al. Fine root mercury heterogeneity: metabolism of lower-order roots as an effective route for mercury removal. Environmental science & technology 46, 769–777 (2011).
Guo, Y. Y. et al. Fine root branch orders contribute differentially to uptake, allocation and return of potentially toxic metals. Environmental Science & Technology 47, 11456–11472 (2013).
Zadworny, M. & Eissenstat, D. M. Contrasting the morphology, anatomy and fungal colonization of new pioneer and fibrous roots. New Phytologist 190, 213–221 (2011).
Acknowledgements
We thank Prof. Shenglei Fu and Dr. Jiacun Gu for their assistance in field sampling and Ke Zhao and Junfei Guo for their nice work in processing root samples. We are also grateful to Dr. Junjian Wang, Dan Flynn and Huifang Wu for their careful revision and comments in improving this study. Thanks are also given to Prof. Dali Guo and Hui Zeng for their encouraging us to finish this work. This study was funded by Natural Science Foundation of China (NSFC Grants 31200344), Postdoctoral Science Foundation of China (2013M530333) and open fund of Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
D.L.K., C.E.M.: Conceived and designed the experiments. C.E.M., D.L.K.: Performed the experiments. C.E.M.: Analyzed the data. D.L.K.: Contributed reagents/materials/analysis tools. C.E.M., D.L.K.: Wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kong, D., Ma, C. Acquisition of ephemeral module in roots: a new view and test. Sci Rep 4, 5078 (2014). https://doi.org/10.1038/srep05078
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05078
This article is cited by
-
Responses of absorptive root and mycorrhizal colonization of Chinese fir (Cunninghamia lanceolata) to varied environmental conditions
Plant Ecology (2022)
-
Do root modules still exist after they die?
Forest Ecosystems (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.