Abstract
Study design:
We introduced an adenoviral vector expressing interleukin-1β (IL-1β) small-hairpin RNA (shRNA) into the injured spinal cords to evaluate the therapeutic potential of IL-1β downregulation in a rat model of spinal cord injury (SCI).
Objectives:
The purpose of this study was to investigate the possible protective effects of the IL-1β downregulation on traumatic SCI in rats.
Setting:
Department of Orthopedic Surgery, The Second Affiliated Hospital, Fujian Medical University, Quanzhou, People’s Republic of China.
Methods:
An adenoviral shRNA targeting IL-1β was constructed and injected at the T12 section 7 days before SCI. The rats’ motor functions were evaluated by the Basso–Beattie–Bresnahan (BBB) rating scale. Immunofluorescence, enzyme-linked immunosorbent assay, flow-cytometric analysis and western blots were also performed.
Results:
Animals downregulating IL-1β had significantly better recovery of locomotor function and less neuronal loss after SCI. In addition, IL-1β downregulation significantly decreased tumor necrosis factor-alpha (TNF-α) level and Bax expression, reduced the activity of caspase-3 and increased Bcl-2 expression after SCI.
Conclusion:
This study demonstrated that the IL-1β downregulation may have potential therapeutic benefits for both reducing secondary damages and improving the outcomes after traumatic SCI.
Similar content being viewed by others
Introduction
Traumatic spinal cord injury (SCI) includes primary and secondary injury mechanisms. Primary injury is caused by the mechanical impact leading to direct contusion, shearing injury or laceration of the spinal cord. After the primary injury, the spinal cord will undergo a few sequential pathologic changes including ischemia, free radical release, lipid peroxidation, inflammation and apoptosis.1 In contrast to the primary and irreversible trauma, the secondary effects can potentially be influenced, thereby offering possibilities for therapeutic approaches. Although some therapeutic agents are being used to protect the injured spinal cords from those secondary pathological processes,2 there is still no effective treatment for SCI.
Interleukin-1β (IL-1β) has a crucial role in SCI. It was demonstrated that IL-1β is a key inflammatory mediator that is increasingly expressed after SCI,3, 4, 5 and the inflammatory process may impede neuronal repair leading to secondary spinal cord damage.6 Furthermore, increased IL-1β in the spinal cord has been associated with neuronal apoptosis.7, 8 In addition, injection of an IL-1 receptor antagonist (IL-1Ra) reduces IL-1β production in the spinal cord and thereby promotes functional recovery following SCI.9
RNA interference is a method of blocking gene function by inserting short sequences of RNA that match part of the target gene’s sequence.10, 11 There are two RNA interference approaches: small-interfering RNA and small-hairpin RNA (shRNA).12 ShRNA has proved to be an effective and a long-term silencing mechanism.13 It allows stable suppression of functions not only in cell culture but also in animals.14, 15 In the current study, we used adenovirus-mediated shRNA technology to inhibit the expression of IL-1β in spinal cord and then observed the therapeutic effect of rats with SCI.
Materials and methods
Production of the Ad-shIL-1β
To produce adenoviral shRNA targeting IL-1β (Ad-shIL-1β), shRNA sequence containing small-interfering RNA target 5′-ATGAACAACAAAAATGCCTCG-3′ was synthesized and inserted into pDC311-U6-MCMV-EGFP vector (purchased from Hanbio Co. Ltd, Shanghai, China). The pDC311-IL-1β-shRNA and pBHGlox E1, 3Cre16, 17 were co-transfected into HEK293 cells by using LipoFiter transfection reagent (Hanbio) to generate the recombinant adenoviruses (Ad-shIL-1β). Adenoviruses harboring green fluorescent protein (Ad-GFP) were used as a control. Ad-shIL-1β and Ad-GFP were propagated in HEK293 cells. The propagated recombinant adenoviruses in the HEK293 cells were purified, and the titer of virus was measured by plaque assays. Titer of Ad-shIL-1β was 1.2 × 1011 plaque formation unit per ml and that of Ad-GFP was 1.1 × 1011 plaque formation unit per ml.
The SCI model and experimental groups
Seventy-two adult male Sprague-Dawley rats (280–320 g) were used in this study. The animal experiments were performed in accordance with the guidelines of Laboratory Animals and were approved by the Animal Care and Use Committee of Fujian Medical University.
Spinal cord compression at T12 was performed following a previously established static compression model, as described in our previous paper.18 Briefly, animals were anesthetized by inhalation of 2–3% isoflurane administered at a flow rate of 1 l min−1. Midline skin incisions were performed to expose the T12 spinous processes. A laminectomy was performed at T12. The compression was applied by suspending the base of a compression platform (area 2 × 5 mm2) onto the exposed cord. A weight of 50 g was applied statically to the platform for exactly 5 min. After removing the platform, the muscles and skins were sutured.
The rats were randomly assigned into four groups (n=18 per group) including the Sham, the Vehicle, the Ad-GFP and the Ad-shIL-1β groups. In the Sham group, a laminectomy was performed only. In the Vehicle group, 3 μl normal saline was injected into the cord at T12 using a 5-μl micro-syringe with a 33-gauge needle (Hamilton, Reno, NV, USA) at a rate of 0.2 μl min−1,19, 20 7 days before SCI. In the Ad-GFP group, 3 μl Ad-GFP was injected 7 days before SCI. In the Ad-shIL-1β group, 3 μl Ad-shIL-1β was injected 7 days before SCI. After removal of the injectors, muscles and skins were sutured in separate layers.
Neurologic evaluation
The hindlimb locomotor function was assessed at pre-injury and 1, 3, 7, 14 and 21 days after SCI using the Basso–Beattie–Bresnahan (BBB) locomotor test developed by Basso et al.21 The hindlimb movements during locomotion were quantified using a scale ranging from 0 to 21. The rats were observed for 5 min at each time point by two observers who were blinded to the experimental protocol.
Immunochemical staining
The spinal cord sections from the compression epicenter were incubated with antibodies against NeuN (1:100; Invitrogen Life Technologies, Carlsbad, CA, USA) overnight at 4 °C. The sections were then incubated with Alexa Fluor 488-conjugated goat anti-rat IgG (Invitrogen Life Technologies). Fluorescent microscopy was conducted using an Axio Observer Z1 fluorescent microscope (Zeiss, Baden-Württemberg, Germany). Six sections were randomly selected from each animal, and six microscopy fields of each section were randomly selected for quantification of NeuN+ cells. A total of six rats were analyzed in each group. The positive staining in each section was measured using the Image J software (National Institutes of Health, Bethesda, MD, USA).
Measurement of tumor necrosis factor-alpha levels
Spinal cord segments with lesions were harvested at 24 h after SCI. Spinal cords were lysed and homogenized. The levels of tumor necrosis factor-alpha (TNF-α) were analyzed in the supernatants of tissue homogenates using enzyme-linked immunosorbent assay kits (Abcam, Cambridge, MA, USA) according to the manufacturer’s guidelines.
Isolation of leukocytes from spinal cord and flow-cytometric analysis
Spinal cord was harvested and then mechanically dissociated with the edge of a syringe. Cells were suspended in phosphate-buffered saline (PBS) buffer and then passed through a 70-μm nylon cell strainer (Becton Dickinson, San Jose, CA, USA) to isolate tissue debris. The cell suspension was centrifuged at 300 g, 4 °C for 5 min. The cell pellet was re-suspended in PBS and then lysed in 1 × RBC lysis buffer. Cells were then washed with PBS and incubated with the following anti-rat antibodies on ice for 15 min: FITC anti-Gr-1 (RP-1, BD Bioscience, San Jose, CA, USA), PE/Cy7 anti-CD45 (OX-1, Biolegend, San Diego, CA, USA), PE anti-CD11b/c (OX-42, Biolegend) and FITC anti-CD3 (eBioG4.18, eBioscience, San Diego, CA, USA). Samples were washed with PBS and loaded on a BD Calibur flow cytometer (BD Biosciences). To avoid debris, prepared spinal cord cells were stained with 7-Amino-Acinomycin D (BD Bioscience), which is a dye for dead cells. The data were analyzed using the FlowJo software (BD Bioscience).
Western blot analysis
The protein homogenates of the spinal cord samples were prepared by rapid homogenization in lysis buffer, and the lysates were separated by 10% SDS–PAGE. The proteins were transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). After incubation with rabbit anti-IL-1β (1:400; Santa Cruz Biotechnology, Paso Robles, CA, USA), rabbit anti-Bax (1:500; Abcam), rabbit anti-Bcl-2 (1:500; Abcam), rabbit anti-cleaved caspase-3 (1:500; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-GFAP (1:400; Santa Cruz Biotechnology) or rabbit anti-β-actin (1:400; Cell Signaling Technology), the membrane was incubated with HRP-conjugated secondary antibody in TBS-T for 1 h at room temperature. Immunoreactive complexes were visualized by ECL and exposed to X-ray film. The protein signals were quantified by scanning densitometry using AlphaEaseFC (AlphaInnotech, San Leandro, CA, USA). The results from each experimental group were expressed as the relative integrated intensity compared with β-actin.
Statistical analysis
All data were presented as the mean±s.d. One-way analysis of variance (ANOVA) was used to compare the IL-1β, TNF-α, Bax, Bcl-2, cleaved caspase-3 and glial fibrillary acidic protein (GFAP) levels and neuron density among the groups. The BBB scale data were analyzed with repeated-measures ANOVA. A P-value of less than 0.05 was considered as significant. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Ad-shIL-1β decreases IL-1β expression in the spinal cord
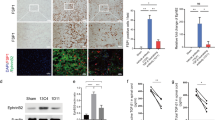
As shown in Figure 1, the levels of the IL-1β protein expression were determined by western blot analysis. The levels of IL-1β were low in the spinal cord of the Sham group, which increased 24 h after SCI. Compared with the Sham group, the levels of IL-1β were significantly higher in the spinal cord of the Vehicle, the Ad-GFP and the Ad-shIL-1β groups (P<0.05). However, the levels of the IL-1β in the Ad-shIL-1β group were significantly lower compared with the levels in the Vehicle and the Ad-GFP groups (P<0.05).
Detection of IL-1β protein in the spinal cords. (a) IL-1β and β-actin detected by western blot in the spinal cord lysates obtained from four groups 24 h after SCI. The blots are representative examples from six experiments each group. (b) The quantitative mean±s.d. data show the level of IL-1β protein expression. The band density is normalized as the ratio of IL-1β:β-actin (n=6). *P <0.05.
Neurological outcomes
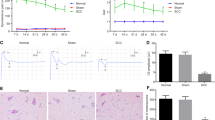
The evaluations of the hindlimb locomotor functions at pre-injury and 1, 3, 7, 14 and 21 days after SCI were shown in Figure 2. All the rats initially showed a slight decrease in the BBB score, and there was no significant difference among the four groups before SCI. The Sham group showed full recovery 3 days after SCI. The Vehicle, the Ad-GFP and the Ad-shIL-1β groups showed a sharp decrease in the BBB score 1 day after SCI, and there was no significant difference among the three groups. Partial improvements were observed, and there was no significant difference among the Vehicle, the Ad-GFP and the Ad-shIL-1β groups 3 days after SCI. However, the neurological improvements were significantly greater in the Ad-shIL-1β group compared with the improvements in the Vehicle and the Ad-GFP groups at 7, 14 and 21 days after SCI (P<0.05).
Ad-shIL-1β increases neuronal survival after SCI
Neuron survival was examined by an immunofluorescence technique using a specific antibody for neuron (neuN) (Figure 3). Qualitatively, neuron density was decreased in the lesion sections of the Vehicle group, the Ad-GFP group and the Ad-shIL-1β group when compared with the corresponding sections of the Sham group. However, Ad-shIL-1β significantly attenuated the decrease in neuron survival compared with vehicle animals.
After NeuN staining, six sections were randomly selected from each animal, and six microscopy fields of each section were randomly selected for quantification of NeuN+ cells. A total of six rats were analyzed in each group. The density of neurons at day 21 after SCI in the Sham (a), Vehicle (b), Ad-GFP (c) and Ad-shIL-1β (d) groups was determined by immunofluorescent labeling with anti-neuN antibody. (e) The data were plotted as the mean±s.d. *P <0.05. A full color version of this figure is available at the Spinal Cord journal online.
TNF-α expression after SCI
The TNF-α levels in the spinal cord 24 h after SCI were shown in Figure 4. The levels of TNF-α in the Sham group were significantly lower compared with the levels in the Vehicle, the Ad-GFP and the Ad-shIL-1β groups (P<0.05). However, the levels of the TNF-α in the Ad-shIL-1β group were significantly lower compared with the levels in the Vehicle and the Ad-GFP groups (P<0.05).
The TNF-α levels in the spinal cord tissues 24 h after SCI. Spinal cord homogenates from six rats of each group were used. Each sample was triplicated in the 96-well enzyme-linked immunosorbent assay (ELISA) plate. A standard curve was generated by applying the standard TNF-α protein provided in the kit. The data were plotted as the mean±s.d. *P<0.05.
Bax, Bcl-2 and active caspase-3 protein detection
Figures 5, 6, 7 show the western blot analysis of Bax, Bcl-2 and active caspase-3 in all groups of animals 24 h after SCI. The expressions of Bax and active caspase-3 were low in the spinal cord of the Sham group. In contrast, the expression of Bcl-2 was high. SCI induced increased expressions of Bax and active caspase-3 and decreased expression of Bcl-2. Ad-shIL-1β treatment greatly reduced the expressions of Bax and active caspase-3 and increased the expression of Bcl-2.
Detection of Bax protein in the spinal cords. (a) Bax and β-actin detected by western blot in the spinal cord lysates obtained from four groups 24 h after SCI. The blots are representative examples from six experiments each group. (b) The quantitative mean±s.d. data show the level of Bax protein expression. The band density is normalized as the ratio of Bax:β-actin (n=6). *P<0.05.
Detection of Bcl-2 protein in the spinal cords. (a) Bcl-2 and β-actin detected by western blot in the spinal cord lysates obtained from four groups 24 h after SCI. The blots are representative examples from six experiments each group. (b) The quantitative mean±s.d. data show the level of Bcl-2 protein expression. The band density is normalized as the ratio of Bcl-2:β-actin (n=6). *P<0.05.
Detection of caspase-3 protein in the spinal cords. (a) Caspase-3 and β-actin detected by western blot in the spinal cord lysates obtained from four groups 24 h after SCI. The blots are representative examples from six experiments each group. (b) The quantitative mean±s.d. data show the level of caspase-3 protein expression. The band density is normalized as the ratio of caspase-3:β-actin (n=6). *P<0.05.
Ad-shIL-1β decreases leukocyte infiltration in the spinal cord
To further evaluate the post-SCI inflammation in each group, we isolated infiltrating leukocytes from spinal cord segments with lesions and analyzed distinct leukocyte populations using flow cytometry. Here, we did not analyze the vehicle group because the data-mentioned above indicated that there was no remarkable difference between the vehicle and the Ad-GFP groups. As shown in Figure 8, the number of Gr1+ granulocytes at day 1 after SCI in Ad-GFP group was significantly higher in comparison with the sham group, and, Ad-shIL-1β profoundly reduced the granulocyte number, suggesting inhibition of granulocyte infiltration. At day 7 after SCI, the number of CD45loCD11b+ microglia was not significantly changed among each group. The numbers of CD45hiCD11b+ macrophages and CD3+ T cells were remarkably increased in the Ad-GFP group, compared with the sham group. However, their numbers were significantly decreased in the Ad-shIL-1β group in comparison with the Ad-GFP group, suggesting inhibition of infiltration of macrophages and T cells. Taken together, our data indicated that Ad-shIL-1β restrained infiltration of inflammatory leukocytes into post-SCI spinal cords.
Ad-shIL-1β decreases leukocyte infiltration in the spinal cord. (a) Flow-cytometric gating strategy for detection of leukocyte populations in isolated spinal cord cells. Single cells were selected using side scatter-height (SSC-H) and scatter-width (SSC-W). Then, Forward scatter (FSC) and side scatter-area (SSC-A) were used to gate total leukocytes. Gr1+ granulocytes were gated in the total leukocytes isolated from spinal cords at 24 h after SCI. For detection of microglia/macrophages and T cells, total leukocytes isolated from spinal cords at 7 days after SCI. CD45hiCD11b+ macrophages, CD45loCD11b+ microglia and CD3+ T cells were gated, respectively. Numbers in the dot plots are the percentages of corresponding cell populations. (b) Cell number of each leukocyte population in the spinal cord after SCI. *P<0.05.
GFAP detection
To evaluate the astrocyte reaction after SCI, we detected GFAP expression in the injured spinal cord sections using western blot. We found that GFAP expression was increased by about two-folds after SCI, and there was no significant difference of GFAP expression between the Ad-GFP and the Ad-shIL-1β groups (Figure 9), suggesting that Ad-shIL-1β might not influence reactive astrocytes after SCI.
Discussion
It is important to develop a therapy that can reduce the evolution of the secondary damages in injured spinal cords. A number of recent studies have focused on the effects of gene therapies on secondary injury.22 The data from the present study demonstrated a protective effect of the adenovirus-mediated shRNA against IL-1β gene on traumatic SCI.
The gene transfer of specific genes for therapeutic purposes offers a valuable approach to the treatment of SCI.22 It has been proven that adenoviruses efficiently transfer exogenous gene expression in the central nervous system including the spinal cord.23 Therefore, in the present study, we used an adenoviral vector expressing shRNA to IL-1β, followed by transfection into the spinal cords of the rats in vivo. As a result, the downregulation of IL-1β in the spinal cords promotes function recovery and increases neuronal survival after SCI.
Secondary injury in traumatic SCI is believed to be a result of a several destructive process such as inflammation, apoptosis, ischemia, hemorrhage and edema, all of them can cause dysfunction and death in neuronal cells.6 Previous reports stated that one of the most important factors precipitating post-traumatic degeneration in the spinal cord is inflammation.24 Furthermore, apoptosis is believed to have an important role in the pathogenesis of secondary injury.25 Consequently, reducing inflammation or apoptosis may improve neurological recovery or facilitate nerve regeneration.
TNF-α is a major initiator of inflammation and is released early after SCI.26 There is evidence that TNF-α also has an important role in the recruitment of inflammatory and immune cells to the injured site.27 Moreover, the infiltration of inflammatory cell to SCI sites is a major contributor to secondary degeneration.28 The inhibition of TNF-α promotes functional recovery after a traumatic SCI.29 The results presented in this study indicated that the TNF-α levels increased significantly in the spinal cord tissues 24 h after SCI. However, the IL-1β downregulation could significantly attenuate the increase in the TNF-α level, due to its ability to reduce inflammation. Consistent with the decreased pro-inflammatory cytokine levels in the spinal cord, our data indicated that Ad-shIL-1β significantly reduced leukocyte infiltration into the spinal cord after SCI. As an index of inflammation, inflammatory leukocyte infiltration has a critical role in the secondary damage of central nervous system tissues. Therefore, Ad-shIL-1β might alleviate the detrimental effect of inflammatory leukocytes through inhibition of their recruitment into the spinal cord. In addition, we did not observe significant changes in reactive astrocytes, suggesting that astrocyte reaction to SCI might not be tightly regulated by IL-1β. However, we do not know whether astrocyte-derived cytokines were altered by application of Ad-shIL-1β. Our ongoing study is checking the expression of both pro- and anti-inflammatory cytokines, as well as neurotrophic factors, in astrocytes after SCI.
Bcl-2 family proteins are the key step on regulation of apoptosis in the nervous system,30 which are composed of anti-apoptotic (Bcl-2) and pro-apoptotic (Bax) proteins that regulate the mitochondrial pathway of programmed cell death.31 Bcl-2 is the best-understood protein in a cell death pathway, inhibiting apoptosis and extending cell survival.32 Bax is a protein that promotes cell death by forming a heterodimer with Bcl-2 and blocking its anti-apoptotic actions.33 Caspases, especially caspase-3, are known to act downstream of Bax/Bcl-2 control and have a key role in the execution of apoptosis.34 In the present study, we detected the expressions of Bcl-2, Bax and cleaved-caspase-3 at 24 h after SCI and found that SCI upregulated the expressions of Bax and active caspase-3, and downregulated the expression of Bcl-2, which were consistent with the findings from previous studies.18, 35 However, adenovirus-mediated shRNA against IL-1β gene on traumatic SCI suppressed apoptosis by decreasing the expressions of Bax and active caspase-3, and increasing the expression of Bcl-2, thereby reducing cell death, and protected the injured spinal cord neurons.
Conclusion
In summary, the present study shows that IL-1β downregulation clearly had the ability to ameliorate inflammation and prevent apoptosis after the traumatic SCI. Our results indicated that the IL-1β downregulation may have potential therapeutic benefits not only for reducing the secondary damages but also for improving the outcomes after traumatic SCI.
Data archiving
There were no data to deposit.
References
Park E, Velumian AA, Fehlings MG . The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 2004; 21: 754–774.
Blesch A, Lu P, Tuszynski MH . Neurotrophic factors, gene therapy, and neural stem cells for spinal cord repair. Brain Res Bull 2002; 57: 833–838.
Wang CX, Olschowka JA, Wrathall JR . Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res 1997; 759: 190–196.
Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E . Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma 2000; 17: 203–218.
Pan JZ, Ni L, Sodhi A, Aguanno A, Young W, Hart RP . Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res 2002; 68: 315–322.
Hausmann ON . Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003; 41: 369–378.
Ehrlich LC, Peterson PK, Hu S . Interleukin (IL)-1beta-mediated apoptosis of human astrocytes. Neuroreport 1999; 10: 1849–1852.
Wang XJ, Kong KM, Qi WL, Ye WL, Song PS . Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol Sin 2005; 26: 934–942.
Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R . IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma 2001; 18: 947–956.
Lee SK, Kumar P . Conditional RNAi: towards a silent gene therapy. Adv Drug Deliv Rev 2009; 61: 650–664.
Birmingham A, Anderson E, Sullivan K, Reynolds A, Boese Q, Leake D et al. A protocol for designing siRNAs with high functionality and specificity. Nat Protoc 2007; 2: 2068–2078.
Shao Y, Chan CY, Maliyekkel A, Lawrence CE, Roninson IB, Ding Y . Effect of target secondary structure on RNAi efficiency. RNA 2007; 13: 1631–1640.
Chumakov SP, Kravchenko JE, Prassolov VS, Frolova EI, Chumakov PM . Efficient downregulation of multiple mRNA targets with a single shRNA-expressing lentiviral vector. Plasmid 2010; 63: 143–149.
Brummelkamp TR, Bernards R, Agami R . A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553.
Tiscornia G, Singer O, Ikawa M, Verma IM . A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci USA 2003; 100: 1844–1848.
Ng P, Parks RJ, Cummings DT, Evelegh CM, Sankar U, Graham FL . A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther 1999; 10: 2667–2672.
Ng P, Parks RJ, Cummings DT, Evelegh CM, Graham FL . An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther 2000; 11: 693–699.
Lan WB, Lin JH, Chen XW, Wu CY, Zhong GX, Zhang LQ et al. Overexpressing neuroglobin improves functional recovery by inhibiting neuronal apoptosis after spinal cord injury. Brain Res 2014; 1562: 100–108.
Hall JC, Priestley JV, Perry VH, Michael-Titus AT . Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J Neurochem 2012; 121: 738–750.
Wang YF, Fan ZK, Cao Y, Yu DS, Zhang YQ, Wang YS . 2-Methoxyestradiol inhibits the up-regulation of AQP4 and AQP1 expression after spinal cord injury. Brain Res 2011; 1370: 220–226.
Basso DM, Beattie MS, Bresnahan JC . A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12: 1–21.
Nakajima H, Uchida K, Yayama T, Kobayashi S, Guerrero AR, Furukawa S et al. Targeted retrograde gene delivery of brain-derived neurotrophic factor suppresses apoptosis of neurons and oligodendroglia after spinal cord injury in rats. Spine (Phila Pa 1976) 2010; 35: 497–504.
Toyooka T, Nawashiro H, Shinomiya N, Shima K . Down-regulation of glial fibrillary acidic protein and vimentin by RNA interference improves acute urinary dysfunction associated with spinal cord injury in rats. J Neurotrauma 2011; 28: 607–618.
Donnelly DJ, Popovich PG . Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 2008; 209: 378–388.
Beattie MS . Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med 2004; 10: 580–583.
Yan P, Li Q, Kim GM, Xu J, Hsu CY, Xu XM . Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma 2001; 18: 563–568.
Mao L, Wang H, Qiao L, Wang X . Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-alpha, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm 2010; 2010: 238321.
Holtz A, Nystrom B, Gerdin B . Spinal cord injury in rats: inability of nimodipine or anti-neutrophil serum to improve spinal cord blood flow or neurologic status. Acta Neurol Scand 1989; 79: 460–467.
Wang L, Wei FX, Cen JS, Ping SN, Li ZQ, Chen NN et al. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res 2014; 1575: 87–100.
Merry DE, Korsmeyer SJ . Bcl-2 gene family in the nervous system. Annu Rev Neurosci 1997; 20: 245–267.
Gross A, McDonnell JM, Korsmeyer SJ . BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999; 13: 1899–1911.
Yang B, Johnson TS, Thomas GL, Watson PF, Wagner B, Furness PN et al. A shift in the Bax/Bcl-2 balance may activate caspase-3 and modulate apoptosis in experimental glomerulonephritis. Kidney Int 2002; 62: 1301–1313.
Mooney SM, Miller MW . Expression of bcl-2, bax, and caspase-3 in the brain of the developing rat. Brain Res Dev Brain Res 2000; 123: 103–117.
Bullock ED, Johnson EM Jr. . Nerve growth factor induces the expression of certain cytokine genes and bcl-2 in mast cells. Potential role in survival promotion. J Biol Chem 1996; 271: 27500–27508.
Zhang J, Li D, Shen A, Mao H, Jin H, Huang W et al. Expression of RBMX after spinal cord injury in rats. J Mol Neurosci 2013; 49: 417–429.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (Nos. 81201403 and 81171114), the Scientific Research Project of Fujian Provincial Department of Education (No. JB12093), the Scientific Research Project of Quanzhou City Administration of Science and Technology (No. 2012Z31) and the Special Fund for Training Outstanding Talents of Quanzhou City.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, WP., Lin, JH., Cai, B. et al. Effect of adenovirus-mediated RNA interference of IL-1β expression on spinal cord injury in rats. Spinal Cord 54, 778–784 (2016). https://doi.org/10.1038/sc.2016.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.20
This article is cited by
-
Topotecan Reduces Neuron Death after Spinal Cord Injury by Suppressing Caspase-1-Dependent Pyroptosis
Molecular Neurobiology (2022)
-
Lithium alleviated spinal cord injury (SCI)-induced apoptosis and inflammation in rats via BDNF-AS/miR-9-5p axis
Cell and Tissue Research (2021)
-
HBO-PC Promotes Locomotor Recovery by Reducing Apoptosis and Inflammation in SCI Rats: The Role of the mTOR Signaling Pathway
Cellular and Molecular Neurobiology (2021)