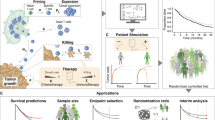
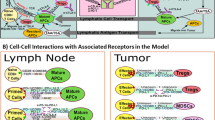
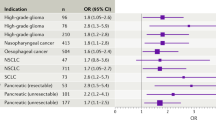
Abstract
Encouraging advances are being made in cancer immunotherapy modeling, especially in the key areas of developing personalized treatment strategies based on individual patient parameters, predicting treatment outcomes and optimizing immunotherapy synergy when used in combination with other treatment approaches. Here we present a focused review of the most recent mathematical modeling work on cancer immunotherapy with a focus on clinical translatability. It can be seen that this field is transitioning from pure basic science to applications that can make impactful differences in patients’ lives. We discuss how researchers are integrating experimental and clinical data to fully inform models so that they can be applied for clinical predictions, and present the challenges that remain to be overcome if widespread clinical adaptation is to be realized. Lastly, we discuss the most promising future applications and areas that are expected to be the focus of extensive upcoming modeling studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019).
Vesely, M. D., Kershaw, M. H., Schreiber, R. D. & Smyth, M. J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 29, 235–271 (2011).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Disis, M. L. Mechanism of action of immunotherapy. Semin. Oncol. 41, S3–S13 (2014).
Choudhry, H. et al. Prospects of IL-2 in cancer immunotherapy. BioMed. Res. Int. 2018, 9056173 (2018).
Belardelli, F., Ferrantini, M., Proietti, E. & Kirkwood, J. M. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 13, 119–134 (2002).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Rosenberg, S. A., Restifo, N. P., Yang, J. C., Morgan, R. A. & Dudley, M. E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Vaddepally, R. K., Kharel, P., Pandey, R., Garje, R. & Chandra, A. B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 12, 738 (2020).
Emens, L. A. & Middleton, G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol. Res. 3, 436–443 (2015).
Wang, Y. et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front. Pharmacol. 9, 185 (2018).
A to Z List of Cancer Drugs (National Cancer Institute, 2021); https://www.cancer.gov/about-cancer/treatment/drugs
Altrock, P. M., Liu, L. L. & Michor, F. The mathematics of cancer: integrating quantitative models. Nat. Rev. Cancer 15, 730–745 (2015).
Konstorum, A., Vella, A. T., Adler, A. J. & Laubenbacher, R. C. Addressing current challenges in cancer immunotherapy with mathematical and computational modelling. J. R. Soc. Interface 14, 20170150 (2017).
Malinzi, J., Basita, K. B., Padidar, S. & Adeola, H. A. Prospect for application of mathematical models in combination cancer treatments. Inform. Med. Unlocked 23, 100534 (2021).
Eftimie, R., Bramson, J. L. & Earn, D. J. Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull. Math. Biol. 73, 2–32 (2011).
Lim, C. et al. Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials? J. Thorac. Oncol. 11, 79–84 (2016).
Artzrouni, M. et al. The first international workshop on the role and impact of mathematics in medicine: a collective account. Am. J. Transl. Res 3, 492–497 (2011).
Barbolosi, D., Ciccolini, J., Lacarelle, B., Barlési, F. & André, N. Computational oncology—mathematical modelling of drug regimens for precision medicine. Nat. Rev. Clin. Oncol. 13, 242–254 (2016).
Hoffmann, K. et al. Integration of mathematical model predictions into routine workflows to support clinical decision making in haematology. BMC Med Inf. Decis. Mak. 20, 28 (2020).
Deisboeck, T. S., Wang, Z., Macklin, P. & Cristini, V. Multiscale cancer modeling. Annu. Rev. Biomed. Eng. 13, 127–155 (2011).
Hamis, S., Powathil, G. G. & Chaplain, M. A. J. Blackboard to bedside: a mathematical modeling bottom-up approach toward personalized cancer treatments. JCO Clin. Cancer Inform. 3, 1–11 (2019).
Yankeelov, T. E. et al. Multi-scale modeling in clinical oncology: opportunities and barriers to success. Ann. Biomed. Eng. 44, 2626–2641 (2016).
Wang, Z., Butner, J. D., Kerketta, R., Cristini, V. & Deisboeck, T. S. Simulating cancer growth with multiscale agent-based modeling. Semin. Cancer Biol. 30, 70–78 (2015).
Yin, A., Moes, D., van Hasselt, J. G. C., Swen, J. J. & Guchelaar, H. J. A review of mathematical models for tumor dynamics and treatment resistance evolution of solid tumors. CPT Pharmacomet. Syst. Pharm. 8, 720–737 (2019).
Kuznetsov, M., Clairambault, J. & Volpert, V. Improving cancer treatments via dynamical biophysical models. Phys. Life Rev. 39, 1–48 (2021).
Bull, J. A. & Byrne, H. M. The hallmarks of mathematical oncology. Proc. IEEE 110, 523–540 (2022).
Cristini, V., Koay, E. & Wang, Z. An Introduction to Physical Oncology: How Mechanistic Mathematical Modeling Can Improve Cancer Therapy Outcomes (CRC Press, 2017).
Stylianopoulos, T., Munn, L. L. & Jain, R. K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 4, 292–319 (2018).
Ulmschneider, M. B. & Searson, P. C. Mathematical models of the steps involved in the systemic delivery of a chemotherapeutic to a solid tumor: from circulation to survival. J. Control. Release 212, 78–84 (2015).
Dogra, P. et al. Mathematical modeling in cancer nanomedicine: a review. Biomed. Microdevices 21, 40 (2019).
Dewhirst, M. W. & Secomb, T. W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 17, 738–750 (2017).
Kim, M., Gillies, R. J. & Rejniak, K. A. Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues. Front. Oncol. 3, 278 (2013).
Sahai, N., Gogoi, M. & Ahmad, N. Mathematical Modeling and simulations for developing nanoparticle-based cancer drug delivery systems: a review. Curr. Pathobiol. Rep. 9, 1–8 (2021).
Dogra, P. et al. Image-guided mathematical modeling for pharmacological evaluation of nanomaterials and monoclonal antibodies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12, e1628 (2020).
Tran, A. P. et al. Delicate balances in cancer chemotherapy: modeling immune recruitment and emergence of systemic drug resistance. Front. Immunol. 11, 1376 (2020).
Mahlbacher, G. E., Reihmer, K. C. & Frieboes, H. B. Mathematical modeling of tumor-immune cell interactions. J. Theor. Biol. 469, 47–60 (2019).
Metzcar, J., Wang, Y., Heiland, R. & Macklin, P. A review of cell-based computational modeling in cancer biology. JCO Clin. Cancer Inform. https://doi.org/10.1200/cci.18.00069 (2019).
Wang, Z., Butner, J. D., Cristini, V. & Deisboeck, T. S. Integrated PK-PD and agent-based modeling in oncology. J. Pharmacokinet. Pharmacodyn. 42, 179–189 (2015).
Pappalardo, F., Palladini, A., Pennisi, M., Castiglione, F. & Motta, S. J. M. M. N. P. Mathematical and computational models in tumor. Immunol. Math. Model Nat. Phenom. 7, 186–203 (2012).
Dréau, D., Stanimirov, D., Carmichael, T. & Hadzikadic, M. An agent-based model of solid tumor progression. In Bioinformatics and Computational Biology, BICoB 2009, vol. 5462, 187–198 (Springer, 2009).
Chiacchio, F., Pennisi, M., Russo, G., Motta, S. & Pappalardo, F. Agent-based modeling of the immune system: NetLogo, a promising framework. BioMed. Res. Int. 2014, 907171 (2014).
Gong, C. et al. A computational multiscale agent-based model for simulating spatio-temporal tumour immune response to PD1 and PDL1 inhibition. J. R. Soc. Interface 14, 20170320 (2017).
Ghaffarizadeh, A., Heiland, R., Friedman, S. H., Mumenthaler, S. M. & Macklin, P. PhysiCell: an open source physics-based cell simulator for 3-D multicellular systems. PLoS Comput. Biol. 14, e1005991 (2018).
Ozik, J. et al. High-throughput cancer hypothesis testing with an integrated PhysiCell-EMEWS workflow. BMC Bioinf. 19, 483 (2018).
de Pillis, L. G., Radunskaya, A. E. & Wiseman, C. L. A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res. 65, 7950–7958 (2005).
dePillis, L. G., Eladdadi, A. & Radunskaya, A. E. Modeling cancer-immune responses to therapy. J. Pharmacokinet. Pharmacodyn. 41, 461–478 (2014).
Stalidzans, E. et al. Mechanistic modeling and multiscale applications for precision medicine: theory and practice. Netw. Syst. Med. 3, 36–56 (2020).
Stepanova, N. V. Course of the immune reaction during the development of a malignant tumour. Biophysics 24, 917–923 (1979).
Łuksza, M. et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 (2017).
Serre, R. et al. Mathematical modeling of cancer immunotherapy and Its synergy with radiotherapy. Cancer Res. 76, 4931–4940 (2016).
Sung, W., Hong, T. S., Poznansky, M. C., Paganetti, H. & Grassberger, C. Mathematical modeling to simulate the effect of adding radiation therapy to immunotherapy and application to hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 112, 1055–1062 (2022).
Adhikarla, V. et al. A mathematical modeling approach for targeted radionuclide and chimeric antigen receptor T cell combination therapy. Cancers 13, 5171 (2021).
Elpiniki, N., Steffen, E. E., Jana, L. G. & Yang, K. Mathematical modeling of an immune checkpoint inhibitor and its synergy with an immunostimulant. Discret. Continuous Dynamical Syst. B 26, 2133–2159 (2021).
West, J. et al. The immune checkpoint kick start: optimization of neoadjuvant combination therapy using game theory. JCO Clin. Cancer Inform. 3, 1–12 (2019).
Lindauer, A. et al. Translational pharmacokinetic/pharmacodynamic modeling of tumor growth inhibition supports dose-range selection of the anti-PD-1 antibody pembrolizumab. CPT Pharmacomet. Syst. Pharm. 6, 11–20 (2017).
Shah, D. K. & Betts, A. M. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J. Pharmacokinet. Pharmacodyn. 39, 67–86 (2012).
Simeoni, M. et al. Predictive pharmacokinetic–pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res. 64, 1094–1101 (2004).
Chen, X. et al. Mechanistic projection of first-in-human dose for bispecific immunomodulatory P-cadherin LP-DART: an integrated PK/PD modeling approach. Clin. Pharmacol. Ther. 100, 232–241 (2016).
Reigner, B. G. & Blesch, K. S. Estimating the starting dose for entry into humans: principles and practice. Eur. J. Clin. Pharmacol. 57, 835–845 (2002).
Betts, A. M. et al. The application of target information and preclinical pharmacokinetic/pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J. Pharmacol. Exp. Ther. 333, 2–13 (2010).
Escors, D. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J. Sci. 2014, 734515 (2014).
Kather, J. N. et al. High-throughput screening of combinatorial immunotherapies with patient-specific in silico models of metastatic colorectal cancer. Cancer Res. 78, 5155–5163 (2018).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Ruiz-Martinez, A. et al. Simulations of tumor growth and response to immunotherapy by coupling a spatial agent-based model with a whole-patient quantitative systems pharmacology model. PLoS Comput. Biol. 18, e1010254 (2022).
Wang, H., Zhao, C., Santa-Maria, C. A., Emens, L. A. & Popel, A. S. Dynamics of tumor-associated macrophages in a quantitative systems pharmacology model of immunotherapy in triple-negative breast cancer. iScience 25, 104702 (2022).
Ribba, B. et al. Prediction of the optimal dosing regimen using a mathematical model of tumor uptake for immunocytokine-based cancer immunotherapy. Clin. Cancer Res. 24, 3325–3333 (2018).
Schmidt, M. M. & Wittrup, K. D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 8, 2861–2871 (2009).
Griffiths, J. I. et al. Circulating immune cell phenotype dynamics reflect the strength of tumor-immune cell interactions in patients during immunotherapy. Proc. Natl Acad. Sci. USA 117, 16072–16082 (2020).
Tsur, N. et al. Predicting response to pembrolizumab in metastatic melanoma by a new personalization algorithm. J. Transl. Med 17, 338 (2019).
Tsur, N., Kogan, Y., Rehm, M. & Agur, Z. Response of patients with melanoma to immune checkpoint blockade—insights gleaned from analysis of a new mathematical mechanistic model. J. Theor. Biol. 485, 110033 (2020).
Butner, J. D. et al. Mathematical prediction of clinical outcomes in advanced cancer patients treated with checkpoint inhibitor immunotherapy. Sci. Adv. 6, eaay6298 (2020).
Butner, J. D. et al. A mathematical model for the quantification of a patient’s sensitivity to checkpoint inhibitors and long-term tumour burden. Nat. Biomed. Eng. 5, 297–308 (2021).
Tang, C. et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin. Cancer Res. 23, 1388–1396 (2017).
Butner, J. D. et al. Early prediction of clinical response to checkpoint inhibitor therapy in human solid tumors through mathematical modeling. eLife 10, e70130 (2021).
Mueller-Schoell, A. et al. Early survival prediction framework in CD19-specific CAR-T cell immunotherapy using a quantitative systems pharmacology model. Cancers 13, 2782 (2021).
Das, P. et al. Optimal control strategy for cancer remission using combinatorial therapy: a mathematical model-based approach. Chaos Solitons Fractals 145, 110789 (2021).
Barish, S., Ochs, M. F., Sontag, E. D. & Gevertz, J. L. Evaluating optimal therapy robustness by virtual expansion of a sample population, with a case study in cancer immunotherapy. Proc. Natl Acad. Sci. USA 114, e6277–e6286 (2017).
Mpekris, F. et al. Normalizing tumor microenvironment with nanomedicine and metronomic therapy to improve immunotherapy. J. Control. Release 345, 190–199 (2022).
Rodrigues, D. S., Mancera, P. F. A., Carvalho, T. & Gonçalves, L. F. A mathematical model for chemoimmunotherapy of chronic lymphocytic leukemia. Appl. Math. Comput. 349, 118–133 (2019).
Mpekris, F. et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl Acad. Sci. USA 117, 3728–3737 (2020).
Coletti, R., Leonardelli, L., Parolo, S. & Marchetti, L. A QSP model of prostate cancer immunotherapy to identify effective combination therapies. Sci. Rep. 10, 9063 (2020).
Liu, Q., Yin, X., Languino, L. R. & Altieri, D. C. Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat. Biopharm. Res. 10, 112–122 (2018).
Benchaib, M. A., Bouchnita, A., Volpert, V. & Makhoute, A. Mathematical modeling reveals that the administration of EGF can promote the elimination of lymph node metastases by PD-1/PD-L1 blockade. Front. Bioeng. Biotechnol. 7, 104 (2019).
Wei, H. C., Yu, J. L. & Hsu, C. Y. Periodically pulsed immunotherapy in a mathematical model of tumor, CD4+ T cells, and antitumor cytokine interactions. Comput. Math. Methods Med. 2017, 2906282 (2017).
Lai, X. & Friedman, A. How to schedule VEGF and PD-1 inhibitors in combination cancer therapy? BMC Syst. Biol. 13, 30 (2019).
Pozzi, G. et al. T cell therapy against cancer: a predictive diffuse-interface mathematical model informed by pre-clinical studies. J. Theor. Biol. 547, 111172 (2022).
Welsh, J. et al. Abscopal effect following radiation therapy in cancer patients: a new look from the immunological point of view. J. Biomed. Phys. Eng. 10, 537–542 (2020).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Kosinsky, Y. et al. Radiation and PD-(L)1 treatment combinations: immune response and dose optimization via a predictive systems model. J. Immunother. Cancer 6, 17 (2018).
Raies, A. B. & Bajic, V. B. In silico toxicology: computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 6, 147–172 (2016).
Butner, J. D. & Wang, Z. Predicting immune checkpoint inhibitor response with mathematical modeling. Immunotherapy 13, 1151–1155 (2021).
Reticker-Flynn, N. E. & Engleman, E. G. Cancer systems immunology. eLife https://doi.org/10.7554/eLife.53839 (2020).
Kiran, K. L. & Lakshminarayanan, S. Global sensitivity analysis and model-based reactive scheduling of targeted cancer immunotherapy. Bio Syst. 101, 117–126 (2010).
Dogra, P. et al. A mathematical model to predict nanomedicine pharmacokinetics and tumor delivery. Comput. Struct. Biotechnol. J. 18, 518–531 (2020).
Dogra, P. et al. Translational modeling identifies synergy between nanoparticle-delivered miRNA-22 and standard-of-care drugs in triple-negative breast cancer. Pharm. Res. 39, 511–528 (2022).
Shirasawa, M. et al. Prognostic impact of peripheral blood neutrophil to lymphocyte ratio in advanced-stage pulmonary large cell neuroendocrine carcinoma and its association with the immune-related tumour microenvironment. Br. J. Cancer 124, 925–932 (2021).
Tanaka, R. et al. Preoperative neutrophil-to-lymphocyte ratio predicts tumor-infiltrating CD8+ T cells in biliary tract cancer. Anticancer Res. 40, 14264 (2020).
Seymour, L. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18, e143–e152 (2017).
Jiang, P. & Liu, X. S. Big data mining yields novel insights on cancer. Nat. Genet. 47, 103–104 (2015).
Olivier, M., Asmis, R., Hawkins, G. A., Howard, T. D. & Cox, L. A. The need for multi-omics biomarker signatures in precision medicine. Int. J. Mol. Sci. 20, 4781 (2019).
Yu, K. H. & Snyder, M. Omics profiling in precision oncology. Mol. Cell. Proteom. 15, 2525–2536 (2016).
Zhang, W., Chien, J., Yong, J. & Kuang, R. Network-based machine learning and graph theory algorithms for precision oncology. npj Precis. Oncol. 1, 25 (2017).
Richter, A. N. & Khoshgoftaar, T. M. A review of statistical and machine learning methods for modeling cancer risk using structured clinical data. Artif. Intell. Med. 90, 1–14 (2018).
Cuplov, V., Sicard, G., Barbolosi, D., Ciccolini, J. & Barlesi, F. Harnessing tumor immunity with chemotherapy: mathematical modeling for decision-making in combinatorial regimen with immune-oncology drugs. J. Clin. Oncol. 38, e14095–e14095 (2020).
Ardila, D. et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 25, 954–961 (2019).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Landhuis, E. Deep learning takes on tumours. Nature 580, 551–553 (2020).
Azuaje, F. Artificial intelligence for precision oncology: beyond patient stratification. npj Precis. Oncol. 3, 6 (2019).
Nagy, M., Radakovich, N. & Nazha, A. Machine learning in oncology: what should clinicians know? JCO Clin. Cancer Inform. 4, 799–810 (2020).
Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 1, 206–215 (2019).
Karniadakis, G. E. et al. Physics-informed machine learning. Nat. Rev. Phys. 3, 422–440 (2021).
Raissi, M., Perdikaris, P. & Karniadakis, G. E. Physics-informed neural networks: a deep learning framework for solving forward and inverse problems involving nonlinear partial differential equations. J. Comput. Phys. 378, 686–707 (2019).
Wieland, F.-G., Hauber, A. L., Rosenblatt, M., Tönsing, C. & Timmer, J. On structural and practical identifiability. Curr. Opin. Syst. Biol. 25, 60–69 (2021).
Okuneye, K. et al. A validated mathematical model of FGFR3-mediated tumor growth reveals pathways to harness the benefits of combination targeted therapy and immunotherapy in bladder cancer. Comput Syst. Oncol. 1, e1019 (2021).
Bekisz, S. & Geris, L. Cancer modeling: from mechanistic to data-driven approaches, and from fundamental insights to clinical applications. J. Comput. Sci. 46, 101198 (2020).
Clarke, M. A. & Fisher, J. Executable cancer models: successes and challenges. Nat. Rev. Cancer 20, 343–354 (2020).
Roudko, V., Greenbaum, B. & Bhardwaj, N. Computational prediction and validation of tumor-associated neoantigens. Front. Immunol. 11, 27 (2020).
Garner, H. & de Visser, K. E. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat. Rev. Immunol. 20, 483–497 (2020).
Irvine, D. J. & Dane, E. L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 20, 321–334 (2020).
Bekker, R. A. et al. Rethinking the immunotherapy numbers game. J. Immunother. Cancer 10, e005107 (2022).
ForyŚ, U., Waniewski, J. & Zhivkov, P. Anti-tumor immunity and tumor anti-immunity in a mathematical model of tumor immunotherapy. J. Biol. Syst. 14, 13–30 (2006).
Acknowledgements
This research has been supported in part by the National Science Foundation Grant DMS-1930583 (V.C. and Z.W.), the National Institutes of Health (NIH) Grants 1R01CA253865 (V.C. and Z.W.), 1R01CA226537 (R.P., W.A., V.C. and Z.W.), 1R01CA222007 (V.C. and Z.W.), 1R01AI165372 (Z.W.), 1R01DK132104 (Z.W.) and 1R01DK133610 (Z.W.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Z.W. conceived and coordinated this work. J.D.B. and Z.W. drafted the structure of the paper, reviewed the literature and contributed to figures. P.D., C.C., R.P,, W.A. and V.C. provided critical feedback on figures. All authors contributed to the writing and revision of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Computational Science thanks Walter Koch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ananya Rastogi, in collaboration with the Nature Computational Science team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Butner, J.D., Dogra, P., Chung, C. et al. Mathematical modeling of cancer immunotherapy for personalized clinical translation. Nat Comput Sci 2, 785–796 (2022). https://doi.org/10.1038/s43588-022-00377-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43588-022-00377-z
This article is cited by
-
Mathematical modeling and bifurcation analysis for a biological mechanism of cancer drug resistance
Acta Mathematica Scientia (2024)