Abstract
It has been postulated that associative memory is formed by at least two sets of external stimuli, CS and US, that are transmitted to the memory centers by distinctive conversing pathways. However, whether associative memory can be induced by the activation of only the olfactory CS and a biogenic amine-mediated US pathways remains to be elucidated. In this study, we substituted the reward signals with dTrpA1-mediated thermogenetic activation of octopaminergic neurons and the odor signals by ChR2-mediated optical activation of a specific class of olfactory neurons. We show that targeted activation of the olfactory receptor and the octopaminergic neurons is indeed sufficient for the formation of associative olfactory memory in the larval brain. We also show that targeted stimulation of only a single type of olfactory receptor neurons is sufficient to induce olfactory memory that is indistinguishable from natural memory induced by the activation of multiple olfactory receptor neurons.
Similar content being viewed by others
Introduction
Appetitive olfactory memory is formed in Drosophila by at least two sets of external stimuli, conditioned odor stimuli (CS) mediated by the olfactory receptor neurons (ORNs) and unconditioned reward stimuli (US) mediated by the octopaminergic (OA) neurons. Previous studies demonstrated that neural signaling of OA neurons is required for the acquisition of appetitive memory in both adult flies and larvae1,2. It has also been shown that induced activation of OA neurons substitutes appetitive US3,4. Moreover, recent studies suggest that distinctive sets of DA neurons differentially mediate appetitive and aversive US information during memory acquisition in the adult flies4,5,6,7. While these studies have uncovered the functional neural circuitries that mediate US information during the course of associative memory induction, the contribution of CS pathway in memory acquisition is yet to be addressed by targeted activation approaches. In particular, whether associative memory can be induced by targeted activation of only the olfactory CS and the OA-mediated US pathways remains to be elucidated. Moreover, whether the complex combinatorial signals mediated by the activation of multiple ORNs are necessary to induce distinctive associative memory has not been addressed so far.
With its simple neuroanatomical design, the larval brain of Drosophilamelanogaster can be utilized as an excellent model system for the elucidation of the neurocircuitry mechanism of memory. The architecture of the larval brain is a simple extension of the embryonic axonal plan and the neuronal pathway of olfactory information is straightforward without redundancy8. Using a single-odor paradigm, we described previously that sugar-reward training produces appetitive olfactory memory that persists medium term in the third instar larvae2,9.
In this study, we applied optogenetic and thermogenetic techniques to the analysis of the neurocircuitry mechanism of memory induction in the larval brain. We substituted sugar reward stimuli by thermogenetic activation of OA neurons with the dTrpA1 channel10 and odor stimuli by optical activation of a specific class of olfactory receptor neurons (ORNs) with Channelrhodopsin-2 (ChR2)11. We show that targeted activation of the converging memory circuitry with blue light and heat produces associative memory in the transgenic larvae. Furthermore, we also show that memory thus produced is specific to the odorant determined by the type of the activated ORNs and as stable as natural memory produced by the activation of multiple ORNs using a real odorant.
Results and Discussion
Optogenetic and thermogenetic constructs
The Drosophila larva has simple and straightforward olfactory system with only 21 olfactory receptors (Ors)8. Odors are sensed by the larval dorsal organ innervated by the dendrites of ORNs, each of which express one or a small number of Ors and projects to specific glomerulus of the larval antennal lobe (AL)8,12,13. Olfactory information sensed by ORNs is further conveyed by projection neurons to mushroom body (MB) (Figure 1a). Previous studies12,13,14 have elucidated the 21-dimentinal-odor space of fruit fly larvae demonstrating distinctive selectivity of each of the larval receptors for different odorants. We chose four receptors (Or24a, Or42b, Or82a, Or83b); Or24a and Or42b show strong affinity for acetophenone and ethyl acetate, respectively, at low dose while Or82a is activated by geranyl acetate only at high dose12,13. All three odorants are moderate attractants for Drosophila larvae and have been used effectively as CS in appetitive conditioning using sucrose9. Or83b is broadly expressed in all types of ORNs15. We made direct fusion constructs that express the coding sequence of ChR2 under an Or-transcriptional promoter sequence (Or-ChR2 in Figure 1b) that have been characterized to drive distinctive expression in a specific set of larval ORNs12,14,16.
Larval appetitive memory circuit and the transgenic constructs.
(a) Schematic presentation of the larval appetitive memory circuit. The odor CS sensed by ORNs is transmitted to specific sets of AL glomeruli. From there, projection neurons (PN) further convey the information to the MB calyx. Sucrose reward stimuli sensed by sugar-responsive gustatory neurons is firstly transmitted to subesophageal ganglia (SOG) and then transmitted to the AL glomeruli and the calyx of MB via the modulatory OA neurons. (b) Structures of the Or-ChR2 and Tdc2-dTrpA1 constructs. Or-ChR2 construct; the coding sequence of ChR2 is directly placed under the transcriptional promoter sequence of an Or gene, which is expressed in a specific set of the larval ORNs. Tdc2-dTrpA1 construct; the coding sequence of dTrpA1 is fused with the transcriptional promoter sequence of the Tdc2 gene, which is expressed in the larval OA neurons.
To examine functionality of Or-ChR2 constructs and behavioral consequences of their stimulation, we tested phototactic responses of Or-ChR2 larvae on an agar plate with diametrically-opposed quadrants illuminated with blue light (468 nm) or red light (625 nm) (Supplemental Figure 1b). Compared to the w(CS10) control larvae, all Or-ChR2 larvae showed increased preference for blue light (Supplemental Figure 2a) resulting in significant suppression of negative phototaxis (Supplemental Figure 2b). By contrast, Or-ChR2 larvae showed normal photophobic response toward white light as did the control larvae (Supplemental Figure 3).
To substitute reward US with artificial stimuli, we made a fusion construct (Tdc2-dTrpA1 in Figure 1b) that expresses dTrpA1-coding sequence under the promoter of the tyrosine decarboxylase 2 (Tdc2) gene17. The Tdc2 promoter sequence utilized in this construct has been characterized to drive specific expression in the larval OA neurons2,18,19. Whereas control larvae preferred cooler temperature, transgenic larvae carrying the Tdc2-dTrpA1 construct showed positive thermotactic response when assayed on a 21–28°C temperature gradient (Figure 2 and Supplemental Movies 1, 2). By contrast, transgenic larvae carrying Or-ChR2 but not Tdc2-dTrpA1 construct preferred cooler temperatures exhibiting negative thermotactic RI, indistinguishable from the w (CS10) control larvae (Supplemental Figure 4).
Thermotactic behaviors of Tdc2-dTrpA1 larvae.
(a) Thermotactic behavior test. Thermotactic behavior of larvae was tested on a 2.5% agar plate with temperature gradient between 21°C and 28°C (shown on the right). Typically, ~50 larvae were aligned along the medial line and the numbers of animals moved in the warm and cool halves were counted after 3 min. Response index (RI) was calculated as indicated. (b) Thermotactic response indices of Tdc2-dTrpA1 larvae. While the w (CS10) control larvae preferred cooler temperatures exhibiting negative thermotactic RI, all the larvae carrying the Tdc2-dTrpA1 construct preferred warm temperatures exhibiting positive thermotactic RI. ***p < 0.001 by Mann-Whitney U-test with w (CS10). Number of sample is indicated in the bar.
Targeted activation of ORNs and OA neurons causes enhanced larval phototaxis toward blue light
In order to examine the effects of targeted activation of a specific set of ORNs and OA neurons, we examined phototactic behaviors of transgenic larvae that carry both the Or-ChR2 and the Tdc2-dTrpA1 constructs. We stimulated the double-transgenic larvae with blue light for 60 sec on a 28°C agar plate and then tested their phototactic behaviors on a agar plate with diametrically-opposed quadrants illuminated with blue or red light (Figure 3a). Dual stimulation with blue light and heat resulted in no alteration for control larvae (Figure 3b, 3c). By contrast, it caused significant increase in the number of larvae on the blue area for all the larvae carrying an Or-ChR2 construct (Figure 3b), resulting in suppression of the negative phototactic response indices (Figure 3c). Only 45 sec stimulation was enough to suppress larval photophobic response while stimulation ≥ 90 sec failed to induce the behavioral alteration probably due to hyper activation (Supplemental Figure 5a).
Artificial induction of larval memory with blue light and heat.
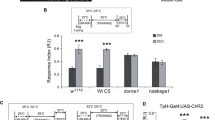
(a) Dual stimulation and phototaxis test. Transgenic larvae carrying both Or-ChR2 and Tdc2-dTrpA1 were illuminated with blue light for 60 sec on a 28°C agar plates. Larvae were then transferred to the center of a 21°C agar plate with diametrically opposed quadrants illuminated with blue (468 nm) or red (625 nm) light. Typically, ~50 larvae were used and the numbers of animals moved in the blue and the red areas were counted after 60 sec. Response index (RI) was calculated as indicated. (b) Phototactic responses of the transgenic larvae on the blue/red quadrants plate. For the Or-ChR2;Tdc2-dTrpA1 larvae, the number of animals moved in the blue area was significantly increased after dual stimulation (Trained: 60 sec blue light at 28°C) while no alteration was detected for the w (CS10) larvae. ***p < 0.001 by Mann-Whitney U-test between trained and naïve larvae. Number of sample is indicated in the bar. (c) Phototactic response indices toward blue light. Dual stimulation with blue light and heat suppressed negative phototactic response toward blue light for the Or-ChR2;Tdc2-dTrpA1 but not the w (CS10) control larvae. ***p < 0.001 by Mann-Whitney U-test between trained and naïve larvae. Number of sample is indicated above the bar.
To clarify the exact requirements of artificial conditioning, we stimulated Or-ChR2;Tdc2-dTrpA1 larvae for 60 sec with either blue or red light in combination with cool (21°C) or warm temperature (28°C) (Figure 4a). The phototactic behavior of the double-transgenic larvae was not altered by red light at either temperature (Figure 4b). By contrast, blue light stimulation at 28°C but not at 21°C caused significant suppression of negative phototaxis toward blue light. No difference was detected for the w (CS10) control larvae by either conditioning.
Induction of associative memory requires both blue light and heat.
(a) Larval conditionings and phototaxis test. Or-ChR2;Tdc2-dTrpA1 larvae were conditioned for 60 sec at 28°C or 21°C in combination with blue or red light. Larval phototactic behavior was then tested at 21°C on the blue/red quadrants plate. Response index (RI) was calculated as indicated. (b) Phototactic responses of transgenic larvae differentially conditioned with blue light or heat. Only the combined stimulation with blue light and heat caused suppression of negative phototaxis toward blue light for the double transgenic larvae (Or24a-ChR2;Tdc2-dTrpA1 or Or42b-ChR2;Tdc2-dTrpA1) while no difference was induced with red light at either temperature. None of the conditionings altered the phototactic response of the w (CS10) control larvae. ***p < 0.001 Kruskal-Wallis ANOVA followed by Dunn’s post hoc test. Number of sample is indicated above the bar. (c) Phototactic behaviors of single and double transgenic larvae. Larvae carrying either Tdc2-dTrpA1, Or-ChR2 or both were conditioned with blue light at 28°C for 60 sec and then tested on the blue/red quadrants plate at 21°C. Compared to the larvae carrying either dTrpA1 or Or-ChR2 or to the w (CS10) control larvae, the transgenic larvae carrying both Tdc2-dTrpA1 and Or-ChR2 exhibited significant suppression of negative phototaxis toward blue light ***p < 0.001 by Mann-Whitney U-test. Number of sample is indicated above the bar.
To further confirm the requirements of artificial conditioning, we then conditioned different transgenic larvae carrying either Or-ChR2 or Tdc2-dTrpA1 or both. We conditioned the larvae with blue light at 28°C for 60 sec and examined their behavior on the blue/red quadrants plate. Compared to the control w (CS10) larvae, no difference was observed for the transgenic larvae carrying Tdc2-dTrpA1 alone (Figure 4c). Larvae carrying the Or-ChR2 construct alone showed reduced photophobic response toward blue light as described above. However, no difference was found between the naïve and trained Or-ChR2 larvae (Supplemental Figure 2).
By contrast, larvae carrying both Or-ChR2 and Tdc2-dTrpA1 transgenes exhibited significant suppression of negative phototaxis toward blue light compared to the w(CS10) control larvae and to the transgenic larvae carrying either Tdc2-dTrpA1 or Or-ChR2 alone (Figure 4c). These results thus clearly indicated that the targeted activation of both the olfactory CS and the appetitive US pathways is required to alter the larval phototactic behavior on the blue/red quadrants plate.
Targeted activation of ORNs and OA neurons produces odor-specific memory
While the above experiments examined associative requirements of the dual stimuli for larval conditioning, they fail to exclude the possibility that the combined effect could merely be caused by general desensitization toward blue light. To exclude such possibility, we stimulated the Or-ChR2;Tdc2-dTrpA1 double transgenic larvae with blue light at 28°C and examined their olfactory response using a real odorant known to activate each receptor: acetophenone for Or24a and ethyl acetate for Or42b (Figure 5a). Similarly to the photophobic responses, only 45 sec was enough to alter larval olfactory response while stimulation ≥ 90 sec failed to do so (Supplemental Figure 5b). Consistent with the predicted Or specificity, dual stimulation of Or24a-ChR2;Tdc2-dTrpA1 larvae resulted in enhanced olfactory response for acetophenone but not for ethyl acetate. By contrast, dual stimulation of Or42b-ChR2;Tdc2-dTrpA1 larvae resulted in enhanced olfactory response for ethyl acetate but not for acetophenone (Figure 5b). Associative conditionings at 28°C caused no difference in the olfactory responses of the larvae carrying the Or24a-ChR2, Or42b-ChR2 or Tdc2-dTrpA1 construct alone (Supplemental Figure 6). These results thus demonstrated that targeted activation of a specific set of ORNs and the OA neurons indeed produced distinctive olfactory memory that is determined by the specificity of the Or promoter introduced into the test animals.
Olfactory responses of transgenic larvae trained with blue light and heat.
(a) Associative larval conditioning and olfactory response test. Transgenic larvae carrying both Or-ChR2 and Tdc2-dTrpA1 were conditioned with blue light at 28°C for 60 sec. Larvae were then transferred to an olfactory test plate at 21°C, on which specific odorant is spotted on one side. After 3 min, the numbers of animals moved in the indicated semicircular areas were counted. Typically, ~50 larvae were used in a single test. Response index (RI) was calculated as indicated. (b) Odor specificity of artificial memory induced by the targeted stimulation. 60 sec conditioning with blue light at 28°C resulted in significant increase in the olfactory response of Or24a-ChR2;Tdc2-dTrpA1 larvae toward acetophenoe but not ethyl acetate. Similarly, the artificial stimulation resulted in significant increase in the olfactory response of Or42b-ChR2; Tdc2-dTrpA1 larvae toward ethyl acetate but not acetophenoe. **p < 0.01 by Mann-Whitney U-test between 21°C and 28°C. Number of sample is indicated in the bar. (c) Stability of artificial olfactory memory. Temporal changes of olfactory response indices of the conditioned Or24a-ChR2;Tdc2-dTrpA1 larvae toward acetophenoe. Larvae were stimulated for 60 sec with blue light at 21°C or 28°C and then tested at the indicated time. **p < 0.01, ***p < 0.001, by Mann-Whitney U-test between 21°C and 28°C. Each data point represents RI of independent animal groups (average of 10-14 experiments).
Artificial olfactory memory persists for medium term
Previous studies2,9 showed that larval appetitive conditioning with sucrose produces associative olfactory memory that persists for two hours. To determine the stability of artificial olfactory memory produced with blue light and heat, we analyzed the stability of olfactory memory of Or24a-ChR2;Tdc2-dTrpA1 larvae using acetophenone. Similarly to the natural conditioning, blue-light stimulation at 28°C produced olfactory memory that was detected up to 120 min and lost by 180 min (Figure 5c). These results thus demonstrate that targeted activation of ORNs and OA neurons produced olfactory memory that is as stable as natural memory produced with real odorant and sucrose.
By the use of a combined optogenetic and thermogenetic technique, we have demonstrated that targeted activation of a specific set of olfactory CS and reward US neurons is indeed sufficient to induce distinctive olfactory memory that is as stable as natural memory. Since many of the fly Ors are tuned to a variety of odorants, a given odorant tends to activate multiple types of Ors12,13,20. Consequently, conventional olfactory conditioning leads to activation of multiple classes of ORNs transmitting complex combinatorial codes to the memory centers. By contrast, our results demonstrate that targeted stimulation of only a single type of ORNs is sufficient to induce olfactory memory that is indistinguishable from memory induced by the activation of multiple ORNs. Previous studies showed that larvae with only single type ORNs are able to exhibit robust chemotactic responses14. By extending this observation, our results reveal the minimum elementary circuitry that mediates the induction of distinctive olfactory associative memory in the larval brain.
It has bee shown that Drosophila larvae exhibit positive chemotaxis toward variety of odorants and associative conditioning with odor and sucrose produces olfactory memory with enhanced olfactory response to the CS odor2,9. Our result that naïve larvae carrying a Or-ChR2 construct exhibit enhanced phototaxis toward blue light is consistent with the intrinsic response caused by the activation of the larval ORN. Moreover, the result that associative conditioning with blue light at 28°C fails to alter phototactic behaviors of transgenic larvae carrying Or-ChR2 alone (Supplementary Figure 2) argues against the possibility that the warm temperature itself serves as a reward for the larvae.
Studies in the adult flies have indicated that signaling of dopamine neurons is required for the generation of not only aversive memory but also appetitive memory4,5,6,7. In the adult flies, the PPL1 cluster DA neurons mediate US information in aversive learning6,7,21 while the PAM cluster DA neurons mediate US information in appetitive learning with sugar and nutrient value4,5. Studies using mutations of the D1-DA receptor dDA1 suggest that DA signaling is also required for both aversive and appetitive learning in larvae22,23. Given that blockade of synaptic transmission from the DA neurons defined by the TH-GAL4 driver has little effects on appetitive learning in larvae2, reward signals could also be mediated by larval DA neurons that are not specified by the TH-GAL4 driver, which covers the PPL1 but not the PAM cluster in the adult brain4,5. While activation of OA neurons by the Tdc2 promoter is sufficient to produce appetitive memory in larvae (this study and Schroll et al.3), whether the appetitive information of OA neurons is mediated by another set of DA neurons in the larval brain is yet to be answered by further anatomical and behavioral investigations.
In conclusion, we have developed a robust artificial stimulation technique that allows us to produce distinctive olfactory appetitive memory in fruit fly larvae by brief conditioning. Given its simplicity, our technique is applicable for in depth elucidation of the neurocircuitry mechanisms of memory in the fly brain in combination with other molecular genetic techniques such as the GAL4-UAS, RexA-lexAop or QF-QUAS expression systems24 that allow targeted analysis of specific sets of neurons.
Methods
Transgenic fly construction
Or-ChR2 constructs
To make direct fusion between an Or-promoter and ChR2, we amplified the Or promoter sequences that have been utilized to construct Or-GAL4 lines and characterized to show Or-specific expression in the larval brain14,16. The promoter sequence of Or83b (2642 base pairs) was amplified based on the sequence used in Or83b-GAL416. The promoter sequences of Or42b (8039 base pairs), Or24a (8720 base pairs) and Or82a (1865 base pairs) were amplified based on the sequences used in the respective Or-GAL4 lines14.
We inserted the PCR-amplified ChR2 (H134R)25 coding fragment (a gift from Karl Deisseroth) tagged with HA into a P-element based pUAST vector by replacing the vector sequence spanning 5x UAS and Hsp70 basic promoter with ChR2 coding fragment. The Or-promoter fragment was inserted upstream of the ChR2 coding sequence in the derived vector construct.
Tdc2-dTrpA1 construct
Construction of Tdc2-dTrpA1 was done according to the scheme used to construct Tdc2-GAL417. Genomic sequence containing a region from -3459 to +4530 of the dTdc2 locus was amplified through stepwise PCR and cloned into pCaSpeR4. Previous studies2,18,19 demonstrated that this element drives specific expression in the larval OA neurons. A 3.6 kb DNA fragment containing the coding region of dTrpA110 (gift from Paul Garrity) tagged with HA was inserted upstream of the dTdc2 coding sequence17.
We verified all the constructs by complete sequencing prior to germinal transformation, which was performed by Genetics Services, Inc. (Sudbury, MA).
Larval assays
A white (w) stock, w (CS10), outcrossed with Canton S at 10 times was used as the standard stock. To ensure homogeneous genetic background, all the stocks were outcrossed to the w (CS10) stock at least 5 times. Behavioral experiments were performed using early third instar larvae (72–76 h after egg laying) raised with 1 mM all-trans retinal (Sigma) in a standard fly media. Larval thermotactic behavior was tested on a 2.5% agar plate with temperature gradient between 21°C and 28°C on the agar surface as measured with an infrared thermometer. Larval olfactory response was tested as described2.
Optogenetics
Optogenetic stimulation was performed according to the methods26,27 with modifications. For conditioning of larvae, we used an apparatus equipped with light-emitting diodes (LEDs) (3 Watt each) on dual flexible arms (Twin LED Light, RELYON, Tokyo). For blue light, 468 nm LEDs were used with a 490 nm short-path filter. For red light, 625 nm LEDs were used with a 580 nm long-path filter. We placed larvae on an agar plate set at the bottom of a cylinder-like covering made with aluminum foil and illuminated them from 12 cm above the agar surface with the dual LED apparatus (Supplemental Figure 1a). For conditioning, we placed an agar plate on a hot plate to have the agar surface temperature either at 28°C or 21°C (without heating) as determined with an infrared thermometer.
Larval phototactic behaviors were tested on a 2.5% agar plate with diametrically-opposed quadrants, each illuminated with 22 LEDs (Supplemental Figure 1b). For blue light, 468 nm LEDs (2,600 mcd) were used with 490 nm short-path filter of 50% transmission. For red light, 625 nm LEDs (2,600 mcd) were used with 580 nm long-path filter of 50% transmission.
References
Schwaerzel, M. et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502 (2003).
Honjo, K. & Furukubo-Tokunaga, K. Distinctive neuronal networks and biochemical pathways for appetitive and aversive memory in Drosophila larvae. J. Neurosci. 29, 852–862 (2009).
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006).
Burke, C. J. et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492, 433–437 (2012).
Liu, C. et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, 512–516 (2012).
Aso, Y. et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451 (2010).
Aso, Y. et al. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet 8, e1002768 (2012).
Vosshall, L. B. & Stocker, R. F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007).
Honjo, K. & Furukubo-Tokunaga, K. Induction of cAMP response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J. Neurosci. 25, 7905–7913 (2005).
Hamada, F. N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008).
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Kreher, S. A., Kwon, J. Y. & Carlson, J. R. The molecular basis of odor coding in the Drosophila larva. Neuron 46, 445–456 (2005).
Kreher, S. A., Mathew, D., Kim, J. & Carlson, J. R. Translation of sensory input into behavioral output via an olfactory system. Neuron 59, 110–124 (2008).
Fishilevich, E. et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 15, 2086–2096 (2005).
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004).
Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271–282 (2003).
Cole, S. H. et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 280, 14948–14955 (2005).
Zhang, T., Branch, A. & Shen, P. Octopamine-mediated circuit mechanism underlying controlled appetite for palatable food in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 110, 15431–15436 (2013).
Selcho, M., Pauls, D., El Jundi, B., Stocker, R. F. & Thum, A. S. The role of octopamine and tyramine in Drosophila larval locomotion. J. Comp. Neurol. 520, 3764–3785 (2012).
Hallem, E. A., Dahanukar, A. & Carlson, J. R. Insect odor and taste receptors. Annu. Rev. Entomol. 51, 113–135 (2006).
Claridge-Chang, A. et al. Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415 (2009).
Kim, Y. C., Lee, H. G. & Han, K. A. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 27, 7640–7647 (2007).
Selcho, M., Pauls, D., Han, K. A., Stocker, R. F. & Thum, A. S. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE 4, e5897 (2009).
del Valle Rodriguez, A., Didiano, D. & Desplan, C. Power tools for gene expression and clonal analysis in Drosophila. Nat. Methods. 9, 47–55 (2012).
Zhao, S. et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods. 8, 745–752 (2011).
Honjo, K., Hwang, R. Y. & Tracey, W. D., Jr Optogenetic manipulation of neural circuits and behavior in Drosophila larvae. Nat. Protoc. 7, 1470–1478 (2012).
Pulver, S. R., Pashkovski, S. L., Hornstein, N. J., Garrity, P. A. & Griffith, L. C. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 101, 3075–3088 (2009).
Acknowledgements
We thank Drs. Karl Deisseroth, Paul Garrity and Fumika N. Hamada for plasmids. We are grateful to Mr. Kohei Takeda for his help in characterization of transformants and to Dr. Hiromu Tanimoto for helpful comments. We thank the Bloomington Stock Center for fly stocks. Supported by Grants-in-Aid for Scientific Research, MEXT, Japan (K.F.T.).
Author information
Authors and Affiliations
Contributions
T.H. and C.Y.L. performed the behavioral experiments and prepared the figures. M.Y.K. and K.H. constructed the plasmid and the transgenic flies. K.F.T. designed the experiments and wrote the main manuscript text. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Supplementary Information
Video 1
Supplementary Information
Video 2
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Honda, T., Lee, CY., Yoshida-Kasikawa, M. et al. Induction of Associative Olfactory Memory by Targeted Activation of Single Olfactory Neurons in Drosophila Larvae. Sci Rep 4, 4798 (2014). https://doi.org/10.1038/srep04798
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04798
This article is cited by
-
Serotonin receptor 5-HT7 in Drosophila mushroom body neurons mediates larval appetitive olfactory learning
Scientific Reports (2020)
-
Prior activity of olfactory receptor neurons is required for proper sensory processing and behavior in Drosophila larvae
Scientific Reports (2018)
-
DISC1 causes associative memory and neurodevelopmental defects in fruit flies
Molecular Psychiatry (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.