Abstract
The relationships between metabolic rate, body temperature (Tb), body composition and ageing are complex, and not fully resolved. In particular, Tb and metabolic rate often change in parallel, making disentangling their effects difficult. Here we show that in both sexes of mice and hamsters exposure to a temperature of 32.5 °C leads to a reduced lifespan, coincident with lowered metabolic rate and elevated Tb with no change in body composition. We exploit the unique situation that when small mammals are exposed to hot ambient temperatures their Tb goes up, at the same time that their metabolic rate goes down, allowing us to experimentally separate the impacts of Tb and metabolic rate on lifespan. The impact of ambient temperature on lifespan can be reversed by exposing the animals to elevated heat loss by forced convection, which reverses the effect on Tb but does not affect metabolic rate, demonstrating the causal effect of Tb on lifespan under laboratory conditions for these models. The impact of manipulations such as calorie restriction that increase lifespan may be mediated via effects on Tb, and measuring Tb may be a useful screening tool for putative therapeutics to extend the human lifespan.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All additional data supporting the findings of this study are publicly available on the open science framework (http://osf.io.e7baj; https://doi.org/10.17605/OSF.IO/4FUJ7). Source data are provided with this paper.
References
Rubner, M. (ed.) Das Problem det Lebensdaur und seiner beziehunger zum Wachstum und Ernarnhung (Oldenberg, 1908).
Harman, D. Aging: a theory based on free radical and radiation biology. J. Gerontol. 11, 298–300 (1956).
Stark, G., Pincheira-Donoso, D. & Meiri, S. No evidence for the ‘rate of living’ theory across the tetrapod tree of life. Glob. Ecol. Biogeog. 29, 857–884 (2020).
Holmes, D. J., Fluckiger, R. & Austad, S. N. Comparative biology of aging in birds: an update. Exp. Gerontol. 36, 869–883 (2001).
Austad, S. N. & Fischer, K. E. Mammalian aging, metabolism, and ecology – evidence from the bats and marsupials. J. Gerontol. 46, B47–B53 (1991).
Speakman, J. R. Body size, energy metabolism and lifespan. J. Exp. Biol. 208, 1717–1730 (2005).
Speakman, J. R. et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3, 87–95 (2004).
Oklejewicz, M., Hut, R. A., Daan, S., Loudon, A. S. & Stirland, A. J. Metabolic rate changes proportionally to circadian frequency in tau mutant Syrian hamsters. J. Biol. Rhythms 12, 413–422 (1997).
Brand, M. D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 35, 811–820 (2000).
Ramsey, J. J., Harper, M. E. & Weindruch, R. Restriction of energy intake, energy expenditure, and aging. Free Radic. Biol. Med. 29, 946–968 (2000).
Blanc, S. et al. Energy expenditure of Rhesus monkeys subjected to 11 years of dietary restriction. J. Clin. Endocrinol. Metab. 88, 16–23 (2003).
Redman, L. M. et al. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 27, 805–815.e4 (2018).
Daan, S., Deerenberg, C. & Dijkstra, C. Increased daily work precipitates natural death in the kestrel. J. Anim. Ecol. 65, 539–544 (1996).
Pontzer, H. et al. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr. Biol. 26, 410–417 (2016).
Selman, C. et al. Impact of experimentally elevated energy expenditure on oxidative stress and lifespan in the short-tailed field vole, Microtus agrestis. Proc. Biol. Sci. 275, 1907–1916 (2008).
Hollosy, J. O. Minireview – exercise and longevity – studies on rats. J. Gerontol. 43, B149–B151 (1988).
Vaanholt, L. M., Daan, S., Garland, T. & Visser, G. H. Exercising for life? Energy metabolism, body composition and longevity in mice exercising at different intensities. Phys. Biochem. Zool. 83, 239–251 (2010).
Rikke, B. A. et al. Strain variation in the response of body temperature to dietary restriction. Mech. Aging Dev. 124, 663–678 (2003).
Mitchell, S. E. et al. The effects of graded levels of caloric restriction: III. Impact of short-term calorie and protein restriction on mean daily body temperature and torpor use in the C7BL/6 mouse. Oncotarget 6, 18314–18337 (2015).
Cintron-Colon, R. et al. Insulin-like growth factor 1 receptor regulates hypothermia during calorie restriction. Proc. Natl Acad. Sci. USA 114, 9731–9736 (2017).
Froy, O., Chapnik, N. & Miskin, R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing. Dev. 130, 154–160 (2009).
Conti, B. et al. Transgenic mice with a reduced core body temperature have an increased life span. Science 314, 825–828 (2006).
Liao, C. Y. et al. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell 10, 629–639 (2011).
Speakman, J. R. & Keijer, J. Not so nuanced: Reply to the comments of Gaskill and Garner on ‘Not so hot: Optimal housing temperatures for mice to mimic the environment of humans’. Mol. Metab. 3, 337 (2013).
Gordon, C. J. Thermal physiology of laboratory mice: defining thermoneutrality. J. Therm. Biol. 37, 654–685 (2012).
Zhao, Z. J. et al. The shift of thermoneutral zone in striped hamster acclimated to different temperatures. PLoS ONE 9, e84396 (2014).
Conti, B. Considerations on temperature, longevity and aging. Cell. Mol. Life Sci. 65, 1626–1630 (2008).
Rikke, B. A. & Johnson, T. E. Lower body temperature as a potential mechanism of life extension in homeotherms. Exp. Gerontol. 39, 927–930 (2004).
Mitchell, S. E. et al. The effects of graded levels of caloric restriction: VIII. Impact of short term calorie and protein restriction on basal metabolic rate in the C57BL.6 mouse. Oncotarget 8, 17453–17474 (2017).
Guijas, C. et al. Metabolic adaptation to calorie restriction. Sci. Signal. 13, eabb2490 (2020).
Goel, M. K., Khanna, P. & Kishore, J. Understanding survival analysis: Kaplan−Meier estimate. Int. J. Ayurveda Res. 1, 274–278 (2010).
Acknowledgements
We thank J.-X. Yu and G.-M. Deng from Wenzhou University for their assistance with animal care. This work was partly supported by grants (31670417 and 31870388 to Z.Z., and 92057206 to J.R.S.) from the National Natural Science Foundation of China and the National Key R&D Program of China (2019YFA0801900 to J.R.S.).
Author information
Authors and Affiliations
Contributions
Z.Z. and J.R.S. designed research; Z.Z., J.C., C.N., M.B., J.X., D.H., S.L. and W.L. the performed research. Z.Z. and J.C. analysed the data. Z.Z. and J.R.S. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Isabella Samuelson
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Locomotor behavior.
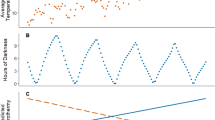
a, b, c and d. The locomotor behaviour over 48 h of striped hamster and Swiss mice. Shown are means ± s.e.m. of female hamsters, 21 °C, n=11, 32.5 °C, n=10, 32.5 °C+W, n=10, and male hamsters, 21 °C, n=10, 32.5 °C, n=12, 32.5 °C +W, n=12; and female mice, n=10 per group, and male mice, 21 °C, n=10, 32.5 °C, n=12, 32.5 °C +W, n=11 of biologically independent samples. e, f, g and h. The average locomotor behaviour over the dark and light phases. Shown are individual values, means ± SD of female hamsters, 21 °C, n=11, 32.5 °C, n=10, 32.5 °C+W, n=10, and male hamsters, 21 °C, n=10, 32.5 °C, n=12, 32.5 °C +W, n=12; and female mice, n=10 per group, and male mice, 21 °C, n=10, 32.5 °C, n=12, 32.5 °C +W, n=11 of biologically independent samples. Statistical significance was determined by one-way ANOVA (one-sided), followed by Tukey post-hoc tests. Black bars, dark phase; blank bars, light phase. Both rodents in the 32.5 °C and 32.5 °C +W groups showed similar circadian pattern of locomotor behavior to those in the 21 °C group, increasing in dark phase in the night, and decreasing in light phase in the day (Extended Data Fig. 1a–d). The average locomotor counts of female hamsters did not differ significantly between the three groups over the dark (F2,28=2.60, P=0.092), but it did over the light (Extended Data Fig. 1e). The average locomotor counts of male hamsters differed significantly among the three groups (Extended Data Fig. 1f). The average locomotor counts of female mice did not differ significantly between the three groups (dark: F2,27=2.58, P=0.094; light: F2,27=0.01, P=0.999, Extended Data Fig. 1g). The average locomotor counts of male mice was significantly lower at 32.5 °C than that at 21 °C over the dark, whereas it was not significantly different between the three groups over the light (F2,31=1.37, P=0.270, Extended Data Fig. 1h).
Extended Data Fig. 2 Serum IL-6 and TNFa concentration.
a, b, c and d. Serum IL-6 concentration of striped hamsters and Swiss mice. Shown are individual values, means ± SD of female hamsters, n=7 per group, and male hamsters, 21 °C, n=7, 32.5 °C, n=7, 32.5 °C+W, n=6; and female mice, n=7 per group, and male mice, 21 °C, n=7, 32.5 °C, n=7, 32.5 °C+W, n=6 biologically independent samples. Statistical significance was determined by one-way ANOVA (one-sided). e, f, g and h. Serum TNFa concentration of striped hamsters and Swiss mice. Shown are individual values, means ± SD of female hamsters, n=7 per group, and male hamsters, 21 °C, n=7, 32.5 °C, n=7, 32.5 °C+W, n=6; and female mice, n=7 per group, and male mice, 21 °C, n=7, 32.5 °C, n=7, 32.5 °C+W, n=6 biologically independent samples. Statistical significance was determined by one-way ANOVA (one-sided). Serum IL-6 concentration did not significantly differ between the 21 °C, 32.5 °C and 32.5 °C+W groups for either hamsters (females, F2,18=0.34, P=0.716, Extended Data Fig. 2a; males, F2,17=0.13, P=0.880, Extended Data Fig. 2b) or mice (females, F2,18=0.26, P=0.772, Extended Data Fig. 2c; males, F2,17=0.25, P=0.781, Extended Data Fig. 2d). Also, there was no significant difference in serum IL-6 concentration between the three groups for either hamsters (females, F2,18=0.04, P=0.964, Extended Data Fig. 2e; males, F2,17=0.11, P=0.898, Extended Data Fig. 2f) or mice (females, F2,18=0.10, P=0.909, Extended Data Fig. 2g; males, F2,17=0.06, P=0.943, Extended Data Fig. 2h).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 1
Survivorship and mortality.
Source Data Fig. 2
Body mass and food intake.
Source Data Fig. 3
Daily energy expenditure (DEE).
Source Data Fig. 4
Body temperature (Tb).
Source Data Extended Fig. 1
Locomotor behavior.
Source Data Extended Fig. 2
Serum IL-6 and TNFα concentration.
Rights and permissions
About this article
Cite this article
Zhao, Z., Cao, J., Niu, C. et al. Body temperature is a more important modulator of lifespan than metabolic rate in two small mammals. Nat Metab 4, 320–326 (2022). https://doi.org/10.1038/s42255-022-00545-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00545-5
This article is cited by
-
Fat accumulation in striped hamsters (Cricetulus barabensis) reflects the temperature of prior cold acclimation
Frontiers in Zoology (2024)
-
Supplementation with autologous adipose stem cell-derived mitochondria can be a safe and promising strategy for improving oocyte quality
Journal of Assisted Reproduction and Genetics (2024)
-
Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes
Nature Aging (2023)
-
Targeting aging with the healthy skeletal system: The endocrine role of bone
Reviews in Endocrine and Metabolic Disorders (2023)
-
Effect of different masses, ages, and coats on the thermoregulation of dogs before and after exercise across different seasons
Veterinary Research Communications (2023)