Key Points
-
Suggests that all procedures, whether therapeutic, diagnostic or screening, that certainly result or are likely to result in bleeding need antibiotic prophylaxis.
-
Recommends the use of a second or third choice antibiotic in each close subsequent surgical session.
-
Highlights that all events concerning antibiotic prophylaxis must be noted in the patient's clinical chart, together with the signed informed consent form, to avoid legal implication.
Abstract
Although quite consistent indications on antibiotic prophylaxis for infective endocarditis (IE) have been reported internationally, several common dental practice issues are still not clear: which dental procedures require antibiotic prophylaxis? In the case of multiple procedures can the same antibiotic be used? How can dentists identify high-risk conditions for IE? Do dentists verify patient antibiotic intake? What are the requirements of antibiotic prophylaxis in cases of coexistence of diseases which involve host defence impairment? What are the modalities of second choice drug administration? And finally, are chlorhexidine mouthwashes before dental procedures combined with antibiotics useful or not? Uncertainty also persists as far as the real need for prophylaxis is concerned and although several sources have suggested that a wide prospective randomised controlled study may be the definitive solution, problems exist in performing such a study.
Similar content being viewed by others
Introduction
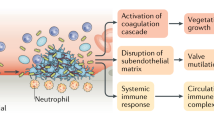
Infective endocarditis (IE) after dental procedures has always been a major topic in international literature as well as in clinical practice since it affects patient health and involves medico-legal implications. Although most international guidelines indicate a single double dose of antibiotic administration before any invasive dental procedures in a selected patient population in both adults and children,1,2,3,4 many controversies continue to trouble the entire scientific community as to the real need for antibiotic prophylaxis (AP). Only recently the National Institute for Health and Clinical Excellence (now National Institute for Health and Care Excellence, NICE)5 modified its guidelines for AP against IE by adding the term 'routinely' after 'antibiotic prophylaxis against infective endocarditis is not recommended', thus emphasising that the use or the non-use of AP is the practitioner's responsibility, after adequately informing the patient about risks and benefits of the treatment and after his/her written approval/refusal. Nevertheless, several issues of uncertainty also exist in common dental practice. These issues mainly address the problem of which invasive procedures require AP and whether the same antibiotic can be used in cases of multiple procedures, planned or not, in a short range of time to avoid the risk of bacterial resistance to that antibiotic. Minor questions concern the dentist's ability to identify high risk conditions for IE, the need to establish a correct antibiotic intake for the patient, the possible AP indication in cases of coexistence of diseases which involve host defence impairment, the modalities of second choice drug administration, and the usefulness of chlorhexidine mouthwashes before dental procedures combined with antibiotics.
Procedures requiring AP
According to the guidelines of the American Heart Association (AHA),1 the European Society of Cardiology (ESC),3 and the Australian expert group on infective endocarditis prophylaxis,2 all dental procedures which may involve bleeding, such as manipulation of gingival tissue or the periapical region of teeth or incision of the oral mucosa, should be preceded by AP. That is, all procedures, whether therapeutic, diagnostic or screening, which certainly result or are likely to result in bleeding,6 since bleeding involves the communication between the vascular tree and the oral environment with the consequent risk of bacteraemia and then the risk of distant site infections such as IE, although there is no scientific evidence of such a correlation. A complete list of such procedures has never been provided by institutional societies internationally, however, it would be useful for clinical and legal purposes (Box 1). Actually, an almost complete list was previously reported by the 1997 AHA guidelines,7 by a 2006 Spanish consensus document,8 and by the 2008 Australian guidelines.2 In the latter, dental procedures were divided into two subgroups, one in which AP is always required and another in which AP is required only if multiple procedures are being conducted, or if the procedure is prolonged or periodontal disease is present due to the higher risk of bacteraemia in these conditions.2
Suture removal requires particular attention since it is listed among those procedures for which AP is not recommended both in the Australian and in the updated 2015 European guidelines,2,3 whereas it is recommended in the 2007 AHA guidelines,1 although no explanations were given for these opposing indications. Oddly, AP was not recommended for suture removal in the previous AHA guidelines. Actually, since sutures are an unavoidable site of plaque accumulation, all efforts should be made to avoid or reduce microorganism penetration of the tissues during their removal. Therefore, the thread should be previously cleaned and it must be cut just near the tissue surface so that only a minimal portion of the contaminated suture material passes through the soft tissues during its removal. Moreover, since bleeding during suture removal was found to be related to bacteraemia,9 in the most frequent case of loose suture removal, where bleeding usually does not occur, AP does not seem to be indicated. In some cases, however, some suture knots are so invaginated within the soft tissue that their removal involves bleeding of a certain intensity and, therefore, AP would seem absolutely necessary. If the risk of bleeding can be predicted based on the type of surgical procedure, the use of absorbable sutures can be proposed.9 Sutures should be avoided whenever possible, and AP should be administered within two hours, preferably by the parenteral route if significant bleeding occurs after suture removal.9 Lastly, since a correlation was found between the number of sutures and bacteraemia incidence, patients undergoing removal of more than five sutures should undergo AP.10 AP is therefore recommended before suture removal in high risk patients, depending on the number of sutures to be removed and the predictable risk of bleeding.1,11
AP for multiple close procedures
Amoxicillin is considered the first choice antibiotic worldwide for IE prophylaxis in patients without a history of allergy to β-lactam antibiotics. Actually, it belongs to the wide β-lactam family of antibiotics (penicillin, first to fourth generation cephalosporins, carbapenems, and monobactams) which are the most widely used antibiotics, especially in many European countries (Italy, France, Spain, Poland, Holland).12 The production of β-lactamase enzymes, which are able to separate the antibiotic β-lactam ring, thus neutralising antibacterial activity, is the most common mechanism of resistance to penicillins. Furthermore, although amoxicillin has an extremely short half-life (1-1.5 hours) while remaining above the minimum inhibitory concentration (T >MIC = four hours) for a long period,13 it was previously found to determine bacterial resistance after both short-duration therapies (three days)14 and repeated administrations of a single weekly prophylactic dose (1 to 3 g), although it was much more frequent when the administrations were more numerous15,16,17 since the selection of strains with reduced susceptibility to amoxicillin has been shown to be a rapid phenomenon. Actually, since the bactericidal action of amoxicillin is relatively slow, time-dependent, and scarcely correlated to drug concentrations that are greater than the MIC so that it cannot increase more than a certain level (approximately four times the MIC), there will be a residual population of microorganisms, specifically viridians group streptococci, which remains when drug levels decrease to less than the minimum bactericidal concentration (MBC)13 which is about six hours for 2 g of oral amoxicillin, while exceeding the MIC (≤1 μg/mL), and which may develop resistance. The following factors, which are often present simultaneously, may predispose to the persistence of microorganisms in the bloodstream for six hours, precisely when the amoxicillin serum level decreases under the MBC level, even when amoxicillin prophylaxis has been correctly carried out: 1) delay in starting the procedure; 2) excessive duration of the procedure; 3) persistent bleeding after surgery which may prolong the release of microorganisms into the bloodstream; 4) occasional or repeated post-surgical bleeding due to wound trauma; and 5) possible contamination from dental plaque in case of a second intention wound healing. Prolonged post-surgical bleeding due to anticoagulant treatment is also frequent in patients with heart valve prostheses which are those who require AP the most. Moreover, amoxicillin-resistant oral streptococci were identified in dental plaque specimens from healthy adults (5%)18 and especially from children and adolescents at risk for IE (20%).19 IE due to penicillin-resistant oral streptococci was also observed in patients who took amoxicillin before surgery.20,21 In fact, patients for which IE AP is indicated take amoxicillin more frequently than all other patients for prophylactic purposes alone and this may promote resistance mechanisms by their oral cavity microbial flora.
Since repeated administrations of the same antibiotic may cause resistance by the oral microbial flora, not only for amoxicillin,15,16,17 but also to other antibiotics,17,22 nullifying the effectiveness of prophylaxis itself, a clear protocol needs to be defined by scientific societies for multiple invasive close procedures which are often necessary in dentistry, for example in periodontology, where cause-related therapy involves debridement, scaling and root planing, more commonly performed in four different sessions after 1–2 preliminary professional oral hygiene sessions. Similarly, multiple sessions are also required for all oral surgical procedures, in which at least one pre-operative professional oral hygiene session, in order to obtain healthy gingival tissues, and suture removal a few days after surgery, are needed.
In order to reduce the risk of antibiotic resistance due to frequent close repetition of the same molecule for AP, two different solutions may be contemplated. The first involves the use of a second or third choice antibiotic in each subsequent surgical session,15,16 although differences exist in such a drug preference due to territorial differences in microbial resistance. Actually, vancomycin was recommended by the 2008 Australian guidelines as one of the second choice drugs to be used in patients with hypersensitivity to penicillin, those on long-term penicillin therapy or who have taken a β-lactam antibiotic more than once in the previous month,2 although on the contrary, it was discarded by AHA as a second choice drug for IE AP. Particular attention should also be paid to clindamycin since the incidence of adverse reactions to this drug was recently found to be higher in England (13 fatal and 149 non-fatal reactions/one million prescriptions) than that to amoxicillin (zero fatal reactions out of almost three million prescriptions and 22.62 non-fatal reactions out of one million prescriptions).23 Nonetheless, these rates may differ from country to country and may change over time. However, clindamycin is safer than the risk of IE in high-risk patients, although it is less safe than other antibiotics.24 Therefore, in cases of multiple close procedures a different antibiotic should be used before each procedure, choosing among those proposed by the AHA and ESC guidelines: amoxicillin, cephalexin or other first- or second-generation oral cephalosporins, clindamycin and macrolids (azythromycin or clarithromycin) in the case of oral administration, while ampicillin, cefazolin or ceftriaxone and clindamycin are recommended in the case of parenteral administration.1,3 Obviously, allergy to penicillins must be taken into account when choosing and cephalosporins should not be used in individuals with a history of anaphylaxis, angioedema, or urticaria after use of penicillins.1
Alternatively, the association with a β-lactamase competitive inhibitor such as clavulanic acid with amoxicillin and sulbactam with ampicillin (in the case of parenteral administration) may be used in all procedures, especially in countries where this association is not frequent, since resistant microbial strains to amoxicillin/clavulanate (AC) were also found.25
It is obvious that, whenever possible, more than one procedure should be performed in a single session, as in full mouth disinfection f or scaling and root planing, although this implies an unavoidable and often considerable lengthening of operative times, which, on the other hand, cannot be tolerated by patients with increased cardiac risk as those at risk for IE often are.
Identifying high risk conditions
In most cases, high risk conditions which need AP are clear to the dentist from the patient's medical history, as in cases of previous IE or prosthetic valve insertion. In other cases risk conditions cannot be easily definable by the dentist, especially in patients with repaired congenital heart diseases which may have a residual defect with altered endothelialisation at the site or adjacent to the site of a prosthetic patch or device,1,3 so that he/she may need to request that the patient's cardiologist writes a report on the patient's risk of IE, which should be attached to the patient's clinical chart.24
The role of dental care providers
An additional problem that dentists and dental hygienists are facing is verifying the actual and correct patient intake of AP prescriptions since this must take place one hour before the procedure when taken orally. If the antibiotic has not been taken, immediate administration should be performed, preferably through an intramuscular injection if the procedure is to be performed sooner and if there are no contraindications to this kind of administration, such as an anticoagulant therapy. On the other hand, if the procedure has already started or been completed and the antibiotic has not been taken, it should be administered within two hours of the procedure.1 All these events must be noted in the patient's clinical chart, together with the signed informed consent form to avoid legal implications, although, after an adequate explanation and prescription, taking the antibiotic is the patient's responsibility and not the prescriber's.26
Host defence impairment
Another issue in IE prevention is to establish whether and when the existence of any co-morbidity conditions which may imply a reduction of host defences, such as older age, diabetes mellitus, immunosuppressive conditions or therapies, and dialysis, indicates AP in cardiac conditions for which, although at risk for endocarditis, prophylaxis to date is not indicated. Nevertheless, there is no scientific evidence suggesting that AP prevents distant site infections for most medical conditions which imply an immune system impairment.27,28,29 However, these conditions may complicate IE. Each of them independently increases the risk of an adverse outcome from IE and they often occur in combination, which further increases morbidity and mortality rates.1 Moreover, nonspecific immune mediators, such as complement and phagocytes, are important factors that protect against bacteraemia, at first exposure to a microorganism, early in bacteraemia in an immune host and throughout the course of bacteraemia since they increase the response due to the specific immune mediators.30 Therefore, in absence of any specific indications by international guidelines, it seems reasonable to perform AP in all patients with compromised immune defences having risk conditions for IE,
Second choice drug administration
As for administration modalities, if amoxicillin should be taken one hour before surgery due to its high (74–92%) and quick bioavailability, independently from food intake,13 the same rule does not apply to second and third choice antibiotics, although their high prophylactic dose allows higher drug concentrations than therapeutic doses. Actually, if the bioavailability is high after oral administration, independently from food intake, for the majority of the first generation cephalosporins (cephalexin = 80–95%) and clindamycin (90%), this does not apply to macrolids. The bioavailability is low for azithromycin (40%) and clarithromycin (50%); it is even lower in the case of food intake for azithromycin but only slightly lower for clarithromycin. For this reason azithromycin should be taken on an empty stomach.13
Chlorhexidine mouthwash
Although the effectiveness of chlorhexidine mouthwashes in reducing both oral microbial flora31,32 and post-extraction bacteraemia33,34 have been shown, no published updated guidelines for IE recommend chlorhexidine use in combination with antibiotics,1,2,3,4,5,35 before dental procedures as a preventive measure, and, rather, the AHA described that it is unlikely that topical antiseptics are effective in significantly reducing the frequency, magnitude, and duration of bacteraemia associated with dental procedures.1 It seems reasonable to assume that a reduction of both oral microbial flora and possibly post-procedural bacteraemia is the premise for which host defence mechanisms, associated or not with appropriate AP, may overcome the risk of IE, provided that the patient does not frequently use chlorhexidine as a daily mouthwash since resistance of oral microbes is highly likely.36,37,38 Therefore, a one minute rinse with pure 0.20% chlorhexidine mouthwashes is advisable before any invasive oral procedures in patients at high risk for developing IE.
All these variables in dental treatment give an account of the need for more detailed protocols to be followed in order to protect not only the health of patients but the practitioner's work in the medico-legal field as well.
What to expect?
IE AP is a complex problem which is still not resolved, especially if we consider that its necessity has not been well-established at all. In England, with the 2008 NICE suppression of IE AP, a 78.6% reduction in AP prescribing was found in the first two years after the emanation of the NICE guidelines,39 but a temporal, although not causal, association between the same point of reference time and a significant increase of IE incidence was also found.40 Despite this, in the 2015 NICE update on the use of prophylaxis against IE, no change of any of the existing guidelines was applied in that the longstanding increase in the incidence of IE in the UK and other countries was considered not to be well-understood and possibly due to a number of factors. In July 2016 a radical change was made, which now gives dentists the chance to discuss and decide with the patient the treatment to be performed.5 It is exceedingly clear that a wide prospective randomised controlled study must be the final leg of the AP trip which began almost 60 years ago, when the first AHA guidelines were published.41
The need for a large sample size and, therefore, its multi-centricity, as well as ethics due to problems in control group establishment, have possibly always prevented this kind of study being performed, while it actually represents the missing link which can determine whether AP is effective in IE prevention or not and therefore whether and to whom AP must be prescribed. Nevertheless, obstacles prevent the feasibility of such a study. One has always been, and still is, that dental procedures are usually performed on an outpatient basis and often in private practices where selection, enrolment and monitoring of patients are difficult to carry out. Moreover, since bacteraemia can occur in dentulous patients during routine daily activities such as chewing food and oral hygiene measures, and it is possibly inversely related to the degree of oral hygiene as far as duration and amount are concerned, it cannot be excluded that an IE which may occur after an invasive dental procedure may be related to poor oral hygiene and not to the procedure itself. Simultaneously, a patient registry could be established, with an agreement among the various cardiologic, dental and infective societies all over the world, to collect all cases of IE and to investigate their statistical and temporal association with previous oral invasive procedures with or without AP, as the AHA already did in the early 80s.42 All these difficulties account for the fact that, although ten years have elapsed from the last AHA guidelines and the need for both a case-control study and monitoring studies was already anticipated in such guidelines,1 only monitoring studies have been published. However, they did not find any difference in the incidence of IE before and after the 2007 AHA guidelines,43,44,45,46,47,48 although those guidelines were found not to be completely followed by dental practitioners,49,50 and many patients took antibiotics before dental procedures without any prescription or with a prescription by their family doctors.49 Furthermore, in England, before the present NICE update, not all clinicians followed NICE guidelines. Dentists were found to follow the NICE guidelines (87%) more than the infection specialists (56%) while cardiologists and cardiothoracic surgeons were the least adherent (39%).51 Actually, as early as 2006, some English scientific literature supported AP prescribing for high risk cardiologic conditions in cases of dental procedures which involve dento-gingival manipulation or endodontics,35 and, therefore, a certain number of English professionals certainly followed those guidelines, and the ESC ones afterwards.3,52 In conclusion, on the one hand IE AP continues to be a great dilemma since no evidence exists in favour or against its effectiveness in high risk patients, even in relation to the cost/benefit ratio of its administration.53 On the other hand several issues on AP should be clarified by all cardiologic, infection and dental scientific societies in order to improve drug performance and ensure proper treatment for each clinical condition for both patient safety and medico-legal protection of dental providers, although, to this end, antibiotic treatment or non-treatment for IE prophylactic purpose should always be discussed with the patient and valid consent should always be gained and signed by the patient on his/her clinical chart.
References
Wilson W, Taubert K A, Gewitz M et al. Prevention of infective endocarditis: Guidelines from the American Heart Association. Circulation 2007; 116: 1736–1754.
Infective Endocarditis Prophylaxis Expert Group. Prevention of endocarditis. 2008 update from Therapeutic Guidelines Antibiotic version 13, and Therapeutic Guidelines: Oral and Dental version 1. Melbourne: Therapeutic Guidelines limited. 2008. Available at http://www.csanz.edu.au/wp-content/uploads/2014/12/Prevention_of_Endocarditis_2008.pdf (accessed April 2017).
The task force for the management of infective endocarditis of the European Society of Cardiology. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J 2015; 36: 3075–3123.
Baltimore RS, Gewitz M, Baddour LM et al. Infective endocarditis in childhood: 2015 update. A scientific statement from the American Heart Association. Circulation 2015; 132: 1487–1515.
National Institute for Health and Care Excellence. Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. 2008. Updated July 2016. Available at nice.org.uk/guidance/cg64 (accessed March 2017).
Kaplan E I, Anthony B F, Bisno A et al. Prevention of bacterial endocarditis. AHA Committee report. Circulation 1977; 56: 139A–143A.
Dajani A S, Taubert K A, Wilson W et al. Prevention of bacterial endocarditis. Recommendations of the American Heart Association. J Am Med Ass 1997; 277: 1794–1801.
Gutiérrez J L, Bagán JV, Bascones A et al. Consensus document on the use of antibiotic prophylaxis in dental surgery and procedures. Med Oral Patol Oral Cir Bucal 2006; 11: E188–E205.
Brown A R, Papasian C J, Shultz P, Theisen F C, Shultz R E . Bacteremia and intraoral suture removal: can an antimicrobial rinse help? J Am Dent Assoc 1998; 129: 1455–1461.
Giglio J A, Rowland R W, Dalton H P, Laskin D M . Suture removal-induced bacteremia: a possible endocarditis risk. J Am Dent Assoc 1992; 123: 65–66: 69–70.
K Crawford JJ, Small EW . Bacteremia following intraoral suture removal. Oral Surg Oral Med Oral Pathol 1988; 65: 23–28.
Ferrer P, Sabaté M, Ballarín E et al. Sales of macrolides, lincosamides, streptogramins, and amoxicillin/clavulanate in the in-and outpatient setting in 10 European countries, 2007–2010. Springerplus 2015; 4: 612–621.
Levison M E, Levison J H . Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 2009; 23: 791–819.
Chardin H, Yasukawa K, Nouacer N et al. Reduced susceptibility to amoxicillin of oral streptococci following amoxicillin exposure. J Med Microbiol 2009; 58: 1092–1097.
Southall P J, Mahy N J, Davies R M et al. Resistance in oral streptococci after repeated two-dose amoxycillin prophylaxis. J Antimicrob Chemother 1983; 12: 141–146.
Woodman A J, Vidic J, Newman H N et al. Effect of repeated high dose prophylaxis with amoxycillin on the resident oral flora of adult volunteers. J Med Microbiol 1985; 19: 15–23.
Harrison G A J, Stross W P, Rubin M P et al. Resistance in oral streptococci after repeated three-dose erytromycin prophylaxis. J Antimicrob Chemother 1985; 15: 471–479.
Masuda K, Nemoto H, Nakano K et al. Amoxicillin-resistant oral streptococci identified in dental plaque specimens from healthy Japanese adults. J Cardiol 2012; 59: 285–290.
Nemoto H, Nomura R, Ooshima T et al. Distribution of amoxicillin-resistant oral streptococci in dental plaque specimens obtained from Japanese children and adolescents at risk for infective endocarditis. J Cardiol 2013; 62: 296–300.
Hall G E, Baddour L M . Apparent failure of endocarditis prophylaxis caused by penicillin-resistant streptococcus mitis. Am J Med Sci 2002; 324: 51–53.
Bavunoglu I, Sahin S, Yilmaz M et al. Native triple-valve endocarditis caused by penicillin-resistant streptococcus sanguis. Nat Clin Pract Cardiovasc Med 2007; 4: 340–343.
Seppälä Helena, Al-Juhaish M, Järvinen H et al. Effect of prophylactic antibiotics on antimicrobial resistance of viridans streptococci in the normal flora of cataract surgery patients. J Cataract Refract Surg 2004; 30: 307–315.
Thornhill M H, Dayer M J, Prendergast B et al. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother 2015; 70: 2382–2388.
Thomhill M H, Dayer M, Lockart P B et al. A change in the NICE guidelines on antibiotic prophylahis. Br Dent J 2016; 221: 112–114.
Toussaint K A, Gallagher J C . ß-lactamß-lactamase inhibitor combinations: from then to now. Ann Pharmacother 2015; 49: 86–98.
Martin M V, Longman L P, Forde M P et al. Infective endocarditis and dentistry: the legal basis for an association. Br Dent J 2007; 203: E1–E3.
Lockhart P B, Loven B, Brennan M T . The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J Am Dent Ass 2007; 138: 458–474.
Little J W, Falace D A, Miller C S et al. Antibiotic prophylaxis in dentistry: an update. Gen Dent 2008; 56: 20–28.
Termine N, Panzarella V, Ciavarella D et al. Antibiotic prophylaxis in dentistry and oral surgery. Int Dent J 2009; 59: 263–270.
Cates KL . Host factors in bacteremia. Am J Med 1983; 75: 19–25.
Veksler A E, Kayrouz G A, Newman M G . Reduction of salivary bacteria by pre-procedural rinses with chlorhexidine 0.12%. J Periodontol 1991; 62: 649–651.
Sreenivasan P K, Gittins E . The effects of a chlorhexidine mouthrinse on culturable microorganisms of the tongue and saliva. Microb Res 2004; 159: 365–370.
Jokinen M A . Prevention of postextraction bacteremia by local prophylaxis. Int J Oral Surg 1978; 7: 450–452.
Tomás I, Álvarez M, Limeres J et al. Effect of a chlorhexidine mouthwash on the risk of postextraction bacteremia. Infect Control Hosp Epidemiol 2007; 28: 577–582.
Gould F K, Elliott T S J, Foweraker J et al. Guidelines for the prevention of endocarditis: report of the working party of the British Society for antimicrobial chemotherapy. J Antimicrob Chemother 2006; 57: 1035–1042.
RindomScxhiøtt C, Briner W W, Kirkland J J et al. Two years of oral use of chlorhexidine in man. III. Changes in sensitivity of the salivary flora. J Periodontal Res 1976; 11: 153–157.
Westergren G, Emilson C-G . In vitro development of chlorhexidine resistance in streptococcus sanguis and its transmissibility by genetic transformation. Scand J Dent Res 1980; 88: 236–243.
Grenier D, Bertrand J, Mayrand D . Porphyromonas gingivalis outer membrane vesicles promote bacterial resistance to chlorhexidine. Oral Microbiol Immunol 1995; 10: 319–320.
Thornhill M H, Dayer M J, Forde J M et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ 2011; 342: d2392.
Dayer M, Jones S, Prendergast B et al. Incidence of infective endocarditis in England, 2000–2013: a secular trend, interrupted time-series analysis. Lancet 2015; 385: 1219–1228.
Jones T D, Baumgartner L, Bellows M T et al. (Committee on Prevention of Rheumatic Fever and Bacterial Endocarditis, American Heart Association). Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1955; 11: 317–320.
Durack D T, Kaplan E L, Bisno A L . Apparent failures of endocarditis prophylaxis. Analysis of 52 cases submitted to a National Registry. J Am Med Ass 1983; 250: 2318–2322.
Pasquali S K, He X, Mohamad Z et al. Trends in endocarditis hospitalizations at US children's hospitals: impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J 2012; 163: 894–899.
Desimone D C, Tleyjeh I M, Correa de Sa D D et al. Incidence of infective endocarditis due to viridans group streptococci before and after publication of the 2007 American Heart Association's Endocarditis Prevention Guidelines. Circulation 2012; 126: 60–64.
Pant S, Patel N J, Deshmukh A et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65: 2070–2076.
Mackie A S, Liu W, Savu A, Marelli A J, Kaul P . Infective endocarditis hospitalization before and after the 2007 American Heart Association prophylaxis guidelines. Can J Cardiol 2016; 32: 942–948.
Khan O, Shafi A M A, Timmis A . International guideline changes and the incidence of infective endocarditis: a systematic review. Open Heart 2016; 3: e000498.
Bates H E, Hall M, Shah S S et al. Trends in infective endocarditis hospitalisations at United States children's hospitals from 2003 to 2014: impact of the 2007 American Heart Association antibiotic prophylaxis guidelines. Cardiol Young 2016; 15: 1–5.
Lockhart P B, Hanson NB, Ristic H, Menezes A R, Baddour L . Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. J Am Dent Assoc 2013; 144: 1030–1035.
Jain P, Stevenson T, Sheppard A et al. Antibiotic prophylaxis for infective endocarditis. Knowledge and implementation of American Heart Association Guidelines among dentists and dental hygienists in Alberta, Canada. J Am Dent Ass 2015; 146: 743–749.
Dayer M J, Chambers J B, Prendergast B et al. NICE guidance on antibiotic prophylaxis to prevent infective endocarditis: a survey of clinicians' attitudes. Q J Med 2013; 106: 237–243.
The task force on prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Eur Heart J 2009; 30: 2369–2413.
Glenny A M, Oliver R, Roberts G J et al. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry (Review). Cochrane Database Syst Rev 2013; 10: CD003813.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Pippi, R. Antibiotic prophylaxis for infective endocarditis: some rarely addressed issues. Br Dent J 222, 583–587 (2017). https://doi.org/10.1038/sj.bdj.2017.356
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2017.356