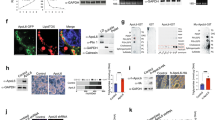
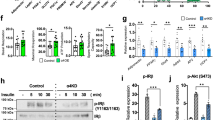
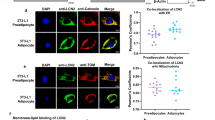
Abstract
Patatin-like phospholipase-domain-containing 2 (PNPLA2; also known as adipose triglyceride lipase, ATGL) and PNPLA3 (also known as adiponutrin) are encoded by close paralogues and appear to have opposite functions on triacylglycerol mobilization and storage. PNPLA2 is a major triglyceride lipase in adipose tissue and liver, whereas a common human variant of PNPLA3, I148M, greatly increases risk of hepatosteatosis. Nonetheless, the function of PNPLA3 and the mechanism by which the I148M variant promotes triacylglycerol accumulation are poorly understood. Here we demonstrate that PNPLA3 strongly interacts with α/β-hydrolase-domain-containing 5 (ABHD5; also known as CGI-58), an essential co-activator of PNPLA2. Molecular imaging experiments demonstrate that PNPLA3 effectively competes with PNPLA2 for ABHD5 and that PNPLA3 I148M competes even more effectively. Inducible overexpression of PNPLA3 I148M greatly suppressed PNPLA2-dependent lipolysis and triggered massive triacylglycerol accumulation in brown adipocytes, with these effects dependent on ABHD5. The interaction of PNPLA3 and ABHD5 can be regulated by fatty acid supplementation and synthetic ABHD5 ligands, raising the possibility that this interaction might be targeted for the treatment of fatty liver disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article and its supplementary information files or are available from the corresponding authors upon reasonable request.
References
Haemmerle, G. et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 (2006).
Lass, A. et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin–Dorfman syndrome. Cell Metab. 3, 309–319 (2006).
Sztalryd, C. & Brasaemle, D. L. The perilipin family of lipid droplet proteins: gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta 1862, 1221–1232 (2017).
Schweiger, M., Lass, A., Zimmermann, R., Eichmann, T. O. & Zechner, R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297, E289 (2009).
Lord, C. C. et al. Regulation of hepatic triacylglycerol metabolism by CGI-58 does not require ATGL co-activation. Cell Rep. 16, 939–949 (2016).
Xie, M. & Roy, R. The causative gene in Chanarian–Dorfman syndrome regulates lipid droplet homeostasis in C. elegans. PLoS Genet. 11, e1005284 (2015).
Holmes, R. Comparative studies of adipose triglyceride lipase genes and proteins: an ancient gene in vertebrate evolution. Open Access Bioinformatics 4, 15–29 (2012).
Baulande, S., Lasnier, F., Lucas, M. & Pairault, J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J. Biol. Chem. 276, 33336–33344 (2001).
Kershaw, E. E. et al. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 55, 148–157 (2006).
He, S. et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 285, 6706–6715 (2010).
Huang, Y., Cohen, J. C. & Hobbs, H. H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem. 286, 37085–37093 (2011).
Kumari, M. et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 15, 691–702 (2012).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
BasuRay, S., Smagris, E., Cohen, J. C. & Hobbs, H. H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 66, 1111–1124 (2017).
Smagris, E. et al. Pnpla3 I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 61, 108–118 (2015).
Basantani, M. K. et al. Pnpla3/adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res. 52, 318–329 (2011).
Chen, W., Chang, B., Li, L. & Chan, L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology 52, 1134–1142 (2010).
Li, J. Z. et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Invest. 122, 4130–4144 (2012).
Perttila, J. et al. PNPLA3 is regulated by glucose in human hepatocytes, and its I148M mutant slows down triglyceride hydrolysis. Am. J. Physiol. Endocrinol. Metab.. 302, E1063–E1069 (2012).
Patel, S., Yang, W., Kozusko, K., Saudek, V. & Savage, D. B. Perilipins 2 and 3 lack a carboxy-terminal domain present in perilipin 1 involved in sequestering ABHD5 and suppressing basal lipolysis. Proc. Natl Acad. Sci. USA 111, 9163–9168 (2014).
Granneman, J. G. & Moore, H.-P. H. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol. Metab. 19, 3–9 (2008).
Yamaguchi, T., Omatsu, N., Matsushita, S. & Osumi, T. CGI-58 interacts with perilipin and is localized to lipid droplets: possible involvement of CGI-58 mislocalization in Chanarin–Dorfman syndrome. J. Biol. Chem. 279, 30490–30497 (2004).
Granneman, J. G., Moore, H.-P. H., Mottillo, E. P. & Zhu, Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem. 284, 3049–3057 (2009).
Kerppola, T. K. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 1, 1278–1286 (2006).
Huang, Y. et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl Acad. Sci. USA 107, 7892–7897 (2010).
Qiao, A. et al. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology 54, 509–521 (2011).
Sanders, M. A. et al. Endogenous and synthetic ABHD5 ligands regulate ABHD5–perilipin interactions and lipolysis in fat and muscle. Cell Metab. 22, 851–860 (2015).
Rondini, E. A. et al. Novel pharmacological probes reveal ABHD5 as a locus of lipolysis control in white and brown adipocytes. J. Pharmacol. Exp. Ther. 363, 367–376 (2017).
Cohen, J. C., Horton, J. D. & Hobbs, H. H. Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 (2011).
Dongiovanni, P. et al. PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol. 19, 6969–6978 (2013).
Ghosh, A. K., Ramakrishnan, G., Chandramohan, C. & Rajasekharan, R. CGI-58, the causative gene for Chanarin–Dorfman syndrome, mediates acylation of lysophosphatidic acid. J. Biol. Chem. 283, 24525–24533 (2008).
Chakrabarti, P. et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 52, 1693–1701 (2011).
Dubuquoy, C. et al. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J. Hepatol. 55, 145–153 (2011).
Chamoun, Z., Vacca, F., Parton, R. G. & Gruenberg, J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol. Cell 105, 219–233 (2013).
Donati, B. et al. The rs2294918 E434K variant modulates patatin-like phospholipase domain-containing 3 expression and liver damage. Hepatology 63, 787–798 (2016).
Kumashiro, N. et al. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology 57, 1763–1772 (2013).
Mitsche, M. A., Hobbs, H. H. & Cohen, J. C. Patatin-like phospholipase domain–containing protein 3 promotes transfers of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. J. Biol. Chem. 293, 6958–6968 (2018).
Montero-Moran, G. et al. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J. Lipid Res. 51, 709–719 (2010).
Edens, N. K., Leibel, R. L. & Hirsch, J. Mechanism of free fatty acid re-esterification in human adipocytes in vitro. J. Lipid Res. 31, 1423–1431 (1990).
Leibel, R. L., Hirsch, J., Berry, E. M. & Gruen, R. K. Alterations in adipocyte free fatty acid re-esterification associated with obesity and weight reduction in man. Am. J. Clin. Nutr. 42, 198–206 (1985).
Jenkins, C. M. et al. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 (2004).
Ohno, Y., Nara, A., Nakamichi, S. & Kihara, A. Molecular mechanism of the ichthyosis pathology of Chanarin–Dorfman syndrome: stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD5. J. Dermatol. Sci. 92, 245–253 (2018).
Kien, B. et al. ABHD5 stimulates PNPLA1-mediated ω-O-acylceramide biosynthesis essential for a functional skin permeability barrier. J. Lipid Res. 59, 2360–2367 (2018).
Stender, S. et al. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 49, 842–847 (2017).
Schweiger, M. et al. Measurement of lipolysis. Methods Enzymol. 538, 171–193 (2014).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Acknowledgements
We thank J. Wang and M. Sanders for critical discussions and W. Liu for providing plasmids containing human PNPLA3. This work was supported by National Institutes of Health (NIH) grants, F30-DK116529-01A1 to A.Y., K99-DK114471 to E.P.M., and DK76629 and DK105963 to J.G.G. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center grant P30 CA022453 to the Karmanos Cancer Institute at Wayne State University and the Perinatology Research Branch of the National Institutes of Child Health and Development at Wayne State University. The Lipidomics Core Facility at Wayne State University was supported in part by National Center for Research Resources, National Institutes of Health grant S10RR027926.
Author information
Authors and Affiliations
Contributions
J.G.G. and E.P.M. conceived the experiments. J.G.G., E.P.M. and A.Y. designed the experiments. A.Y., E.P.M., L.Z. and L.M.L. conducted the experiments. A.Y., E.P.M. and J.G.G. analysed the data and wrote the manuscript. All authors read the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7
Rights and permissions
About this article
Cite this article
Yang, A., Mottillo, E.P., Mladenovic-Lucas, L. et al. Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes. Nat Metab 1, 560–569 (2019). https://doi.org/10.1038/s42255-019-0066-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0066-3
This article is cited by
-
Lipid droplets and cellular lipid flux
Nature Cell Biology (2024)
-
Lipid droplet biogenesis and functions in health and disease
Nature Reviews Endocrinology (2023)
-
Structural and functional insights into ABHD5, a ligand-regulated lipase co-activator
Scientific Reports (2022)
-
Shared genetic loci for body fat storage and adipocyte lipolysis in humans
Scientific Reports (2022)
-
Lipolysis regulates major transcriptional programs in brown adipocytes
Nature Communications (2022)