Abstract
This study aimed to evaluate the safety and efficiency of hybrid endoscopic submucosal dissection (H-ESD) using a newly developed ALL IN ONE (AIO) snare. This was a matched control study in a porcine model. Five paired simulated stomach lesions 2–2.5 cm in size were removed by H-ESD using an AIO snare or conventional ESD (C-ESD) using an endoscopic knife. The outcomes of the two procedures were compared, including en-bloc resection rates, procedure times, intraprocedural bleeding volumes, muscular injuries, perforations, thicknesses of the submucosal layer in resected specimens, and stomach defects. All simulated lesions were resected en-bloc. Specimens resected by H-ESD and C-ESD were similar in size (7.68 ± 2.92 vs. 8.42 ± 2.42 cm2; P = 0.676). H-ESD required a significantly shorter procedure time (13.39 ± 3.78 vs. 25.99 ± 4.52 min; P = 0.031) and submucosal dissection time (3.99 ± 1.73 vs. 13.1 ± 4.58 min; P = 0.003) versus C-ESD; H-ESD also yielded a faster dissection speed (241.37 ± 156.84 vs. 68.56 ± 28.53 mm2/min; P = 0.042) and caused fewer intraprocedural bleeding events (0.40 ± 0.55 vs. 3.40 ± 1.95 times/per lesion; P = 0.016) than C-ESD. The thicknesses of the submucosal layer of the resected specimen (1190.98 ± 134.07 vs. 1055.90 ± 151.76 μm; P = 0.174) and the residual submucosal layer of the stomach defect (1607.94 ± 1026.74 vs. 985.98 ± 445.58 μm; P = 0.249) were similar with both procedures. The AIO snare is a safe and effective device for H-ESD and improves the treatment outcomes of gastric lesions by shortening the procedure time.
Similar content being viewed by others
Introduction
Endoscopic submucosal dissection (ESD) is a microinvasive technique for the en-bloc removal of precancerous lesions or early-stage gastrointestinal cancer with limited lymph node metastasis risk1. Compared with endoscopic mucosal resection (EMR), ESD achieves a high en-bloc resection rate but increases the risk of perforation and bleeding2. Moreover, ESD requires a longer learning curve due to technique complexity3. The standard ESD protocol consists of the following steps: lesion marking, submucosal injection, mucosal incision, submucosal dissection, and hemostasis using electrical equipment or devices, such as needles, knives, and hemostatic forceps1.
Hybrid ESD (H-ESD) was developed to simplify the en-bloc resection of selected lesions. Briefly, after mucosal incision and partial submucosal dissection, the lesion is removed using a snare4. H-ESD has been demonstrated safe and effective for the resection of colorectal lesions, with a shorter procedure duration, fewer complications, and no difference in recurrence versus conventional ESD (C-ESD)5. New devices with multiple functions, including marking, incision, dissection, and hemostasis, have been developed to increase the efficiency and lower the cost of H-ESD, such as the SOUTEN snare (ST1850-20, Kaneka, Medix, Tokyo, Japan)6 and Flat Adenoma Resection Instruments (FARIn; Endox-Feinmechanik GmbH, Bad Urach, Germany)7. Recently, a novel multifunctional snare [ALL IN ONE (AIO); LeoMed, Changzhou, China] was introduced for efficient H-ESD (Fig. 1). AIO performs integrated injection and argon plasma coagulation (APC) functions, enabling selective initial range marking, submucosal injection, mucosal incision and dissection, and hemostasis to ensure complete, independent, and safe ESD operation8,9. In this study, we investigated the safety and efficiency of the AIO snare for H-ESD in a porcine model.
Methods
Study design
This was a matched-control study to evaluate the safety and efficiency of the AIO snare for H-ESD versus a traditional endoscopic knife for C-ESD in a porcine model in vivo. The study was conducted at the experimental animal center of Pengli Testing Technology (Shanghai, China) Co., Ltd. (Shanghai, China) in accordance with Animal Research Reporting In vivo Experiments guidelines10. This study was approved by the Institutional Review Board of Pengli Testing Technology (Shanghai, China) Co., Ltd. Animal experiments were conducted according to guidelines for the ethical review of laboratory animal welfare (People’s Republic of China National Standard GB/T 35892-2018) and the institutional guidelines.
Animals, devices, and procedure
Two matched healthy white pigs with pre-procedure weights of 35–40 kg were used. The animals underwent fasting, general anesthesia with endotracheal intubation, and monitoring. The experiment was conducted using a GIF-Q260J (Olympus, Japan) endoscope with a transparent cap at the tip. A high-frequency generator (VIO 200 S, ERBE, Germany) and water jet (Olympus, Japan) were used. A total of ten (matched 2 × 5) simulated lesions 2–2.5 cm in length were marked using the transparent cap as reference (3 in the stomach body and 2 in the antrum at similar locations in each pig. and dissected by 3 endoscopists (Dr. Peng Jin, and Dr. Lang Yang, and Dr. Hui Su), all the endoscopists were trained but with a little experience of needle-type knife (MK-T-2-195) and AIO snare (less than 5 cases for each). For C-ESD, a traditional endoscopic needle-type knife (MK-T-2-195, Micro-Tech, Nanjing, China) was used for marking, mucosal incision, and dissection. A 3 cm AIO snare with a 2 mm tip was used for H-ESD. In addition, submucosal injection with saline plus indigo was conducted by using a disposable needle (M00518351, Boston Scientific, USA) in both H-ESD and C-ESD group to decrease bias. In H-ESD, the lesion was marked by AIO snare, followed by submucosal injection by using disposable needle. Then, circumferential mucosal incision deep into the submucosal layer was made by protruded tip of AIO snare, the lesion was removed appropriately using the AIO snare after partial submucosal dissection depending on experience (Supplementary Video). Intraprocedural bleeding was controlled by knife/AIO snare or hemostatic forceps (HBF-23/2000, Micro-Tech, Nanjing, China), depending on the amount of bleeding. The specimens were fixed and cut for histological analysis.
Outcome measures and definitions
The primary outcome was procedure time, defined as the time from the start of the mucosal incision to the completion of lesion resection, including submucosal injection, mucosal incision, dissection/snaring, and homeostasis in each procedure. The time of submucosal dissection was measured from the beginning to the end of submucosal dissection, including dissection using a knife or snare and the time required for additional submucosal injection and homeostasis.
The secondary outcomes were as follows: en-bloc resection rates, submucosal resection speeds, adverse events (intraprocedural bleeding, perforation, and muscular injury), thicknesses of the submucosal layer in the resected specimen, and stomach defects. Intraprocedural bleeding was defined as bleeding during submucosal dissection, and the number of intraoperative bleedings, hemostasis using hemostatic forceps were recorded. Perforation was observed and recorded during the experimental procedure. Muscular injury was defined as destroyed muscle bundle observed under endoscopy or degeneration or necrosis of muscle cells in hematoxylin–eosin-stained slides. Dissection speed was calculated as the specimen size divided by the time of submucosal dissection. The depth of the submucosal layer of resected specimens and stomach defects was measured as previously described11. Briefly, the depth of the submucosal layer was calculated as the area of the submucosal layer divided by the corresponding muscular length11. In addition, Dr. PJ and LY had experience of more than 150 gastric ESD cases were classified as senior endoscopists, and Dr. HS had experience of less than 50 gastric ESD cases was classified as junior endoscopist.
Sample size calculation
According to a preliminary experiment, the mean C-ESD procedure time is approximately 35 min with a standard deviation (SD) of 5 min, while that for H-ESD is 18 min for resecting lesions 2–3 cm in size. We used PASS 11.0 software for sample size estimation. With a power of 0.90 and two sides alpha of 0.05, the sample size should be 4 in each group. To compensate for a 20% dropout rate, we aimed to include 5 lesions in each group.
Statistical analysis
Categorical data are represented by the number of cases and percentage, and comparisons between the 2 procedures were performed by the chi-square or Fisher’s exact tests. Normally distributed quantitative data are shown as the mean and SD and were analyzed using Student’s t test. The generalized linear model was used to analyze the factors influencing procedure time. P values < 0.05 were considered to indicate significance. All statistical analyses were performed using SPSS 26.0 software (IBM Corporation, Armonk, NY).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Pengli Testing Technology (Shanghai, China) Co., Ltd.
Results
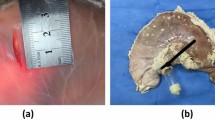
All lesions were resected en-bloc, and no perforations occurred (Fig. 2). Specimens resected by H-ESD and C-ESD were similar (7.68 ± 2.92 vs. 8.42 ± 2.42 cm2; P = 0.676). The H-ESD procedure time was significantly shorter than that for C-ESD (13.39 ± 3.78 vs. 25.99 ± 4.52 min; P = 0.031), as was the submucosal dissection time (3.99 ± 1.73 vs. 13.1 ± 4.58 min; P = 0.003), but similar mucosal incision time was observed in H-ESD and C-ESD (9.48 ± 5.14 vs. 12.88 ± 6.64 min; P = 0.382); a faster dissection speed was also obtained using H-ESD (241.37 ± 156.84 vs. 68.56 ± 28.53 mm2/min; P = 0.042). No perforation occurred; however, H-ESD resulted in fewer number of intraprocedural bleeding than C-ESD (0.40 ± 0.55 vs. 3.40 ± 1.95; P = 0.016), but similar number of hemostasis events using hemostatic forceps (0.20 ± 0.45 vs. 0.40 ± 0.89; P = 0.267). Two tiny muscular injuries were observed under endoscopy in C-ESD group, but there is no statistic difference between the two procedures (P = 0.444). In addition, the histological analysis of the resected specimens showed intact muscularis mucosae and muscularis propria without degeneration and necrosis corresponding to stomach defects in hybrid and conventional ESD. Moreover, the thickness of submucosal layer of the resected specimen was similar in H-ESD and C-ESD (1190.98 ± 134.07 vs. 1055.90 ± 151.76 μm; P = 0.174), as was the thickness of the residual submucosal layer of the stomach defect (1607.94 ± 1026.74 vs. 985.98 ± 445.58 μm; P = 0.249) (Table 1 and Fig. 3).
A generalized linear regression model was used to analyze the effects of AIO, operator, location, intraprocedural bleeding, and specimen size on procedure time. The results show that AIO is an independent factor affecting procedure time, and the use of AIO can significantly reduce the procedure time (P = 0.004) (Table 2).
Discussion
This study evaluated the safety and efficiency of the AIO snare for H-ESD in the resection of gastric lesions in a live porcine model. In these simulated gastric lesions, the AIO snare yielded a comparable en-bloc resection rate to that achieved with the traditional endoscopic knife but required a shorter procedure time.
Endoscopic resection is a less invasive treatment for early gastric cancer with limited lymph node metastasis or precancerous lesions2. EMR and polypectomy were initially introduced for early-stage gastric neoplasms, which are safe and effective. However, conventional gastric EMR for larger lesions often results in an insufficient resection margin due to snare slippage, potentially leading to piecemeal resection, which complicates the pathological evaluation of specimens and is associated with a high local recurrence rate12,13. To overcome the technique limitation of EMR, ESD adopts a mucosal lesion incision followed by submucosal dissection instead of a snare, theoretically allowing preplanned en-bloc resection of target lesions of any size. ESD is significantly superior to EMR in achieving higher curative resection rates and lower recurrence rates14. Thus, the European guidelines recommend ESD as the first-line endoscopic treatment for gastric superficial lesions with null/very low lymph node metastasis risk and EMR as an alternative for elevated lesions < 10 mm with a low likelihood of advanced histology15. However, ESD requires a longer procedure time and is associated with a higher rate of adverse events since it is more technically difficult than EMR and requires more specialized skills.
H-ESD is an alternative method for endoscopic resection that bridges the gap between ESD and EMR. H-ESD involves mucosal incision, partial submucosal dissection of the lesion, and snare resection. H-ESD was initially used as a rescue method for resecting colorectal lesions and later for the planned resection of target lesions16. In H-ESD, a mucosal incision is made by an endoscopic knife or SOUTHEN snare17. A systemic analysis showed that H-ESD is safe and effective for removing colorectal lesions, with a shorter procedure duration, fewer adverse events, and no difference in recurrence rates compared to C-ESD; however, H-ESD is associated with a lower en-bloc resection rate5. ESD is the best endoscopic modality for achieving en-bloc resection for lesions larger than 2 cm. However, regarding colorectal lesions, those 2–3 cm in size were found to be most suitable for H-ESD18. H-ESD has also been used for esophageal lipoma19, anal canal fibroma20, rectal neuroendocrine tumor21,22 resection, and even for full-thickness resection of T2 colorectal cancer23.
H-ESD effectively reduces the procedure time for gastric dysplastic lesions. A randomized trial showed that H-ESD with SOUTHEN requires a significantly shorter procedure time, with high and comparable curability rates and safety profiles to those of C-ESD for differentiated intramucosal lesions without ulceration < 20 mm in diameter6. Similarly, Chiyo et al. reported that cost-efficient H-ESD using SOUTEN is acceptable for resecting gastric neoplasms < 15 mm17. In the current study, we tested the newly developed, multifunctional AIO snare, which offers the following advantages: (1) the conical head allows the protective tube head end to be easily inserted into the tissue opening, and the linker between the tip and snare are embedded designed for the tube, which stabilize the length of protruded tip for subsequent mucosal incision and submucosal dissections, we found that the AIO snare achieved the similar mucosal incision time than traditional needle-type knife. Those suggest that the cut or dissection ability of AIO snare may not inferior to traditional needle type knife; (2) the knob on the handle ensures that the knife head is positioned accurately and does not move when the hand is moved by mistake, ensuring a stable operational process; and (3) the AIO is equipped with argon gas and water injection ports that can selectively inject low viscoelasticity liquid such as water or saline and APC, and perform injections, electroincisions, electrocoagulation, and edge electroincision and electrocoagulation to achieve selective initial range marking, submucosal injection, mucosal incision and stripping, and hemostasis functions, but the function of APC and injection was not involved in test to decrease bias. In the current study, the AIO snare was tested for H-ESD for lesions 2–2.5 cm in size, which is larger than previous reports6,17, and en-bloc resection was achieved for all lesions. In addition, H-ESD required a shorter procedure time and achieved a faster dissection speed than C-ESD, indicating that H-ESD can be expanded to include larger lesions and achieve en-bloc resection. During H-ESD, the mucosal incision outside the marking dots and the submucosa is partially dissected, enabling subsequent snaring to easily achieve en-bloc resection. In addition, to prevent muscular injury, it is better to elevate the lesion by adequate submucosal injection and/or lift the snare away from the muscular propria when the snare is cut. In current study, two tiny muscular injuries were observed under endoscopy in C-ESD, but not in pathological slides, possible explanation was that the muscular injuries were too tiny and missed by pathological sampling. Moreover, fewer intraprocedural bleeding events occurred with H-ESD than with C-ESD, which may be one reason that H-ESD simplified the dissection process. Additionally, the resection depths with H-ESD and C-ESD were similar (mean 1190 μm). However, the H-ESD resection depth in the human population requires further investigation. In practice, the dissection depth can adjust and cut just above the muscle layer in C-ESD, whereas, the adjusting the dissection depth is challenging when using snare resection in H-ESD. Thus, carefully pre-resection evaluation and selection of mucosal lesions is essential for successful planned H-ESD. In addition, in case of submucosal invasive lesion suspected, the step of snare resection in H-ESD should be abandoned and transit to conventional dissection by protruded tip of AIO snare.
The strengths of this study are as follows. (1) This was a matched-control study to test the safety and efficiency of a newly developed AIO snare for H-ESD, and this snare was demonstrated to safely achieve en-bloc resection with a shorter procedure time than that of C-ESD using an endoscopic knife. (2) The experiment was conducted by three endoscopists with different experience levels, indicating that the AIO snare is easy to use, even for less experienced endoscopists. (3) H-ESD was achieved using one device (the AIO snare), eliminating the need for device exchange during the procedure and potentially reducing ESD durations and costs. However, this study had some limitations. First, this preliminary study was conducted in a porcine model with small number simulated lesions without the ulceration associated with increased submucosal fibrosis resulting from invasive cancer or pretreatment biopsy. Therefore, the results need to be validated in a population study. In addition, the area of partial submucosal dissection in H-ESD was determined by endoscopists’ experience, which may influence the snare resection difficulties and procedure time. In Bae JH et al.’s study on H-ESD for colorectal lesions, the snared submucosal tissue was resected when the maximum length of the slit on the handle was < 5 mm24. However, how to predict the area of undissected submucosal tissue for safe en-bloc resection in gastric neoplasia remains to be illustrated.
Conclusion
The AIO snare was found to be a safe and effective device for H-ESD, with a shorter procedure time and similar en-bloc resection rate for gastric lesions to that achieved with C-ESD. Those results providing guidance for the next stage of clinical trials.
Data availability
All materials are commercially available, and the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
19 May 2024
The original online version of this Article was revised: In the original version of this Article, the Supplementary Video, which was included with the initial submission, was omitted from the Supplementary Information file. The correct Supplementary Video file now accompanies the original Article.
References
Esaki, M., Ihara, E. & Gotoda, T. Endoscopic instruments and techniques in endoscopic submucosal dissection for early gastric cancer. Expert. Rev. Gastroenterol. Hepatol. 15, 1009–1020. https://doi.org/10.1080/17474124.2021.1924056 (2021).
Vasconcelos, A. C., Dinis-Ribeiro, M. & Libânio, D. Endoscopic resection of early gastric cancer and pre-malignant gastric lesions. Cancers (Basel) 15, 1. https://doi.org/10.3390/cancers15123084 (2023).
Yoshida, M. et al. Learning curve and clinical outcome of gastric endoscopic submucosal dissection performed by trainee operators. Surg. Endosc. 31, 3614–3622. https://doi.org/10.1007/s00464-016-5393-9 (2017).
Okamoto, Y. et al. Indications and outcomes of colorectal hybrid endoscopic submucosal dissection: A large multicenter 10-year study. Surg. Endosc. 36, 1894–1902. https://doi.org/10.1007/s00464-021-08471-5 (2022).
McCarty, T. R., Bazarbashi, A. N., Thompson, C. C. & Aihara, H. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: A systematic review and meta-analysis. Endoscopy 53, 1048–1058. https://doi.org/10.1055/a-1266-1855 (2021).
Esaki, M. et al. Hybrid and conventional endoscopic submucosal dissection for early gastric neoplasms: A multi-center randomized controlled trial. Clin. Gastroenterol. Hepatol. 21, 1810-1818.e8. https://doi.org/10.1016/j.cgh.2022.10.030 (2023).
Gölder, S. K., Schaller, T., Farin, G., Messmann, H. & Probst, A. Partially insulated cutting instruments for hybrid endoscopic submucosal dissection—the Flat Adenoma Resection Instruments (FARIn). Endoscopy 48(Suppl 1), E218-219. https://doi.org/10.1055/s-0042-109058 (2016).
Kou, P., Ge, Q., & Zhang, Z. The utility model relates to a tool head assembly of a medical electric knife and a medical electric knife. CHINA, 202222751354[P], 2023–10–20. https://pss-system.cponline.cnipa.gov.cn/retrieveList?prevPageTit=changgui.
Zhou, P., Cai, M., Ge, Q. et al. The utility model relates to a tool head assembly of a new medical electric knife and a new medical electric knife. CHINA, CN202111337053.9 [P]. 2022–08–05. https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=changgui.
PercieduSert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Ito, A. et al. Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection. J. Gastroenterol. 53, 1171–1178. https://doi.org/10.1007/s00535-018-1446-2 (2018).
Ono, H. et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48, 225–229. https://doi.org/10.1136/gut.48.2.225 (2001).
Horiki, N. et al. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg. Endosc. 26, 72–78. https://doi.org/10.1007/s00464-011-1830-y (2012).
Ono, H. et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second). Dig. Endosc. 33, 4–20. https://doi.org/10.1111/den.13883 (2021).
Pimentel-Nunes, P. et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy 54, 591–622. https://doi.org/10.1055/a-1811-7025 (2022).
Gostout, C. J. & Knipschield, M. A. Submucosal endoscopy with mucosal resection: A hybrid endoscopic submucosal dissection in the porcine rectum and distal colon. Gastrointest. Endosc. 76, 829–834. https://doi.org/10.1016/j.gie.2012.05.037 (2012).
Chiyo, T. et al. Acceptability of hybrid endoscopic submucosal dissection using multifunctional snare for small-sized gastric neoplasms: A prospective observational study. J. Gastrointest. Liver Dis. 31, 390–395. https://doi.org/10.15403/jgld-4524 (2022).
Gorospe, E. C. & Wong Kee Song, L. M. Hybrid endoscopic submucosal dissection in the colon: Cutting corners or trimming fat. Gastrointest. Endosc. 83, 593–595. https://doi.org/10.1016/j.gie.2015.08.059 (2016).
Deshmukh, A., Elmeligui, A., Parsa, N., Tejedor-Tejada, J. & Nieto, J. Successful removal of a giant esophageal lipoma with hybrid endoscopic submucosal dissection. VideoGIE 6, 398–400. https://doi.org/10.1016/j.vgie.2021.05.020 (2021).
Okamoto, T., Ikeya, T. & Fukuda, K. Hybrid endoscopic submucosal dissection for anal canal fibroma. VideoGIE 7, 154–157. https://doi.org/10.1016/j.vgie.2022.01.003 (2022).
Nasu, T. et al. Traction-assisted hybrid endoscopic submucosal dissection for small rectal neuroendocrine tumors. Endoscopy 54, E550–E551. https://doi.org/10.1055/a-1662-4965 (2022).
Gravito-Soares, M. et al. Endoscopic resection of a rectal neuroendocrine tumor: Hybrid endoscopic submucosal dissection. GE Port. J. Gastroenterol. 26, 131–133. https://doi.org/10.1159/000487550 (2019).
Wilson, N., Abdallah, M. & Bilal, M. Hybrid endoscopic submucosal dissection and endoscopic full-thickness resection for complete resection of a T2 colorectal adenocarcinoma in a nonsurgical candidate. Gastrointest. Endosc. 98, 136–137. https://doi.org/10.1016/j.gie.2023.01.045 (2023).
Bae, J. H. et al. Optimized hybrid endoscopic submucosal dissection for colorectal tumors: A randomized controlled trial. Gastrointest. Endosc. 83, 584–592. https://doi.org/10.1016/j.gie.2015.06.05 (2016).
Funding
This study was supported by the Beijing Municipal Science and Technology Commission (BMSTC, Fund No. D171100002617001).
Author information
Authors and Affiliations
Contributions
Lang Yang and Peng Jin conceived and designed the study. Lang Yang, Peng Jin, Xian-zong Ma, Hui Su, Jie Zhang, Jian-qiu Sheng participated in data acquisition. Lang Yang, Peng Jin and Hui Su gave technical or material support. Lang Yang, Xian-zong Ma performed data analysis and interpretation. Lang Yang, Xian-zong Ma participated in preparing the manuscript. Lang Yang, Peng Jin, Jian-qiu Sheng reviewed and edited the manuscript. Peng Jin and Jian-qiu Sheng supervised the study. All authors critically revised the manuscript for important intellectual property and approved submitting this manuscript. The manuscript was revised and corrected for the English presentation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, L., Ma, Xz., Su, H. et al. Safe and effective hybrid endoscopic submucosal dissection with ALL IN ONE snare in porcine gastric model (with video). Sci Rep 14, 10060 (2024). https://doi.org/10.1038/s41598-024-61031-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61031-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.