Abstract
Mass spectrometry is a powerful analytical tool used for the analysis of a wide range of substances and matrices; it is increasingly utilized for clinical applications in laboratory medicine. This Primer includes an overview of basic mass spectrometry concepts, focusing primarily on tandem mass spectrometry. We discuss experimental considerations and quality management, and provide an overview of some key applications in the clinic. Lastly, the Primer discusses significant challenges for implementation of mass spectrometry in clinical laboratories and provides an outlook of where there are emerging clinical applications for this technology.
Similar content being viewed by others
Introduction
Mass spectrometry has been used since the mid-twentieth century in basic science laboratories and various industries for quantitative and qualitative analysis. For many years, the sophisticated instrumentation and specialized knowledge required to develop analytical methods, as well as the complexity of sample preparation, kept the use of mass spectrometry confined to highly specialized laboratories. However, the operation of the instruments has since been simplified, and the instruments themselves have become more robust, which has made the technology more accessible to a broader user base. Although mass spectrometry continues to be used in research applications for biomarker discovery and complex protein characterization, it is increasingly common to find these instruments in the routine clinical laboratory as well.
Mass spectrometric analysis is based on the separation of ions by their mass to charge ratio (m/z). There are several different types of mass analysers used for mass spectrometric analyses including quadrupole, magnetic sector, radio-frequency ion trap, time of flight (TOF), orbitrap and ion cyclotron resonance1. There are also numerous approaches to create ions in the gas phase prior to analysis by mass spectrometry including electron ionization, chemical ionization, electrospray ionization (ESI), photoionization, inductively coupled plasma and matrix-assisted laser desorption ionization2.
Mass analysers can be combined to perform tandem mass spectrometry (MS/MS), which is a relatively simple concept — two mass analyses occur in series, often with a fragmentation step in between. One approach to MS/MS is to combine two or more identical types of mass analysers in series, such as a triple quadrupole analyser or a TOF/TOF analyser. Alternatively, two (or more) types of mass analysers can be combined to create hybrid instruments for MS/MS; for example, a quadrupole and TOF combined to create Q-TOF analysers or a quadrupole and linear ion trap put together to form a Q-Trap instrument. For any of these configurations, mass spectrometry analysis is accomplished by performing the first mass analysis or filtering, passing selected ions into a collision cell where fragmentation occurs, followed by a second mass analysis. For some configurations that utilize a trapping mass analyser, several cycles of mass spectrometry and fragmentation can be performed to generate structural information for an unknown compound. MS/MS is useful for both qualitative determinations (for example, structural characterization or presence) and targeted quantitative analysis with the use of stable isotope-labelled standards.
In liquid chromatography, molecules are separated based on differential equilibrium between a mobile phase and a stationary phase — the equilibrium is driven by their solubility or strength of interaction with the mobile and stationary phases. Initially, analytes interact with functionalized particles (the stationary phase) and are eluted when they have a stronger interaction with the solvent flowing through the chromatographic column (the mobile phase) relative to the stationary phase. The optimal characteristics of the solvent and stationary phase are dependent on the analysis performed3. The use of liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) aids the separation of the analyte(s) of interest from matrix components, improving sensitivity and imprecision and improving specificity by separating interferences, such as isobaric compounds, that cannot be differentiated by the mass spectrometer4.
The strength of LC-MS/MS lies in the high analytic selectivity that is inherent to this technique. For quantitative analysis, a specimen is pretreated via dilution or by purification to remediate some complexities of the matrix, and then injected onto the liquid chromatography system. The liquid chromatography eluent carrying the analyte is introduced into the source of the mass spectrometer, where gas phase ions are produced. The first mass analyser filters incoming ions, only allowing precursor ions to move forward towards the collision cell. In the collision cell, precursor ions are fragmented to product ions, which are analysed in the last stage of the tandem mass spectrometer. The resulting mass spectrum reflects the product ions only; when focusing only on particular precursor/product ion pairs, quantitative analyses can be performed using a technique called selected reaction monitoring.
Utilization of mass spectrometry in a clinical laboratory is very different from work performed in research laboratories owing to regulatory requirements in place for clinical testing to ensure robustness, reproducibility and accuracy, such as the Clinical Laboratory Improvement Amendment or accreditation requirements from the College of American Pathologists5. These regulations typically characterize LC-MS/MS tests as laboratory developed tests and establish specific requirements for performance of laboratory developed tests along with the quality management of the associated processes. It should be noted that additional regulation is looming, as there is pending legislation that would require submission of laboratory developed tests for review and approval by the US Food and Drug Administration (FDA)6 — however, this is both a fluid and a contentious topic and enactment of this legislation is not a given. In addition to regulatory requirements, there is also a time-sensitive element to this testing — patient diagnosis or treatment can be pending, waiting for the LC-MS/MS result to become available. These techniques of clinical LC-MS/MS will be the focus of the current Primer, with most examples utilizing ESI triple quadrupole-based mass spectrometry; a typical set-up for this LC-MS/MS system can be seen in Fig. 1.
This Primer includes an overview of some basic mass spectrometry concepts, focusing primarily on MS/MS using quadrupole mass analysers. We discuss experimental considerations and quality management, and provide an overview of some key applications for small molecules. We do not discuss other forms of MS/MS with non-quadrupole mass analysers, nor do we address metabolomics or proteomics. Lastly, the Primer discusses significant challenges for implementation of mass spectrometry in clinical laboratories and provides an outlook of where there are emerging clinical applications for this technology.
Experimentation
Sample preparation
Sample preparation is required before LC-MS/MS analysis. This can be simple (for example, dilution or protein precipitation) or more complex (for example, solid phase extraction (SPE), liquid–liquid extraction (LLE) or supported liquid extraction (SLE))7. Although simple sample preparation is rapid, sample extracts are not as clean as with more complex preparation. Extracts will still contain significant amounts of sample matrix that could cause issues with the robustness and reproducibility of the LC-MS/MS method. An internal standard is added in the same amount to all calibrators, quality control materials and patient samples as a first step in sample preparation to ensure that the internal standard goes through all steps of analysis along with the analyte of interest.
Samples can be diluted with water or a mobile phase before injection onto the LC-MS/MS system. The diluted sample should be centrifuged and/or filtered to remove particulates before injection. Dilutions are commonly used for urine drug analysis8,9 owing to the concentration of drugs in urine being high and the protein content normally being low. Although this technique is simple and fast, it cannot be used to concentrate analytes and no matrix is removed from the sample. To minimize matrix effects, it is recommended to use as high a dilution as possible while still retaining the required sensitivity7.
For protein precipitation, an organic solvent (such as acetonitrile) or acid (such as trichloroacetic acid) and sometimes a salt (for example, zinc sulfate) are added to a body fluid sample such as whole blood or serum/plasma in order to precipitate the proteins out of solution10. After centrifugation, the supernatant can be evaporated and reconstituted to concentrate the analyte if required8. Although this technique is simple, only a fraction of proteins are removed; this may result in issues with robustness and reproducibility. Protein precipitation is a sample preparation strategy often employed for therapeutic drug monitoring (TDM) owing to the requirement for a fast turn-around time for such assays11,12,13.
SPE uses a sorbent, for which there are various mechanisms of interaction, to retain the analyte(s) while matrix components are washed away before the analyte(s) are eluted7. The sorbent is commonly conditioned with elution solvent and equilibrated with buffer prior to addition of the sample. Mixing the sample with an application buffer will maximize retention of the analyte(s) to the sorbent. The sorbent can be washed with additional solvents to remove matrix and other compounds before the measurand(s) are released from the sorbent bed using an elution solvent. This elution mixture can be evaporated and reconstituted in an appropriate injection matrix7. SPE can be optimized for the analyte(s) in the method based on the buffers and the wash solutions used, and creates a cleaner sample extract with the ability to concentrate the analyte(s). However, SPE is complex and time-consuming. This technique is commonly used for analysis of urine and serum drugs and steroid hormones14,15,16,17.
LLE involves use of an organic solvent (such as hexane/ethyl acetate) that can separate hydrophobic analytes from the aqueous body fluid phase. Samples are mixed with the organic solvent to allow the analytes of interest to partition into the organic phase, which is then transferred to a clean vessel. Once evaporated, the analytes are reconstituted in an LC-MS/MS-compatible solvent7. This extraction method creates a sample extract in which analytes can be further concentrated and so is commonly applied in analysis of steroid hormones18,19. However, it is time-consuming and fairly complex to perform. SLE uses the same principle as LLE but the partition is performed in solid media rather than in liquid. The sample mixed with a buffer is allowed to disperse into a sorbent bed (commonly diatomaceous earth) and is then eluted using an organic solvent mixture (for example, ethyl acetate and hexane) as the hydrophobic analyte(s) partition preferentially into the solvent. SLE produces a cleaner sample extract similar to LLE, but can be formatted for higher throughput than LLE. SLE has been used for analysis of steroid hormones and methylmalonic acid (following derivitization to increase lipophilicity)20,21,22,23. For all of these sample preparation strategies, the chemical properties of the analyte(s), for example lipophilicity and pKa, must be taken into account during selection7.
Liquid chromatography
Liquid chromatography is a technique that separates analytes based on the interaction between a stationary phase (the chromatography column) and a liquid phase (mobile phases)4. These interactions are based on multiple mechanisms including polarity, ionic interactions and size exclusion. Reversed-phase chromatography is most commonly used whereby the stationary phase is less polar than the mobile phase so non-polar molecules adsorb to the stationary phase until a less polar mobile phase passes through. In normal-phase chromatography, the stationary phase is more polar than the mobile phase at sample introduction4. When coupled with mass spectrometry, chromatographic mobile phases also may contain a proton donor species that contributes to ionization of the analytes.
In clinical LC-MS/MS analysis, once the patient sample has undergone sample preparation, the sample extract is injected onto the chromatography column. High-pressure pumps deliver the mobile phases to the column at reproducible flow rates. Gradient elution is most commonly used where the composition of the mobile phase (percentage of polar versus non-polar) is varied over the total run time, for example the time required to elute the analytes of interest, clean and then re-equilibrate the column. A typical analysis time ranges from 2 to 5 min for a routine clinical LC-MS/MS test4. If the conditions are tightly controlled, the time that analyte(s) elute from the column (retention time) should remain constant. Chromatographic separation is particularly important when there are isobaric compounds involved; if these are not sufficiently separated from one another there can be interference when an isobaric species mimics the selected reaction monitoring transition of another analyte in the same analytical window of time. The choice of chromatography column in terms of length, internal diameter and packing material is dependent upon the analyte(s) being measured and the pressure limitations of the liquid chromatography system24.
Ion sources
In order for mass spectrometry analysis to take place, the components of the flow from the liquid chromatography system have to be ionized. There are three main types of atmospheric pressure ionization: ESI, atmospheric pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI)4,25. ESI (see Supplementary Fig. 1) is the most common type of ionization used in clinical diagnostics, as it is most useful for polar compounds that form ions in solution. APCI (see Supplementary Fig. 2) tends to be used for analysis of more non-polar compounds that do not form ions in solution but are able to be ionized in the gas phase, such as steroids or cannabinoids. APPI can be important for weakly polar or non-polar compounds that are challenging with ESI or APCI. APPI greatly reduces matrix effects and background signal; however, it is rarely used. In ESI, the liquid flow from the liquid chromatography system, containing the analyte and solvent molecules, becomes charged as it flows out of an electrode at high voltage. This charged eluent forms a spray which can be heated by gas flows to evaporate solvent. Molecular ions are derived from small droplets and enter the mass spectrometer in the gas phase4,25. In APCI, the liquid flow from the liquid chromatography system flows through a heated nebulizer tube creating a gaseous mixture of analyte and solvent molecules. Upon exit from the tube, the gaseous mixture passes a charged corona discharge needle creating reactant gas. The reactant gas and analyte interact; the analytes become charged based on gas phase basicity and these ions enter the inlet of the mass spectrometer4,25. In APPI, the liquid flow from the liquid chromatography system is subjected to heated gas creating a vapour. This vapour and, commonly, a dopant (for example, toluene) pass by an ultraviolet lamp emitting photons causing photoionization and the analyte ions enter the front of the mass spectrometer4,25. The charged species are generated as a function of the addition of a proton ([M + H]+, positive ion) or as an anion ([M – H]–, negative ion). Additionally, adducts may be formed of the analyte and solvent/salt species as well as multiply charged species (for example, [M + 2H]2+).
Types of mass analysis
Triple quadrupole mass spectrometers (or tandem mass spectrometers) are most commonly used for clinical diagnostics. Two mass-filtering quadrupoles can transmit ions with a specific m/z value based on the applied radio frequency and direct current voltage. They can also allow all ions to pass through (radio frequency only). Commonly, another quadrupole is employed to act as a collision cell where fragmentation of ions can take place (radio frequency and collision gas applied)4,25 (Fig. 2a). Quantitative clinical LC-MS/MS methods commonly employ selected reaction monitoring. In this type of analysis, quadrupole 1 provides a stable trajectory for ions of a specific m/z value, and only ions of the specific m/z value enter quadrupole 2 where they are fragmented to form a charged product and a neutral loss. These fragment ions enter quadrupole 3, which is set to filter ions of a specific m/z value, and these ions strike the detector4,25 (Fig. 2b). The precursor ion/product ion pair is called a transition; using testosterone as an example, the transitions for testosterone have the precursor ion/product ion pair 289.1/97.1 m/z and 289.1/109.1 m/z.
Optimization of transitions for selected reaction monitoring
Optimization of transitions when using ESI is commonly performed by directly infusing a pure standard into the mass spectrometer using a syringe pump and investigating and varying the compound-dependent parameters (for example, precursor ion scan followed by product ion scan, varying collision energy)4. Examples of these optimization experiments are given in Table 1.
Most instruments have automated functions that can perform optimization but it is recommended to also perform this task manually in an iterative manner. Although commonly the transition with the highest signal-to-noise ratio is chosen for quantification, it is possible that this transition is not optimal for use in patient samples owing to interferences. Therefore, during method development, it is common to identify and use more transitions so that they can all be investigated for potential issues. It is recommended that product ions corresponding to a water loss from the precursor ion (–18 atomic mass units (amu)) are avoided owing to lack of specificity and, therefore, higher background noise or interfering signals26. Further, product ions with a mass below 100 amu may also be relatively non-specific, although when analysing small molecules it is not always possible to avoid such transitions. After validation, it is common to use two transitions per analyte (one quantifier transition to determine the amount of analyte present, and one qualifier transition to confirm analyte specificity). Two transitions per internal standard are often used for routine clinical applications.
Liquid flow from the liquid chromatography system can be added to the direct infusion set-up in an experiment known as post-column infusion or T-infusion27. Post-column infusion can be used to optimize mass transitions when using APCI, and it is used in both ESI and APCI to optimize the source parameters (for example, temperature and gas flow)4. Examples of these experiments are provided in Table 2. Once all of these parameters are established, the entire LC-MS/MS method can be run to ensure that adequate sensitivity and specificity are achieved. If not, these experiments can be repeated to further optimize the parameters.
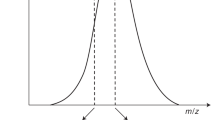
Once these mass spectrometer parameters are optimized, the liquid chromatography method should be run to ensure that the method performs adequately. An important consideration is the dwell time or the time that the mass spectrometer spends monitoring each mass transition. An appropriate dwell time is chosen to ensure adequate data points are collected across the chromatographic peak. If the dwell time is too long, the peak will be poorly defined; if the dwell time is too short, the peak will be jagged (Fig. 3). Ideally, the dwell time should be fixed to obtain a minimum of 15–20 data points across the peak4.
Internal standardization
The use of an internal standard in clinical mass spectrometry is highly recommended. The internal standard helps normalize for any differences in analyte recovery during sample preparation or ionization efficiency4,24. The internal standard should be added to all samples at a concentration that is within the analytical measurement range of the assay, and that gives a reproducible mass spectrometry response; suggested concentrations are 10–50 times the lower limit of quantitation4.
Stable isotope-labelled internal standards should be used for LC-MS/MS analysis, most commonly labelled with deuterium (D or 2H) or carbon-13 (13C). This enables the internal standard to have theoretically the same chromatographic properties as the analyte (for example, retention time), but the mass spectrometer can distinguish between the analyte and the internal standard by the difference in mass. A minimum mass difference of 3 amu between the analyte and internal standard is recommended for analytes up to 1,000 amu to ensure separation from the isotopic envelope, but in compounds with a large number of chlorines, for example, a larger mass difference may be required4. The response of analyte in an internal standard-only solution should be less than 20% of the analyte’s lower limit of quantitation (caused by inefficient isotopic labelling). The response of the internal standard in high analyte-only concentrations should be below 5% of the typical internal standard response (caused by naturally occurring isotopes)4.
Quality control
Clinical LC-MS/MS assays tend to be run in batch format whereby a calibration curve, quality control samples and patient samples are run and analysed together. It is recommended that quality control samples are matrix-matched to the patient samples (for example, if the patient matrix is serum, then the quality control samples are prepared using serum)4. Conservatively, a minimum of 5% of the total batch size should consist of quality control, and if the batch size is large it is recommended that the quality control samples are spaced throughout the batch. However, the number of quality control samples needed is, ultimately, application-dependent. For quantitative assays, quality control samples should be at concentrations either throughout the analytical measurement range of the assay or at important medical decision limits, with a minimum of two liquid levels. For semi-qualitative assays, as a minimum there should be one quality control around 25% below the cut-off concentration (weak negative) and one around 25% higher than the cut-off concentration (weak positive)4. During method development, acceptance criteria are established for the quality control and once the method is in production, the patient samples can only be reported if the quality control samples pass these criteria.
For methods that will be used over an extended period of time, such as for clinical analysis, it is important to determine the stability of the quality control material and calibrators. In general, large batches of quality control material are prepared or purchased so that the same quality control material is used over an extended period of time (for example, 2 years). Therefore, it is important to know the storage requirements for the quality control material during this time period, at what temperature and whether freeze/thawing cycles are allowed4. Additionally, commutability of the matrix used for calibrators and quality control must be assessed — it can be problematic if the calibrators and quality control behave differently compared with clinical specimens.
Results
Data analysis
LC-MS/MS data are represented as a chromatogram, which is a plot of analyte abundance versus time (Fig. 4a). LC-MS/MS data points making up these individual chromatograms are sequentially scanned for each selected transition (quadrupole 1/quadrupole 3 pair) and averaged across the transition’s assigned dwell time. As the system is not continuously scanning all masses, individual data points are connected to generate a near-Gaussian shape of a chromatographic peak. For a peak, the abundance is proportional to the number of pseudo-molecular ions that survive ionization and subsequent collisional dissociation to reach the detector.
There are typically multiple chromatograms for each assayed analyte in a specimen. At least one or, preferably, two or more distinct transitions are monitored for the analyte of interest. The additional transitions are utilized in an intra-sample determination of specificity described as transition ratios or ion ratios28. These transitions can be distinct fragments if the molecule can provide multiple stable product ions of appropriate abundance, although other mechanisms exist to assess specificity when a single neutral loss is utilized29. The consistency of the relationship between a quantifying transition (used to generate the reported result) and the qualifying transition (used to assess co-eluting interferences) is derived from calibration standards and quality controls for the assay4. Additionally, the internal standard chromatogram will often utilize a second transition for peak purity assessments. Figure 5 shows demonstrative data from LC-MS/MS analysis of leucine isomers in amino acids exhibiting the multiple transitions utilized as well as the co-detection of isomers.
Example liquid chromatography–tandem mass spectrometry data generated from the analysis of leucine isomers with counts on the y axis (abundance of response) and time on the x axis. a, Dark blue line: transition 132.1 (quadrupole 1) to 44.1 (quadrupole 3) for equimolar concentrations of alloisoleucine (0.16 min), isoleucine (0.25 min) and leucine (0.40 min). Light blue line: transition 132.1 (quadrupole 1) to 43.1 (quadrupole 3), which is used as a qualifying transition for leucine only. The differences in recorded abundance are caused by the ionization and dissociation differences at the applied collision energy for the selected transitions. b, Transition 142.1 (quadrupole 1) to 78.1 (quadrupole 3) for the 10-deuteron labelled (‘D10’) alloisoleucine internal standard (0.14 min), which is used to normalize the recovery of alloisoleucine. c, Transition 142.1 (quadrupole 1) to 96.1 (quadrupole 3) for both D10-alloisoleucine (0.14 min) and D10-leucine (0.37 min); this transition acts as a normalizer for leucine quantification and as a qualifying transition for the alloisoleucine internal standard. The area under the curve for each peak is used for quantification calculations. MRM, multiple reaction monitoring; XIC, extracted ion chromatogram.
Although LC-MS/MS is often described as specific, non-measurand signals can be observed during analysis. These signals can be derived from isomers of the target compound, in-source dissociation of larger molecules, adducts or isotopes of typically lower m/z species or some combination thereof30. Additionally, noise or background signals can be highly variable between different MS/MS transitions and samples (Fig. 4b). Integration of measurand peaks above noise and in the presence of interfering signals remains a highly subjective process, although progress in automated signal interpretation is improving the reliability and reproducibility of data in clinical samples31.
Calibration
Calibration of mass spectrometry assays is performed on an analyte by analyte basis. Note that assay calibration is separate from instrument calibration, in which the latter establishes mass accuracy and resolution of ions with a stable trajectory to the detector. The ionization cross-section and the post-dissociation transmission efficiency for any two compounds are generally non-equivalent. Although relative abundances of signals in a mass spectrometer can be used to indicate certain relationships, such qualitative approaches are less common in clinical LC-MS/MS. According to International Organization for Standardization (ISO) definitions, a quantitative assay requires the use of SI-traceable units32. Moles per volume (for example, picomoles per litre or picomolar) or mass per volume (for example, nanograms per litre) are the most common expressions of quantitative values in clinical LC-MS/MS. Non-liquid matrices such as hair or tissue are generally reported as the mass of analyte per mass of matrix. As with quality controls, best practice is to include matrix-matched calibration standards in order to minimize measurement deviation due to matrix effects.
Calibration curves are generated through the assay of samples of known concentrations. For each analyte in a standard, the area under the curve of the chromatographic peak is assessed, accounting for baseline noise generated in the ionization and detection processes. The peak areas of an analyte’s quantifying transition and its internal standard are divided to generate a normalized response function. These area ratios are then used to derive a linear least squares regression that describes the concentration–area ratio function of the analyte on that instrument (Fig. 4c).
The number of calibration points to generate a standard curve is dependent on the breadth of the intended measurement interval and the assay’s analytical performance. Clinical LC-MS/MS assays can exhibit various measurement intervals, although a 1,000-fold range is an approximate limit of coverage owing to limitations of carry-over in the system33. Calibration points should exist at the extremities of the analytical measurement range with additional points used to provide accuracy of the best-fit line as appropriate; at a minimum, the distribution should include the midpoint of the assay measurement range, and points located halfway between the midpoint and the extremities.
The nature of LC-MS/MS detection leads to heteroscedasticity for calibration curves, or changing variance across the concentration range measured. Thus, weighting of the regression is a standard practice34. The models for the best-fit line are typically either linear or quadratic fits (meaning first-degree and second-degree polynomial equations of the regression); utility of the model is dependent on the desired analytical range and the clinical use of the measurand. Calibration models and weightings are rarely modified from the validated procedure when the assay is put into practice.
Calibration curves can be assessed for back-calculated accuracy of the individual points using established criteria. An allowable accuracy of 85–115% for values greater than the lower limit of quantitation is common. At the lower limit of quantitation, values within 20% of the expected are acceptable35. Points falling outside these criteria may be excluded, dependent on the laboratory’s standard operating procedure. The rate of re-calibration has numerous variables to consider. Changes in response functions of the instrument over time as well as the changes at the sample preparation level (reagent and technical) are the determinants for assay calibration frequency. Calibration per group of co-prepared samples (batch-based calibration) is conventionally practised.
Calibration curves are used to define the analyte’s response function on the instrument of analysis, with sample by sample variations normalized by the internal standard. By itself, a calibration curve is not capable of determining assay bias or imprecision. Thus, quality controls are prepared and measured with specimens to determine whether the assay is performing within expectations. In regulated clinical laboratories, two liquid levels of quality control should be measured per day at a minimum, unless additional internal controls are designed into the instrument36. However, factors which modulate recovery or response of an analyte in LC-MS/MS workflows may be difficult to ascertain under a scheme of two quality controls per day37. The extraction and measurement of quality controls should be coincidental with specimens, with the frequency determined by the nature of the assay (for example, extraction mode) and the risk of error at each step of specimen processing.
Quality controls
Criteria for LC-MS/MS quality control acceptance, and thus batch acceptance, will use one of two approaches. In a model derived from LC-MS/MS-based clinical trials submitted to the FDA, the plurality of quality controls within a batch should be within 15% of the nominal target35. This quality control approach has a long history as being accepted guidance for LC-MS/MS bioanalysis. A different model familiar to clinical laboratories is to assess the mean and the imprecision of each level of quality control via replicate analysis and apply what is commonly known as Westgard rules38. In contrast to the FDA’s quality control analysis, Westgard is largely focused on long-term precision of the analysis. Either approach should be balanced against the expected performance limits for the measurand, with consideration for the nature of LC-MS/MS procedures.
An additional consideration for application of quality control rules is the ability of multiple analytes to be assessed in the same procedure (multiplexed analysis). The application of Westgard rules was derived from statistics based on a single measurand and longitudinal calibration. By contrast, modern LC-MS/MS procedures can measure many chemical species quantitatively in the same analysis; strict application of either Westgard rules or the FDA conditions can lead to unwarranted failures owing to compounding random error39. In these instances, clinical use of the assay and use of results (for example, reference change value from serial monitoring versus acute single measurements) may influence the structure of quality control assessments40.
Data metrics associated with quality assurance are intrinsic in LC-MS/MS. Individual features may indicate degradation of system capabilities, such as a reduced retention time and peak broadening signifying a loss of stationary phase interactions. Comparative elements, such as the internal standard response of a sample measured against the mean internal standard response of quality controls in the same batch, can be evaluated for systematic errors (such as under-pipetting of the internal standard) or random error (sample-specific ion suppression). Limits for each metric can be defined in laboratory standard operating procedures and used to assess data quality prior to result release, which is an important feature of LC-MS/MS41.
Following calibration of an assay, quality control result review and sample quality assurance interrogation, results for samples are posted to a laboratory information management system or other interface for an electronic medical record. Data can be interpreted for critical alert values, compared with reference intervals or evaluated for biomarker level change in the patient over time42.
Applications
The evolution of mass spectrometry from the research setting to the clinical laboratory has continued to grow in the past decade as the advantages of mass spectrometry-based applications have been realized in laboratory medicine. Although not without its challenges, the following sections will highlight some key areas where LC-MS/MS has demonstrated its utility in clinical diagnostics.
Endocrine system
The endocrine system is one area for the laboratory where LC-MS/MS has been emphasized by professional society guidelines such as those of the Endocrine Society and the Urology Society, which provided evidence showing that mass spectrometry-based methods are preferable for the measurement of sex steroid hormones43. Measurement of steroid hormones in the blood often presents a clinical challenge owing to the very low concentrations (for example, picomolar range) and structural similarity of endogenous hormones and metabolites44. For example, the quantification of oestradiol and testosterone is important for the diagnosis, prognosis or treatment monitoring of various disorders such as hypogonadism, amenorrhoea and tumours of the ovary, breast and testes. Although immunoassays are widely available, they suffer from a lack of specificity owing to steroids with similar structures and poor standardization across platforms. In addition, none of the commercially available immunoassays is reliable enough to measure testosterone levels usually found in women and children45. Human heterophilic antibodies and autoantibodies can also lead to false-positive or false-negative results in immunoassays and have been documented (for example, human chorionic gonadotrophin and thyroglobulin)44,46.
Mass spectrometry also enables steroid profiling and the ability to multiplex and analyse a single sample to assess the metabolic signatures of several steroid hormones47. It allows the quantification of individual steroids and metabolites related to the various enzymatic activities. This deeper understanding of the complex metabolic pathways in different diseases can facilitate precision medicine. In addition to multiplexing, mass spectrometry also has a higher throughput especially when coupled with pre-analytical automated liquid handling systems. LC-MS/MS is also better suited over gas chromatography–mass spectrometry as some steroids have poor volatility owing to their higher polarity and lipophilicity47. Lastly, mass spectrometry has been shown to be an effective technique to measure hormones in alternative matrices such as saliva48,49.
In addition to endogenous steroid hormones, biogenic amines, such as catecholamines and metanephrines, are another class of substances where mass spectrometry can excel. Phaeochromocytoma and paraganglioma are neuroendocrine tumours able to secrete catecholamines50. Timely diagnosis is critical and historically relied on measurements of plasma and urinary catecholamines. With LC-MS/MS methods now available to measure free plasma metanephrines or urinary fractionated metanephrines, the Endocrine Society has recommended these as the first-line test in diagnosis51. However, LC-MS/MS is not without its challenges as matrix effects and isobaric interferences need to be properly addressed. In addition, the establishment of new reference intervals for various age-based and sex-based hormones can be difficult.
Therapeutic drug monitoring
TDM is another rapidly expanding area for mass spectrometry. TDM is the quantification and interpretation of drug concentrations in blood to optimize pharmacotherapy. Drugs with a narrow therapeutic index, highly variable pharmacokinetics or toxicity are often measured and include drugs in the following drug classes: antibiotics, antivirals, antifungals, antiepileptics, antipsychotics, antidepressants and anticancer drugs. Immunosuppressive drugs such as tacrolimus, cyclosporine A, everolimus and sirolimus were some of the initial drugs brought into clinical laboratories for LC-MS/MS analysis. Currently, approximately 50% of TDM requests for tacrolimus and cyclosporin A and more than 70% of those for sirolimus and everolimus worldwide are being measured using LC-MS/MS52. However, although immunoassays can offer rapid turnaround times on automated chemistry analysers, they suffer from cross-reactivity to other metabolites or structurally similar compounds.
TDM using LC-MS/MS offers the ability to characterize a patient’s phenotype by measuring the parent and/or active metabolite concentrations with all of the demographic, disease-related and environmental variables that can affect the drug concentrations53. It allows a sensitive, specific and low-cost methodology to be used in precision medicine and patient management. Mass spectrometry has numerous advantages over traditional immunoassays such as superior specificity, expanded linear ranges, ability to multiplex (for example, measure both active parent and metabolites or multiple co-medications) and high throughput relative to existing high performance liquid chromatography methods53. Whereas whole blood or serum represents the most common matrices used, LC-MS/MS assays have also been utilized with dried blood spots54,55 or oral fluid (for example, fluconazole in hospitalized children)56. With the COVID-19 pandemic, these alternative matrices highlight the ability to offer patients an at-home collection method, but still facilitate routine TDM and care at a time when hospitals do not have the resources to collect patient samples. Another promising alternative matrix for TDM using LC-MS/MS is the ability to measure intracellular drug concentrations at the sites of action which more closely correlate to the therapeutic or adverse effects57. LC-MS/MS assays have already been utilized to monitor immunosuppressive drugs in peripheral blood mononuclear cells58. Other applications include intracellular imatinib (tyrosine kinase inhibitor) concentrations in gastrointestinal stromal tumour cells59, intracellular concentrations of ganciclovir60 or 5-fluorouracil nucleotides responsible for the anticancer effects of 5-fluorouracil61.
Toxicology
Clinical toxicology is another challenging area where quick turn-around times, shifting analytical targets and the impact on the clinical decision process all provide an opportunity for mass spectrometry-based techniques. With the opioid epidemic in North America, the use of urine toxicology screening has become standard of care for controlled substance monitoring. It provides unbiased and objective laboratory results to monitor compliance or identify abuse/misuse of medications and illicit substances. Professional and laboratory medicine practice guidelines62 recommend using definitive tests over immunoassays to monitor compliance with over-the-counter, prescribed/non-prescribed medications and illicit substances. Although current immunoassays for urine toxicology screening provide rapid turn-around times for emergency room settings, they primarily detect drug classes and do not identify the specific drugs present. In addition, these immunoassays suffer from poor specificity and sensitivity, so they are susceptible to false-positive and false-negative results. Therefore, definitive testing such as LC-MS/MS or gas chromatography–mass spectrometry is recommended as follow-up testing.
One of the advantages of mass spectrometry for toxicology testing is the ability to multiplex or detect multiple analytes including parent drug and multiple metabolites. With the decriminalization of marijuana and increasing use of cannabinoids in medicine and recreational products, laboratories are required to be able to distinguish between the different patterns of cannabinoid use63. Mass spectrometry methods have been shown to be able to differentiate Δ8-tetrahydrocannabinol (THC) and Δ9-THC in blood and urine64. Δ9-THC is the main psychoactive ingredient in cannabis and is illegal in the United States. Δ8-THC, an isomer of Δ9-THC, has lower potency but has some psychoactive properties and is legal in parts of the United States. Many commercial immunoassays exhibit cross-reactivity between these compounds and their metabolites. Δ8-THC and the Δ8-carboxy-THC metabolite may also cause an interference on some mass spectrometry-based confirmatory tests. However, LC-MS/MS can be used to chromatographically resolve these isomers and allow accurate interpretation of the test results.
Lastly, broad-spectrum toxicology testing using non-targeted mass spectrometry-based methods allows comprehensive screening not only to identify medication usage and trends of illicit drug use but can also identify new and emerging psychoactive substances or designer drugs65. Most mass spectrometry-based methods use targeted analysis, which provides the specificity to identify the drugs that the assay is designed to detect. However, in the ever-changing drug abuse landscape the targets are constantly evolving. As a result, these non-targeted methods allow public health and clinical toxicology laboratories the opportunity to identify new trends. A major challenge for this type of testing is the absence of certified reference materials and standards.
Proteins and newborn screening
LC-MS/MS has also become a leading technology used in newborn screening programmes. Newborn screening programmes are an essential programme to detect, diagnose and mitigate the effect of congenital disorders. In the United States, mandatory screening is performed on around 3.8 million newborns each year66,67. The Recommended Uniform Screening Panel currently suggests screening for a minimum of 60 disorders66. Although other methodologies over the years have been utilized from immunoassays to electrophoresis, mass spectrometry has become the preferred primary testing method because of its high-throughput sampling speed and selectivity. The mass spectrometry-based assays screen for metabolic disorders, amino acid disorders and fatty acid oxidation disorders by measuring the relative abundance of more than 70 amino acids, acylcarnitines and other small molecules. These assays permit rapid measurement directly from filter paper blood spots or biological fluids68, and allow the identification and possible treatment of metabolic disorders even in patients who are asymptomatic. However, expanding panels, isobaric compounds, matrix effects and ion suppression are just a few of the challenges these techniques present, but many can be overcome with chromatography and other separation techniques.
Other areas where mass spectrometry is beginning to gain traction include replacing serum protein electrophoresis to detect and quantify M proteins for multiple myeloma. Multiple myeloma, a cancer of plasma cells, and its precursor condition, monoclonal gammopathy of undetermined significance, lead to dysregulated overproduction of a unique immunoglobulin called the M protein69. Detection of the M protein is crucial for diagnosing multiple myeloma or monoclonal gammopathy of undetermined significance. With increasing demands to distinguish residual disease and new interferences from monoclonal antibody therapies, mass spectrometry methods such as the clonotypic peptide technique70, which may be the most sensitive method to detect M proteins in serum, or the monoclonal immunoglobulin rapid accurate mass measurement (miRAMM)71 method can be utilized. The mass spectrometry techniques offer increased sensitivity and specificity especially for patients under treatment for multiple myeloma69. However, challenges remain for these newer applications, which have only been adopted by a few laboratories to date; therefore, a regulatory approved commercially supported version of the assay along with the support of professional oncology societies may help implementation of these methods.
Reproducibility and data deposition
Reproducibility
Reproducibility of clinical LC-MS/MS results is an important concept that spans intra-laboratory details (both within and between batches) as well as inter-laboratory comparisons. Conceptually, acute LC-MS/MS analysis with normalizing isotopically labelled internal standards is primarily precision-based. The generation of calibration solutions is the predominant step wherein accuracy is of primary importance. In the preparation and analysis of specimens, precision of preparation amongst calibrators, quality controls and patient samples are the only requirements, as the internal standard corrects for subtle inaccuracies that may occur during testing. Longitudinal reproducibility is again reliant on calibration accuracy and stability and should be interrogated in a similar mode to quality control samples. Additionally, proficiency testing is commonly utilized to provide indications of either harmonization or standardization between laboratories testing for the same measurand. Use of certified reference materials or comparison with reference method procedures (when available) is deployed to provide reliable results from distinct vendors, models, platforms or institutions5.
Data deposition
Results for clinical samples are posted to a laboratory information management system or other interface for an electronic medical record. Data can be interpreted for critical alert values, compared with reference intervals or evaluated for biomarker level change in the patient over time42. Long-term storage of reported results is generally mandated by regulatory organizations for all health-care-related details within an electronic medical record. The raw data utilized to produce the result(s) can be retained by a laboratory for interrogations of performance and quality of the assay, instruments and results.
Limitations and optimizations
There are several challenges in implementing LC-MS/MS for clinical diagnostics. These challenges are primarily a result of the manual nature of the assays, lack of integrated automation, relatively low sample throughput, high initial capital expense (several hundred thousand dollars in addition to associated construction and renovation costs), high level of technical proficiency required by technicians, limited assay standardization and lack of widely available commercial standards including quality control material and calibrators72. Given that the majority of clinical LC-MS/MS assays are considered laboratory developed tests, there are limitations related to the significant amount of time, money and technical expertise required to develop, validate and perform the assays. Additionally, data analysis and reporting are time-consuming and are often manual processes.
Clinical LC-MS/MS assays that measure proteins such as apolipoproteins, thyroglobulin, insulin/C-peptide, PTHrP, PTH and angiotensin have many limitations given the multiple steps required for sample preparation73,74, although streamlined sample preparation workflows for some clinical proteomic assays have recently been developed75,76. Consequently, the use of LC-MS/MS for protein-based clinical diagnostics has largely been limited to reference laboratories and large academic centres.
Staffing considerations
Proficient LC-MS/MS technical specialists possess skill sets that are difficult and time-consuming to teach77. Although certified laboratory technicians with a general background in medical or clinical laboratory science can be trained to conduct several of the routine tasks associated with LC-MS/MS assays, specialists are often required to conduct complex troubleshooting and maintenance activities. However, the specialists capable of this complex work often do not have the certification necessary to perform clinical assays and report results. As a significant cost-saving measure, it can be advantageous for clinical laboratories with several LC-MS/MS instrument platforms to have staff who are capable of conducting instrument service as opposed to relying on contracts from the instrument vendors’ service engineers. Smaller laboratories that have a limited number of LC-MS/MS instrument platforms often must rely on external service contracts owing to a lack of advanced in-house expertise. Because they are so few in number, LC-MS/MS technical specialists are often in high demand in various sectors of the clinical laboratory industry. Accordingly, to foster employee retention, it is imperative for employers to carefully consider appropriate compensation and career growth opportunities for LC-MS/MS technical specialists78,79.
Quality management
Careful attention to several parameters when developing clinical LC-MS/MS assays is necessary to mitigate some of the causes of poor assay performance. The lack of optimization of sample preparation, ionization and data processing can significantly compromise data quality and, consequently, patient safety. In this regard, systemic quality management and sustained vigilance are of considerable importance.
The key aspects of quality for clinical LC-MS/MS methods mirror those necessary for all clinical testing methods, and include robust method design and optimization; careful management of all steps of the analytical process to avoid gross handling errors; adequate training of technicians and competency verification; a proactive maintenance plan to limit within-instrument and between-instrument variations; and continuous quality verification based on the periodic analysis of control samples and external quality assessment samples with assigned target concentrations or peer group comparisons80. The quality management plan for LC-MS/MS assays must monitor the entire testing process, including the pre-analytical, analytical and post-analytical phases. The five Clinical Laboratory Improvement Amendments requirements for laboratory accreditation (facility administration, quality systems, proficiency testing, personnel and inspection) apply to all laboratories performing LC-MS/MS testing5.
Proficiency testing and external quality assessment are essential components of the quality management plan for clinical LC-MS/MS assays81. Sources of proficiency testing failures that are unique to LC-MS/MS assays include variable matrix effects, lot-to-lot reagent variation, poor specificity and non-commutability of the testing material82. Different sources and preparation of calibration material can result in distinct concentration assignments and, consequently, disagreement between assigned concentrations83,84.
Assay performance optimization
The process of optimizing an LC-MS/MS assay includes reducing or eliminating interferences, increasing ruggedness and enhancing sample throughput85. Addressing challenges related to interferences is of particular importance for assays that are designed to measure analytes such as steroid hormones, owing to the large number of similar metabolites, some of which are isobars of the analyte(s) of interest86. This is of particular importance for individuals with endocrine disorders owing to altered steroid hormone pathway regulation wherein the presence of analytical interferences can negatively impact assay accuracy, thereby compromising the efficacy of the diagnostic and treatment strategies for the patient87.
Outlook
LC-MS/MS is a highly sensitive and selective technique that can be effectively used for quantitation of many clinically relevant analytes. The appeal of these LC-MS/MS assays is that many can overcome the limitations of other techniques, which include cross-reactivity, low analytical specificity and limited dynamic range88. There are numerous potential advantages for clinical LC-MS/MS assays, which include shorter assay development and optimization time compared with immunoassays; the ability to include multiple analyte analysis in a single method; and wide applicability to various analytes including drugs, hormones, small molecules, carbohydrates, nucleic acids and proteins.
Automated instrumentation
Advances in LC-MS/MS instrumentation and applications are occurring at a rapid pace. Similar to many other methods used in laboratory medicine, the development of front-to-back automation is a goal for clinical LC-MS/MS. Instrument manufacturers are working towards fully automated black box LC-MS/MS systems wherein the entire analytical process from sample preparation to result generation can be accomplished in the absence of manual intervention. The approval of such a device by the FDA as a diagnostic test would greatly facilitate its adoption by clinical laboratories, including those without technical staff who possess specialized LC-MS/MS training.
Recently, the first all-in-one certified clinical mass analyser with regulatory clearance, the Cascadion SM Clinical Analyzer, was developed by Thermo Fisher Scientific. This analyser combines LC and MS/MS in one instrument. The system is compatible with primary specimen collection tubes and operates under full automation with bidirectional communication with the laboratory information system. Currently, the only commercially available application for this analyser is 25-hydroxy vitamin D. The performance of the assay is comparable with that of other FDA-cleared 25-hydroxy vitamin D assays89. Other instrument manufacturers, including Shimadzu, are also developing black box LC-MS/MS solutions that feature end-to-end automation and fully integrated system components so that LC-MS/MS can be performed more similarly to standard automated clinical laboratory assays90.
Automated data processing
The utility of automated LC-MS/MS data processing and analysis will become even greater with the maturation of automated LC-MS/MS instrumentation given the large sizes of the generated data sets. To enable clinical laboratories to fully harness the valuable information contained within these large-scale data sets, novel fit-for-purpose software tools are needed91,92. Some laboratories have developed and implemented dashboard tools to monitor, track, optimize and support specialized laboratory workflows that are not typically well supported by routine laboratory information system functionality.
As an example of one such solution, an open-source software solution to address the LC-MS/MS data review and quality needs of their clinical laboratory was recently developed93. This software solution includes a database and a visualization component to collect LC-MS/MS data and monitor quality assurance parameters. This customizable Web-based dashboard facilitates automated quality control and data analysis for their complex urine opiate and metabolite LC-MS/MS assay. Web-based dashboards such as this are valuable tools that clinical laboratories can utilize when commercial options are not available. When used efficiently, data visualization tools can help maintain high-quality LC-MS/MS instrument and assay performance93.
Novel ionization methods
More recently, novel mass spectrometry ionization methods and direct sampling techniques have been developed that may find use for routine clinical applications in the future. One such technology is rapid evaporative ionization mass spectrometry (REIMS), which enables the direct characterization of biological or environmental samples without a requirement for sample preparation. In this technique, the aerosol generated from the evaporation of the sample by Joule heating or laser irradiation is directly introduced into the inlet of a mass spectrometer. REIMS has promise in the fields of intra-surgical tissue classification, bacterial identification and rapid profiling of cell lines94. For example, the intelligent knife (iKnife) is based on REIMS technology95, and is intended to be used as an alternative to frozen section histology by enhancing near real-time intraoperative decision-making based on surgical margin characterization96. The iKnife combines a tissue dissection tool (handheld electrosurgical device) with a mass spectrometer to analyse the smoke from evaporating tissue during resection. The MasSpec Pen was developed for use as a clinical and intraoperative device for ex vivo and in vivo cancer diagnosis97. Similar to the iKnife, it is based on ambient ionization, but differs in that it is a non-destructive technique, and biomolecules are extracted from tissues using discrete water droplets and delivered to the inlet of the mass spectrometer rather than relying on evaporation of tissue to generate a sample.
There are numerous other ambient ionization techniques that demonstrate potential utility for in vitro analysis or diagnosis. Paper spray mass spectrometry was developed as a direct sampling ionization method for the mass spectrometry analysis of complex mixtures, including dried blood spots for TDM applications and illicit drug detection in urine specimens98,99. This approach performs ESI directly from the tip of the paper used for sample collection, and has potential for rapid point-of-care analysis. Coated blade spray mass spectrometry is a solid phase micro-extraction-based technology that can be directly coupled to mass spectrometry to enable the rapid qualitative and quantitative analysis of complex matrices100. Coated blade spray mass spectrometry is similar to paper spray in that ESI can be performed directly from a sample collection substrate, but it has the advantage of microsample preparation/purification prior to analysis. Laser diode thermal desorption mass spectrometry is another direct sampling technique, but in this case ionization of the sample is achieved using heat to generate gas phase samples rather than electrospray. This technique was developed primarily to increase analytical throughput by eliminating the need for chromatographic separation prior to mass spectrometry detection and has demonstrated numerous potential applications including characterization of illicit drugs and quantification of therapeutic drugs and their metabolites in blood101.
Conclusion
The realization of the full potential of LC-MS/MS in the clinical laboratory requires a thorough understanding of not only the vast opportunities but also the limitations of the technology. Among the barriers to the more widespread implementation of LC-MS/MS in the clinical laboratory is a lack of the ruggedness and robustness of the instrumentation in the context of minimizing downtime and reducing the requisite level of technical expertise for its efficient operation. There is substantial room for improvement of the application of LC-MS/MS in the areas of enhanced automation and the commercial availability of certified reference materials, calibrators and quality control materials. Despite these challenges, LC-MS/MS remains a powerful technology with distinct analytical capabilities that can be applied not only to routine clinical applications but also to unique clinical applications as they present.
References
Haag, A. M. Mass analyzers and mass spectrometers. Adv. Exp. Med. Biol. 919, 157–169 (2016).
Chardwaj, C. & Hanley, L. Ion sources for mass spectrometric identification and imaging of molecular species. Nat. Prod. Rep. 31, 756–767 (2014).
Hage, D.S. in Contemporary Practice in Clinical Chemistry 4th edn Ch. 8 (eds Marzinke, M. A. & Clarke, W.) 135–157 (Elsevier, 2020).
CLSI. Liquid Chromatography–Mass Spectrometry Methods; Approved Guideline CLSI Document C62-A (Clinical and Laboratory Standards Institute, 2014).
Lynch, K. L. Accreditation and quality assurance for clinical liquid chromatography–mass spectrometry laboratories. Clin. Lab. Med. 38, 515–526 (2018).
Graden, K. C., Bennett, S. A., Delaney, S. R., GiLL, H. E. & Willrich, M. A. V. A high-level overview of the regulations surrounding a clinical laboratory and upcoming regulatory challenges for laboratory dDeveloped tests. Lab. Med. 52, 315 (2021).
Stone, J. in Mass Spectrometry for the Clinical Laboratory Ch. 3 (eds Nair, H. & Clarke, W.) 37-62 (Academic, 2017).
Cao, Z., Kaleta, E. & Wang, P. Simultaneous quantitation of 78 drugs and metabolites in urine with a dilute-and-shoot LC-MS-MS assay. J. Anal. Toxicol. 39, 335–346 (2015).
Dahlin, J. L., Palte, M. J., LaMacchia, J. & Petrides, A. K. A rapid dilute-and-shoot UPLC-MS/MS assay to simultaneously measure 37 drugs and related metabolites in human urine for use in clinical pain management. J. Appl. Lab. Med. 3, 974–992 (2019).
Polson, C., Sarkar, P., Incledon, B., Raguvaran, V. & Grant, R. Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 785, 263–275 (2003).
Smith, A., Dowis, J. & French, D. Quantification of serum voriconazole, isavuconazole, and posaconazole by liquid chromatography tandem mass spectrometry (LC-MS/MS). Curr. Protoc. Toxicol. 76, e47 (2018).
Pablo, A., Breaud, A. R. & Clarke, W. Analysis of busulfan in plasma by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Curr. Protoc. Toxicol. 84, e93 (2020).
Pablo, A. H., Breaud, A. R. & Clarke, W. Analysis of immunosuppressant drugs in whole blood by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Curr. Protoc. Toxicol. 84, e92 (2020).
Langman, L. J. et al. Therapeutic monitoring of opioids: a sensitive LC-MS/MS method for quantitation of several opioids including hydrocodone and its metabolites. Ther. Drug. Monit. 35, 352–359 (2013).
Snozek, C. L., Bjergum, M. W. & Langman, L. J. Cocaine and metabolites by LC-MS/MS. Methods Mol. Biol. 902, 91–103 (2012).
Yang, Y., Rogers, K., Wardle, R. & El-Khoury, J. M. High-throughput measurement of 25-hydroxyvitamin D by LC-MS/MS with separation of the C3-epimer interference for pediatric populations. Clin. Chim. Acta 454, 102–106 (2016).
Keevil, B. G. LC-MS/MS analysis of steroids in the clinical laboratory. Clin. Biochem. 49, 989–997 (2016).
French, D. Development and validation of a serum total testosterone liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay calibrated to NIST SRM 971. Clin. Chim. Acta 415, 109–117 (2013).
Van Der Gugten, J. G. et al. Determination of serum aldosterone by liquid chromatography and tandem mass spectrometry: a liquid–liquid extraction method for the ABSCIEX API-5000 mass spectrometry system. J. Clin. Pathol. 65, 457–462 (2012).
Hawley, J. M., Adaway, J. E., Owen, L. J. & Keevil, B. G. Development of a total serum testosterone, androstenedione, 17-hydroxyprogesterone, 11β-hydroxyandrostenedione and 11-ketotestosterone LC-MS/MS assay and its application to evaluate pre-analytical sample stability. Clin. Chem. Lab. Med. 58, 741–752 (2020).
Van Der Gugten, J. G. & Holmes, D. T. Quantitation of aldosterone in serum or plasma using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Methods Mol. Biol. 1378, 37–46 (2016).
Lo, S. Y., Gordon, C., Sadilkova, K., Jack, R. M. & Dickerson, J. A. Quantifying MMA by SLE LC-MS/MS: unexpected challenges in assay development. Clin. Biochem. 49, 967–972 (2016).
Owen, L. J. & Keevil, B. G. Supported liquid extraction as an alternative to solid phase extraction for LC-MS/MS aldosterone analysis? Ann. Clin. Biochem. 50, 489–491 (2013).
Rappold, B. A. Review of the use of liquid chromatography–tandem mass spectrometry in clinical laboratories: part I — development. Ann. Lab. Med. 42, 121–140 (2022).
CLSI. Mass Spectrometry in the Clinical Laboratory: General Principles and Guidance; Approved Guideline CLSI Document C50-A (Clinical and Laboratory Standards Institute, 2007).
Guan, F. et al. Sensitive liquid chromatographic/tandem mass spectrometric method for the determination of beclomethasone dipropionate and its metabolites in equine plasma and urine. J. Mass. Spectrom. 38, 823–838 (2003).
Bonfiglio, R., King, R. C., Olah, T. V. & Merkle, K. The effects of sample preparations methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 13, 1175–1185 (1999).
Wooding, K. M. et al. Measurement of estradiol, estrone, and testosterone in postmenopausal human serum by isotope dilution liquid chromatography tandem mass spectrometry without derivatization. Steroids. 96, 89–94 (2015).
Kushnir, M. M., Rockwood, A. L., Nelson, G. J., Yue, B. & Urry, F. M. Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clin. Biochem. 38, 319–327 (2005).
Rappold, B. A. Mass spectrometry selectivity, specificity. Clin. Chem. 62, 154–156 (2016).
Lytle, F. E. & Julian, R. K. Automatic processing of chromatograms in a high-throughput environment. Clin. Chem. 62, 144–153 (2016).
ISO 15189. Medical Laboratories — Requirements for Quality and Competence (International Organization for Standardization, 2012).
Rappold, B. A. Special considerations for liquid chromatography–tandem mass spectrometry method development. Clin. Lab. Med. 38, 539–551 (2018).
Gu, H., Liu, G., Wang, J., Aubry, A. F. & Arnold, M. E. Selecting the correct weighting factors for linear and quadratic calibration curves with least-squares regression algorithm in bioanalytical LC-MS/MS assays and impacts of using incorrect weighting factors on curve stability, data quality, and assay performance. Anal. Chem. 86, 8959–8966 (2014).
US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Bioanalytical method validation: guidance for industry. US Food and Drug Administration https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf (2018).
Ehrmeyer, S. S. Satisfying regulatory and accreditation requirements for quality control. Clin. Lab. Med. 33, 27–40 (2013).
Kang, Y., Schneider, B. B. & Covey, T. R. On the nature of mass spectrometer analyzer contamination. J. Am. Soc. Mass Spectrom. 28, 2384–2392 (2017).
Westgard, J. O. Statistical quality control procedures. Clin. Lab. Med. 33, 111–124 (2013).
Ellington, A. A., Kullo, I. J., Bailey, K. R. & Klee, G. G. Measurement and quality control issues in multiplex protein assays: a case study. Clin. Chem. 55, 1092–1099 (2009).
Badrick, T. Biological variation: understanding why it is so important? Pract. Lab. Med. 23, e00199 (2021).
Rappold, B. A. Review of the use of liquid chromatography–tandem mass spectrometry in clinical laboratories: part II — operations. Ann. Lab. Med. 42, 531–557 (2022).
Clinical and Laboratory Standards Institute. Managing and Validating Laboratory Information Systems; Approved Guideline CLSI Document AUTO08-A (Clinical and Laboratory Standards Institute, 2006).
Ketha, H. et al. Clinical applications of LC-MS sex steroid assays: evolution of methodologies in the 21st century. Curr. Opin. Endocrinol. Diabetes Obes. 21, 217–226 (2014).
Carvalho, V. M. The coming of age of liquid chromatography coupled to tandem mass spectrometry in the endocrinology laboratory. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 883–884, 50–58 (2012).
Taieb, J. et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography–mass spectrometry in sera from 116 men, women, and children. Clin. Chem. 49, 1381–1395 (2003).
Zendjabil, M., Chellouai, Z. & Abbou, O. Role of mass spectrometry in steroid assays. Ann. Endocrinol. 77, 43–48 (2016).
Choi, M. H. Mass spectrometry-based steroid profiling: meeting unmet clinical needs. Tohoku J. Exp. Med. 253, 171–180 (2021).
Blair, J., Adaway, J., Keevil, B. & Ross, R. Salivary cortisol and cortison in the clinical setting. Curr. Opin. Endocrinol. Diabetes Obes. 24, 161–168 (2017).
Schiffer, L., Adaway, J. E., Arlt, W. & Keevil, B. G. A liquid chromatography–tandem mass spectrometry assay for the profiling of classical and 11-oxygenated androgens in saliva. Ann. Clin. Biochem. 56, 564–573 (2019).
D’Aurizio, F. & Cantu, M. Clinical endocrinology and hormones quantitation: the increasing role of mass spectrometry. Minerva Endocrinol. 43, 261–284 (2018).
Lenders, J. W. M. & Eisenhofer, G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol. Metab. 32, 152–161 (2017).
Christians, U. et al. Impact of laboratory practices on interlaboratory variability in therapeutic drug monitoring of immunosuppressive drugs. Ther. Drug Monit. 37, 718–724 (2015).
Shipkova, M. & Svinarov, D. LC-MS/MS as a tool for TDM services: where are we? Clin. Biochem. 49, 1009–1023 (2016).
Wilhelm, A. J., den Burger, J. C. & Swart, E. L. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin. Pharmacokinet. 53, 961–973 (2014).
Mbughuni, M. M. et al. Volumetric microsampling of capillary blood spot vs whole blood sampling for therapeutic drug monitoring of tacrolimus and cyclosporin a: accuracy and patient satisfaction. J. Appl. Lab. Med. 5, 516–530 (2020).
van der Elst, K. C. et al. Clinical validation of the analysis of fluconazole in oral fluid in hospitalized children. Antimicrob. Agents Chemother. 58, 6742–6746 (2014).
Avataneo, V. et al. LC-MS application for therapeutic drug monitoring in alternative matrices. J. Pharm. Biomed. Anal. 166, 40–51 (2019).
Lemaitre, F. et al. Opportunity to monitor immunosuppressive drugs in peripheral blood mononuclear cells: where are we and where are we going? Pharmacol. Res. 74, 109–112 (2013).
Berglund, E. et al. Intracellular concentration of the tyrosine kinase inhibitor imatinib in gastrointestinal stromal tumor cells. Anticancer. Drugs 25, 415–422 (2014).
Billat, P. A. et al. Liquid chromatography tandem mass spectrometry quantitation of intracellular concentrations of ganciclovir and its phosphorylated forms. Anal. Bioanal. Chem. 407, 3449–3456 (2015).
Derissen, E. J. et al. Development of an LC-MS/MS assay for the quantitative determination of the intracellular 5-fluorouracil nucleotides responsible for the anticancer effect of 5-fluorouracil. J. Pharm. Biomed. Anal. 110, 58–66 (2015).
Jannetto, P. J. et al. Executive summary: American Association of Clinical Chemistry laboratory medicine practice guideline—using clinical laboratory tests to monitor drug therapy in pain management patients. J. Appl. Lab. Med. 2, 489–526 (2018).
Lin, L. et al. Quantitation of Δ8-THC, Δ9-THC, cannabidiol and 10 other cannabinoids and metabolites in oral fluid by HPLC-MS-MS. J. Anal. Toxicol. 46, 76–88 (2022).
Reber, J. D. et al. An enhanced LC-MS-MS technique for distinguishing Δ8- and Δ9-tetrahydrocannabinol isomers in blood and urine specimens. J. Anal. Toxicol. 46, 343–349 (2022).
Pope, J. D. et al. Urine toxicology screening by liquid chromatography time-of-flight mass spectrometry in a quaternary hospital setting. Clin. Biochem. 95, 66–72 (2021).
Dodds, J. N. & Baker, E. S. Improving the speed and selectivity of newborn screening using ion mobility spectrometry–mass spectrometry. Anal. Chem. 93, 17094–17102 (2021).
Kemper, A. R. et al. Decision-making process for conditions nominated to the recommended uniform screening panel: statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet. Med. 16, 183–187 (2014).
Ombrone, D. et al. Expanded newborn screening by mass spectrometry: new tests, future perspectives. Mass. Spectrom. Rev. 35, 71–84 (2016).
Murray, D. L. Bringing mass spectrometry into the care of patients with multiple myeloma. Int. J. Hematol. 115, 790–798 (2022).
Barnidge, D. R. et al. Monitoring M-proteins in patients with multiple myeloma using heavy-chain variable region clonotypic peptides and LC-MS/MS. J. Proteome Res. 13, 1905–1910 (2014).
Barnidge, D. R. et al. Using mass spectrometry to monitor monoclonal immunoglobulins in patients with a monoclonal gammopathy. J. Proteome Res. 13, 1419–1427 (2014).
Clarke, W., Rhea, J. M. & Molinaro, R. Challenges in implementing clinical liquid chromatography–tandem mass spectrometry methods — the light at the end of the tunnel. J. Mass. Spectrom. 48, 755–767 (2013).
van den Broek, I., Sobhani, K. & Van, J. E. Eyk, advances in quantifying apolipoproteins using LC-MS/MS technology: implications for the clinic. Expert. Rev. Proteom. 14, 869–880 (2017).
Van Der Gugten, J. G. & Holmes, D. T. Quantitation of plasma renin activity in plasma using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Methods Mol. Biol. 1378, 243–253 (2016).
Shi, J. et al. A streamlined method for quantification of apolipoprotein A1 in human plasma by LC-MS/MS. Clin. Chem. 64, 1782–1784 (2018).
Fu, Q. et al. Automated proteomic sample preparation: the key component for high throughput and quantitative mass spectrometry analysis. Mass Spectrom. Rev. https://doi.org/10.1002/mas.21750 (2021).
Bell, L. et al. Challenges and opportunities for biological mass spectrometry core facilities in the developing world. J. Biomol. Tech. 29, 4–15 (2018).
Carden, R., Allsbrook, K. & Thomas, R. An examination of the supply and demand for clinical laboratory professionals in the United States. Transfusion 49, 2520–2523 (2009).
Cortelyou-Ward, K., Ramirez, B. & Rotarius, T. The laboratory workforce shortage: a managerial perspective. Health Care Manag. 30, 148–155 (2011).
Vogeser, M. & Seger, C. Quality management in clinical application of mass spectrometry measurement systems. Clin. Biochem. 49, 947–954 (2016).
Rappold, B. A. Review of the use of liquid chromatography–tandem mass spectrometry in clinical laboratories: part II—operations. Ann. Lab. Med. 42, 531–557 (2022).
Miller, W. G. The role of proficiency testing in achieving standardization and harmonization between laboratories. Clin. Biochem. 42, 232–235 (2009).
Siekmann, L. Requirements for reference (calibration) laboratories in laboratory medicine. Clin. Biochem. Rev. 28, 149–154 (2007).
Netzel, B. C. et al. First steps toward harmonization of LC-MS/MS thyroglobulin assays. Clin. Chem. 62, 297–299 (2016).
Rappold, B. A. Review of the use of liquid chromatography–tandem mass spectrometry in clinical laboratories: part I — development. Ann. Lab. Med. 42, 121–140 (2022).
Ponzetto, F. et al. UHPLC-HRMS analysis for steroid profiling in serum (steroidomics). Methods Mol. Biol. 1738, 261–278 (2018).
Vogeser, M. & Seger, C. Irregular analytical errors in diagnostic testing — a novel concept. Clin. Chem. Lab. Med. 56, 386–396 (2018).
Hoofnagle, A. N. & Wener, M. H. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J. Immunol. Methods 347, 3–11 (2009).
Benton, S. C. et al. Evaluation of the 25-hydroxy vitamin D assay on a fully automated liquid chromatography mass spectrometry system, the Thermo Scientific Cascadion SM Clinical Analyzer with the Cascadion 25-hydroxy vitamin D assay in a routine clinical laboratory. Clin. Chem. Lab. Med. 58, 1010–1017 (2020).
Seger, C. & Salzmann, L. After another decade: LC-MS/MS became routine in clinical diagnostics. Clin. Biochem. 82, 2–11 (2020).
Stanfill, B. A. et al. Quality control analysis in real-time (QC-ART): a tool for real-time quality control assessment of mass spectrometry-based proteomics data. Mol. Cell Proteom. 17, 1824–1836 (2018).
Dickerson, J. A. et al. Design and implementation of software for automated quality control and data analysis for a complex LC/MS/MS assay for urine opiates and metabolites. Clin. Chim. Acta 415, 290–294 (2013).
Pablo, A., Hoofnagle, A. N. & Mathias, P. C. Listening to your mass spectrometer: an open-source toolkit to visualize mass spectrometer data. J. Mass. Spectrom. Adv. Clin. Lab. 23, 44–49 (2022).
Jones, E. A. et al. Matrix assisted rapid evaporative ionization mass spectrometry. Anal. Chem. 91, 9784–9791 (2019).
Balog, J. et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci. Transl Med. 5, 194ra93 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03432429 (2022).
Zhang, J. et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci. Transl Med. 9, eaan3968 (2017).
Manicke, N. E. et al. Quantitative analysis of therapeutic drugs in dried blood spot samples by paper spray mass spectrometry: an avenue to therapeutic drug monitoring. J. Am. Soc. Mass. Spectrom. 22, 1501–1507 (2011).
Wang, H. et al. Paper spray for direct analysis of complex mixtures using mass spectrometry. Angew. Chem. Int. Ed. 49, 877–880 (2010).
Gómez-Ríos, G. A., Tascon, M. & Pawliszyn, J. Coated blade spray: shifting the paradigm of direct sample introduction to MS. Bioanalysis 10, 257–271 (2018).
Wang, C. H. et al. Solid phase microextraction combined with thermal-desorption electrospray ionization mass spectrometry for high-throughput pharmacokinetics assays. Anal. Chim. Acta 1021, 60–68 (2018).
Author information
Authors and Affiliations
Contributions
Introduction (W.A.C.); Experimentation (D.F.); Results (B.A.R.); Applications (P.J.J.); Reproducibility and data deposition (B.A.R.); Limitations and optimizations (S.N.T.); Outlook and Conclusion (S.N.T. and W.A.C.); Overview of the Primer (W.A.C.).
Corresponding author
Ethics declarations
Competing interests
W.A.C. declares consulting for and research support from Thermo Fisher Scientific, consulting for Roche Diagnostics, and research support from Shimadzu Scientific Instruments. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Karl Storbeck, Joshua Hayden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Analytic specificity
-
A methodology’s ability to measure only specific analyte(s) of interest, even if they are found alongside several interfering analytes.
- Fragmentation
-
An ion dissociation process during which a molecular ion breaks into smaller fragment ions, radicals and/or neutral molecules.
- Precursor ions
-
Ions that dissociate into smaller fragment ions; originally termed parent ions.
- Product ions
-
Ions formed as the result of a reaction (fragmentation, ion/molecule reaction) involving a precursor ion; originally termed daughter ions.
- Quadrupole
-
A mass analyser consisting of four parallel cylindrical conducting rods wherein ions are separated based on the stability of their trajectories in the oscillating radio frequency and direct current electric fields that are applied to the rods.
- Sorbent
-
Material used to collect molecules of another substance by the process of sorption.
- Time of flight
-
(TOF). A mass analyser within which ions are separated based on their velocity as they travel through a flight tube.
- Transition ratios
-
Ratios between precursor and product ion intensities or integrated peak areas that are monitored for analyte identification and quantification; also referred to as ion ratios.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, S.N., French, D., Jannetto, P.J. et al. Liquid chromatography–tandem mass spectrometry for clinical diagnostics. Nat Rev Methods Primers 2, 96 (2022). https://doi.org/10.1038/s43586-022-00175-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00175-x
This article is cited by
-
Recent developments in mass-spectrometry-based targeted proteomics of clinical cancer biomarkers
Clinical Proteomics (2024)
-
Lessons learned: establishing a CLIA-equivalent laboratory for targeted mass spectrometry assays – navigating the transition from research to clinical practice
Clinical Proteomics (2024)
-
Influence of physico-chemical properties of two lipoxin emulsion-loaded hydrogels on pre-polarized macrophages: a comparative analysis
Drug Delivery and Translational Research (2024)
-
Nanotechnology-based Detection Strategies for Hypertension Biomarkers
Chemical Research in Chinese Universities (2024)