Abstract
To assess the diagnostic performance of three cardiothoracic (CT) ratio techniques, including diameter, circumference, and area, for predicting hemoglobin (Hb) Bart’s disease between 17 and 22 weeks’ gestation, and to create a multivariable scoring system using multiple ultrasound markers. Before invasive testing, three CT ratio techniques and other ultrasound markers were obtained in 151 singleton pregnancies at risk of Hb Bart’s disease. CT diameter ratio demonstrated the highest sensitivity among the other techniques. Significant predictors included CT diameter ratio > 0.5, middle cerebral artery-peak systolic velocity (MCA-PSV) > 1.5 multiples of the median, and placental thickness > 3 cm. MCA-PSV exhibited the highest sensitivity (97.8%) in predicting affected fetuses. A multivariable scoring achieved excellent sensitivity (100%) and specificity (84.9%) for disease prediction. CT diameter ratio exhibited slightly outperforming the other techniques. Increased MCA-PSV was the most valuable ultrasound marker. Multivariable scoring surpassed single-parameter analysis in predictive capabilities.
Similar content being viewed by others
Introduction
Alpha thalassemia is a red blood cell disorder common in Southeast Asia, the Middle East, China, and Africa. Hemoglobin (Hb) Bart’s disease is a severe form that causes fetal tissue hypoxia and severe anemia. Hydrops fetalis usually occurs in the second half of pregnancy and can lead to stillbirth and maternal complications1,2,3,4,5. Nonetheless, Hb Bart's disease is no longer universally regarded as a fatal disorder. The increasing number of survivors can be attributed to intrauterine transfusions and neonatal intensive care2,6,7. Despite these improvements, there remains a high incidence of associated congenital abnormalities and growth impairment among survivors2,6. Some individuals may experience neurodevelopmental delays2,6. Long-term transfusions and iron chelation are necessary for most patients following hematopoietic stem cell transplantation, which is currently the only definitive cure2,7,8. In utero stem cell transplantation or gene therapy/editing shows promise as another approach, but it is still in the clinical trial stage7.
Prenatal diagnosis of Hb Bart’s disease involves invasive procedures such as chorionic villus sampling, amniocentesis, and cordocentesis. However, these procedures carry risks of fetal loss9,10. To minimize these risks, serial ultrasonography is being explored as a noninvasive alternative approach11. Previous studies have identified ultrasound markers, including enlarged fetal heart or increased cardiothoracic (CT) ratio, elevated middle cerebral artery-peak systolic velocity (MCA-PSV), placentomegaly, and hepatosplenomegaly, that hold potential for predicting fetal Hb Bart’s disease3,12,13,14,15.
Fetal CT ratios can be computed using three methods: CT diameter (CTD), CT circumference (CTC), or CT area (CTA)16. Prior studies indicate a slight increase in fetal CT ratios with advancing gestational age (GA)16,17,18. However, only the CTD ratio is commonly employed to assess fetal cardiomegaly for detecting Hb Bart’s disease11,12,13,14,19,20. Limited research exists on evaluating fetal CTC and CTA ratios as sonographic predictors for Hb Bart’s disease. Therefore, this study aimed to determine optimal cutoff values and assess the diagnostic performance of CTD, CTC, and CTA ratios for identifying Hb Bart’s disease at 17–22 gestational weeks. Additionally, we evaluated midpregnancy ultrasonographic features such as MCA-PSV and placental thickness (PT). Furthermore, our secondary objective was to develop a multivariable scoring system incorporating multiple ultrasound markers to enhance disease prediction accuracy.
Materials and methods
This retrospective and prospective cohort study was conducted on pregnant women at risk of fetal Hb Bart’s disease who underwent second-trimester ultrasound examination and invasive prenatal testing at the Maternal-Fetal Medicine (MFM) Unit of Siriraj Hospital, Bangkok, Thailand, from June 2018 to November 2022. Retrospective data from 129 cases (June 2018–November 2021) and prospective data from 22 cases (December 2021–November 2022) were collected to achieve an adequate sample size. The institutional review board of Siriraj Ethics Committee authorized the research protocol (approval no. Si-897/2021), and written informed consent was obtained from all prospective study participants. The study was performed in accordance with the Declaration of Helsinki.
The study included data from singleton pregnant women who were at risk of carrying a fetus with Hb Bart’s disease based on a maternal and paternal thalassemia blood test screening (alpha thal-1 trait, Hb H, and Hb H Constant Spring/Pakse disease). These women underwent invasive diagnostic procedures (amniocentesis or cordocentesis) for fetal alpha DNA testing or hemoglobin typing between 17 and 22 weeks of gestation. Exclusion criteria were fetal structural and/or functional cardiac anomalies, beta thalassemia major, fetal infection, other causes of fetal anemia, major extracardiac defects, chromosomal abnormalities, and abnormal fetal growth. Women with unknown fetal alpha globin gene analysis results and/or Hb typing, or those lost to follow-up for postprocedural outcomes, were excluded.
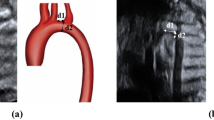
Before prenatal genetic testing, ultrasound examinations were performed by an MFM staff member or an MFM fellow using an abdominal 1–5-MHz curvilinear transducer (Voluson E10, E8, or E6; GE Medical Systems, Chicago, IL, USA). The CTD, CTC, and CTA ratios were measured on cross-sectional images of the fetal chest at the level of the 4-chamber view during end-diastole (Fig. 1A,B). The measurement techniques were described in our previous study16. For each ratio, measurements were repeated three times, with the average calculated from recorded video clips or still images. Intraobserver and interobserver variabilities were not assessed, as our previous study reported good to excellent agreement for the measurements16.
Fetal thorax at the level of four‐chamber view during end‐diastole showing (A) measurement of fetal cardiothoracic diameter ratio and (B) cardiothoracic circumference and area ratios by automated ellipse method. CC‐CA cardiac circumference and cardiac area, CD cardiac diameter, TC‐TA thoracic circumference and thoracic area, TD thoracic diameter.
Additional evaluated ultrasonographic features were MCA-PSV, PT, soft markers of aneuploidy, and signs of hydropic changes. MCA-PSV measurements followed the technique proposed by the Society for Maternal–Fetal Medicine in 201521, with the maximum point of the MCA Doppler waveform selected from still images. MCA-PSV values were expressed as multiples of the median (MoM) by dividing them by the Thai nomogram medians for specific gestational ages reported by Tongsong et al.22 PT was measured by placing the transducer perpendicularly to the placenta and averaging the longitudinal and transverse plane measurements of its center23,24. “Hydrops fetalis” was defined as the presence of at least two abnormal fluid collections (pleural and pericardial effusions, ascites, or fluid accumulation with anasarca)25. All ultrasound findings were obtained before receiving the invasive testing results. Hb Bart’s disease confirmation relied on fetal polymerase chain reaction for alpha globin gene analysis from amniocentesis or Hb typing using high-performance liquid chromatography and alpha-globin gene analysis from cordocentesis.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics, version 29 (IBM Corp, Armonk, NY, USA), and STATA 17.0 software (StataCorp LLC, College Station, TX, USA). Demographic data are presented as numbers (percentages) and means ± standard deviations. The normal distribution was assessed using the Shapiro–Wilk test. Unpaired t-tests compared continuous variables between fetuses affected and unaffected by Hb Bart’s disease. Categorical variables were analyzed using Pearson’s chi-square or Fisher’s exact test. Receiver operating characteristic (ROC) curves determined optimal cutoff values for ultrasonographic markers. Differences in areas under the curve (AUCs) were assessed using the Z-test. Diagnostic parameters (sensitivity, specificity, positive predictive value, negative predictive value, accuracy, false positives [FPs], and false negatives [FNs]) were reported. Univariate analysis identified prenatal predictors associated with Hb Bart’s disease, and multiple logistic regression analysis evaluated the interdependence of significant variables. The most discriminative independent variables were selected. Statistical significance was set at P < 0.05. A multivariable sonographic scoring system was developed, and various cutoffs were evaluated for diagnostic performance. Discriminatory power was assessed using AUC analysis, and calibration plots compared the observed and expected outcome frequencies. The Brier score verified probability forecast accuracy, with a score close to zero indicating optimal model performance. The Hosmer–Lemeshow test assessed calibration, with P > 0.05 indicating good calibration.
Results
During the study period, 151 pregnancies at risk were eligible. Among them, 45 fetuses (29.8%) were diagnosed with Hb Bart’s disease. Frank Hb Bart’s hydrops fetalis and Hb H or Hb H Constant Spring/Pakse disease were detected in 9 cases (6.0%) and 10 cases (6.6%), respectively, the latter being included in the unaffected group. Table 1 shows the baseline characteristics of affected and unaffected pregnancies, including maternal age, parity, GA at ultrasound examination, body mass index, underlying maternal disease, types of parental alpha and beta globin gene analysis, and invasive prenatal diagnostic techniques. No significant differences were found between the groups, except for a significantly higher cordocentesis rate in the affected group (64.4%) than in the unaffected group (0%; P < 0.001). Regarding postprocedural outcomes, all affected pregnancies were safely terminated vaginally, and no procedure-related pregnancy losses occurred.
Measuring fetal CT ratios using all three methods was technically feasible. Fetuses with Hb Bart’s disease exhibited significantly higher mean CTD, CTC, and CTA ratios than unaffected fetuses (0.58 ± 0.06 vs 0.45 ± 0.04, 0.60 ± 0.05 vs 0.50 ± 0.03, and 0.36 ± 0.06 vs 0.24 ± 0.03, respectively; all P < 0.001). The affected group also had significantly higher mean MCA-PSV and PT measurements than the unaffected group (2.01 ± 0.27 vs 1.26 ± 0.24 MoM and 3.60 ± 1.01 vs 2.56 ± 0.54 cm, respectively; all P < 0.001). Among the second-trimester soft markers, fetuses with Hb Bart’s disease demonstrated a significantly higher rate of hyperechoic bowel (P < 0.001), while no significant differences were observed in the remaining markers (P = 0.322–1.000).
Based on ROC curve analysis (Table 2 and Fig. 2), the optimal cutoff values for differentiating affected and unaffected fetuses were as follows: CTD ratio 0.5 (AUC 0.961; 95% CI 0.917–1.000); CTC ratio 0.54 (AUC 0.949; 95% CI 0.908–0.990); and CTA ratio 0.29 (AUC 0.954; 95% CI 0.917–0.992). While the CTD ratio exhibited slightly better AUC and sensitivity (91.1%), there were no significant differences between the AUCs of the three methods. Using a cutoff of 1.5 MoM for MCA-PSV provided the best discrimination (AUC 0.975; 95% CI 0.954–0.996) with the highest AUC and sensitivity (97.8%). Conversely, a PT cutoff of 3 cm had the lowest AUC and sensitivity (AUC 0.839; 95% CI 0.766–0.911; sensitivity 73.3%). All three CT ratio measurement techniques and MCA-PSV had significantly higher AUCs than PT (P < 0.001–0.009). However, there were no significant differences in the AUCs of the three fetal CT ratios and MCA-PSV (P = 0.280–0.558).
Receiver-operating-characteristics curves of three fetal cardiothoracic ratio techniques, middle cerebral artery-peak systolic velocity, and placental thickness for prediction of fetal hemoglobin Bart’s disease. AUC area under the curve, CTA cardiothoracic area ratio, CTC cardiothoracic circumference ratio, CTD cardiothoracic diameter ratio, MCA-PSV middle cerebral artery-peak systolic velocity, PT placental thickness.
We excluded 9 cases of frank hydrops fetalis from the cohort and repeated the statistical analysis. These fetuses exhibited distinct signs of Hb Bart’s disease and may not benefit from predictive sonographic parameters. However, the optimal cutoffs for each ultrasound marker remained unchanged. MCA-PSV maintained the highest sensitivity (97.2%), followed by the CTD ratio (94.4%), CTC ratio (94.4%), CTA ratio (91.7%), and PT (69.4%) (data not shown).
By using multiple logistic regression analysis, significant ultrasonographic parameters and their cutoff values were identified: CTD ratio > 0.5 (P < 0.001), MCA-PSV level < 1.5 MoM (P < 0.001), and PT measurement > 3 cm (P = 0.033). A multivariable scoring system was then developed to predict fetal Hb Bart’s disease. Variables included in the system were selected based on statistical significance and assigned values corresponding to their coefficients. An increased PT > 3 cm received a value of 1, a CTD ratio > 0.5 received a value of 2, and an elevated MCA-PSV MoM > 1.5 received a maximum value of 2.5. Total scores ranged from 0 to 5.5 (Table 3).
Table 4 presents the diagnostic abilities of different cutoffs of the sonographic scoring system for identifying fetuses with Hb Bart’s disease. Two cutoffs were assessed. A threshold score of ≥ 2.5 yielded 100% sensitivity, 84.9% specificity, 89.4% accuracy, 15.1% FP, and 0% FN for disease detection. Alternatively, a threshold score of ≥ 3 achieved higher specificity (90.6%), accuracy (92.7%), and FP (9.4%) but slightly lower sensitivity (97.8%) and FN (2.2%).
The multivariable scoring system was internally validated using the AUC and calibration plot (Fig. 3). The system demonstrated excellent discriminatory ability, with an AUC of 0.990 (95% CI 0.980–1.000; P < 0.001). The calibration plot showed excellent fit (slope 1.027; intercept 0.005). The Hosmer–Lemeshow test yielded a P value of 0.829, indicating good calibration, and the Brier score was 0.035, signifying optimal calibration.
(A) Receiver-operating-characteristics curves of the sonographic scoring system for discriminating between fetuses with and without hemoglobin Bart’s disease (area under the curve = 0.99). (B) The calibration plot of the scoring system demonstrated great consistency between the predicted and observed outcome probabilities.
Discussion
Fetuses with Hb Bart’s disease exhibit cardiovascular adaptations to compensate for anemia and prevent tissue hypoxia. The initial structural change is cardiac chamber enlargement, increasing blood volume and cardiac output for optimal tissue oxygenation3,5,26. The CTD ratio, representing fetal cardiomegaly, has shown excellent predictive ability for Hb Bart’s disease8,11,12,13,14,19,26,27. Our study confirmed the effectiveness of all three fetal CT ratio measurement techniques in distinguishing affected and unaffected pregnancies. While the CTD ratio demonstrated slightly higher sensitivity, there were no significant differences in overall diagnostic accuracy (Fig. 2).
Comparisons with previous publications are summarized in Table 5. Our study’s optimal cutoff for the CTD ratio aligned with previous reports8,11,12,13,14,19,26. The best cutoff for each fetal CT ratio method surpassed or equaled the 95th centile of GA-specific references for normal Thai fetuses, indicating existing fetal cardiomegaly16. Regarding sensitivity, our CTD ratio was slightly lower than that in other studies. This discrepancy may be attributed to the inclusion of 9 hydropic fetuses, some of whom presented with marked pericardial or pleural effusion. Consequently, fetal thoracic diameter appeared longer, resulting in decreased CTD values and potentially reduced sensitivity8,19. Upon excluding all cases of hydrops fetalis, the new sensitivity for the fetal CTD ratio reached 94.4%, similar to earlier studies.
MCA-PSV Doppler assessment is recommended as the primary method for detecting fetal anemia, regardless of etiology21. Our study found that MCA-PSV had the highest sensitivity (97.8%) for identifying Hb Bart’s disease, which was moderately higher than previous reports13,28,29,30. This difference may be attributed to using advanced ultrasound technology with high-resolution probes for 2-dimensional and Doppler ultrasonography and improved MCA-PSV assessment by examiners. We utilized Thai nomograms for median MCA-PSV values22, which appeared lower than those used in a study on the Caucasian population by Mari et al.31 This could result in an increased detection rate of affected fetuses compared to studies using normative data from different populations13. Furthermore, it has been observed that most affected fetuses (92.3%) develop some degree of anemia in midgestation, often presenting with elevated MCA-PSV before 23 weeks of gestation20,27,32. Therefore, elevated MCA-PSV serves as a direct indicator of fetal anemia, the disease’s pathophysiology, during the mid-second trimester.
Comparing optimal cutoffs for MCA-PSV, most previous studies, including ours, used values above 1.5 MoM13,28,29,30. Tongprasert et al.29 proposed a cutoff of 1.3 MoM to detect even mildly anemic-affected fetuses during midpregnancy, achieving exceptional sensitivity (100%) but moderate specificity (69.2%). If we adopted the 1.3 MoM cutoff, our results aligned with the other, demonstrating a high sensitivity of 100% but a lower specificity of 64.2% (data not shown).
Among the midtrimester ultrasound parameters, MCA-PSV exhibited the highest sensitivity, followed by the CTD ratio and PT (Table 2). However, there were no significant differences in the AUCs of the CTD ratio and MCA-PSV, indicating similar diagnostic accuracy for detecting fetal Hb Bart’s disease. PT showed lower sensitivity and specificity than the CTD ratio and MCA-PSV, consistent with previous studies11,14,19,23. This can be attributed to the fact that placental thickening becomes more apparent later in gestation than an increase in the CTD ratio or MCA-PSV3,5,14,27. While some studies have suggested that the CTD ratio is superior to MCA-PSV as a single screening marker throughout gestation8,13,26,27, our findings demonstrate that the CTD ratio and MCA-PSV are equally effective predictors, particularly in the mid-second trimester.
Regarding the association between soft markers and fetal Hb Bart’s disease, we observed a significantly higher prevalence of echogenic bowel among affected pregnancies, consistent with other studies8,33. This could be attributed to bowel hypoperistalsis or bowel wall edema resulting from severe fetal anemia and hypoxia33. However, due to this parameter’s subjective evaluation and limited sensitivity, we did not include it in the multivariable scoring system.
Our study developed a multivariable scoring system using sonographic evaluation to noninvasively predict fetal Hb Bart’s disease. The system included several ultrasound parameters with high diagnostic accuracy and assigned scores based on their significant association with the disease. Unlike previous articles that relied on a single marker or a combination of CTD and MCA-PSV (Table 5), our approach utilized a broader set of markers. Our and other data also revealed that the combination of the CTD ratio and/or MCA-PSV achieved the highest detection rate (100%), outperforming the use of any individual marker13,27,28.
The increase in MCA-PSV above 1.5 MoM was the most significant marker for distinguishing affected and unaffected fetuses, with the highest sensitivity (97.8%) and accuracy (90.7%). An MCA-PSV level exceeding 1.5 MoM, assigned a score of 2.5, was the best single parameter for predicting Hb Bart’s disease (Table 4). In cases where this finding was absent, detecting Hb Bart’s disease required positive results for the remaining two variables in the scoring system. Including multiple markers in the system improved its sensitivity and reduced FNs compared to relying solely on MCA-PSV levels. This was evident in one case where Hb Bart’s disease was genetically confirmed despite an MCA-PSV level of only 1.41 MoM.
The sonographic scoring system serves as a helpful screening tool for differentiating high-risk fetuses requiring invasive procedures from low-risk fetuses suitable for serial ultrasound surveillance. A threshold score of ≥ 2.5 achieved a 100% detection rate for fetal Hb Bart’s disease. However, there were drawbacks, with 15.1% of unaffected fetuses identified as positive, necessitating invasive evaluation (Table 4). Notably, 3 cases (30%) of fetal Hb H disease had only an MCA-PSV MoM value above the cutoff of 1.5, signifying FP results. Alternatively, defining a rating score of ≥ 3 reduced the FP rate to 9.4% but increased the FN rate to 2.2%.
In the clinical implementation of the multivariable scoring system, a score ≥ 2.5 would warrant invasive diagnostic procedures. Conversely, cases with a score < 2.5 can be managed with regular ultrasound monitoring every 2–4 weeks until 24 weeks' gestation to assess sonographic markers and hydropic signs. Studies suggest that additional ultrasound examinations beyond 23–24 weeks gestation may be unnecessary if no positive ultrasound features or hydropic changes are identified3,20,27. If a rating score of ≥ 2.5 and/or hydropic signs emerged during follow-up ultrasounds, confirmatory prenatal diagnosis through invasive genetic testing was pursued. If the scoring system indicates abnormalities that cannot be explained by Hb Bart’s disease or other causes of cardiomegaly, fetal echocardiography is recommended for a more precise and detailed examination to evaluate the cardiac structures.
The study’s strengths lie in measuring all ultrasound parameters during invasive prenatal diagnosis, ensuring accurate fetal diagnosis confirmed by molecular genetic testing. This allowed for precise classification of fetuses with Hb Bart’s disease versus Hb H or Hb H Constant Spring/Pakse disease and the perception of differences in the sonographic markers of these conditions, such as increased MCA-PSV without cardiomegaly in 30% of fetuses with Hb H or Hb H Constant Spring/Pakse disease. Additionally, we determined optimal cutoff values, compared the diagnostic accuracy of different CT ratio techniques and other ultrasound findings in predicting Hb Bart’s disease, and developed a multivariable scoring system with high sensitivity and specificity for clinical decision-making in invasive prenatal diagnosis.
However, our study has several limitations. First, the findings are specific to the mid-second trimester and cannot be generalized to other gestational periods, particularly the first trimester. Second, some retrospective study patients had limitations in image quality. Third, the sensitivity and specificity of the markers for Hb Bart’s disease may vary when assessed by general practitioners, as MFM trainees and specialists performed our assessments. Fourth, the automated ellipse method might exhibit double errors in both CTC and CTA ratios compared to the CTD ratio. Results may differ when using manual tracing. Finally, given the limited sample size in our study, further research is necessary to evaluate the diagnostic accuracy of all three fetal CT ratio methods. Additionally, assessing the effectiveness, obstacles, and cost-effectiveness of the multivariable scoring approach in routine general practice is warranted.
Conclusions
Our study found that all three fetal CT ratio measurement techniques effectively identified Hb Bart’s disease, with the CTD ratio showing slightly better sensitivity. Additionally, elevated MCA-PSV above 1.5 MoM demonstrated the highest sensitivity and accuracy for predicting affected fetuses. The multivariable scoring system enhanced sensitivity and specificity compared to individual ultrasound parameters alone, making it suitable for clinical practice. This noninvasive approach is a valuable tool that improves prenatal detection of Hb Bart’s disease, reduces medical costs, and avoids risks associated with invasive diagnosis in unaffected fetuses.
Data availability
All data generated or analysed during this study are included in this published article.
References
Fucharoen, S. & Winichagoon, P. Hemoglobinopathies in Southeast Asia. Hemoglobin 11, 65–88 (1987).
Jatavan, P., Chattipakorn, N. & Tongsong, T. Fetal hemoglobin Bart’s hydrops fetalis: Pathophysiology, prenatal diagnosis and possibility of intrauterine treatment. J. Matern. Fetal Neonatal Med. 31, 946–957 (2018).
Thammavong, K., Luewan, S., Wanapirak, C. & Tongsong, T. Ultrasound features of fetal anemia lessons from hemoglobin Bart disease. J. Ultrasound Med. 40, 659–674 (2021).
Kwan, W. Y., So, C. H., Chan, W. P., Leung, W. C. & Chow, K. M. Re-emergence of late presentations of fetal haemoglobin Bart’s disease in Hong Kong. Hong Kong Med. J. 17, 434–440 (2011).
Thammavong, K., Luewan, S., Jatavan, P. & Tongsong, T. Foetal haemodynamic response to anaemia. ESC Heart Fail. 7, 3473 (2020).
Songdej, D., Babbs, C., Higgs, D. R., Consortium BI. An international registry of survivors with Hb Bart’s hydrops fetalis syndrome. Blood 129, 1251–1259 (2017).
Horvei, P., MacKenzie, T. & Kharbanda, S. Advances in the management of alpha-thalassemia major: Reasons to be optimistic. Hematol. Am. Soc. Hematol. Educ. Program 2021, 592–599 (2021).
Hui, P. W., Pang, P. & Tang, M. H. Y. 20 years review of antenatal diagnosis of haemoglobin Bart’s disease and treatment with intrauterine transfusion. Prenat. Diagn. 42, 1155–1161 (2022).
Enzensberger, C. et al. Fetal loss rate and associated risk factors after amniocentesis, chorionic villus sampling and fetal blood sampling. Ultraschall Med. 33, E75–E79 (2012).
Ghidini, A., Sepulveda, W., Lockwood, C. J. & Romero, R. Complications of fetal blood sampling. Am. J. Obstet. Gynecol. 168, 1339–1344 (1993).
Lam, Y. H., Ghosh, A., Tang, M. H., Lee, C. P. & Sin, S. Y. Early ultrasound prediction of pregnancies affected by homozygous alpha-thalassaemia-1. Prenat. Diagn. 17, 327–332 (1997).
Tongsong, T., Wanapirak, C., Sirichotiyakul, S., Piyamongkol, W. & Chanprapaph, P. Fetal sonographic cardiothoracic ratio at midpregnancy as a predictor of Hb Bart disease. J. Ultrasound Med. 18, 807–811 (1999).
Leung, K. Y. et al. Ultrasonographic prediction of homozygous alpha0-thalassemia using placental thickness, fetal cardiothoracic ratio and middle cerebral artery Doppler: Alone or in combination?. Ultrasound Obstet. Gynecol. 35, 149–154 (2010).
Tongsong, T., Wanapirak, C., Sirichotiyakul, S. & Chanprapaph, P. Sonographic markers of hemoglobin Bart disease at midpregnancy. J. Ultrasound Med. 23, 49–55 (2004).
Sirichotiyakul, S., Luewan, S., Srisupundit, K., Tongprasert, F. & Tongsong, T. Prenatal ultrasound evaluation of fetal Hb Bart’s disease among pregnancies at risk at 11 to 14 weeks of gestation. Prenat. Diagn. 34, 230–234 (2014).
Sompagdee, N., Anuwutnavin, S., Burapasikarin, C., Ruangvutilert, P. & Thongkloung, P. Nomograms of fetal cardiothoracic ratio from 17 to 37 weeks’ gestation as assessed by three different measurement techniques and their correlation with gestational age. Prenat. Diagn. 41, 1658–1667 (2021).
Gembruch, U., Shi, C. & Smrcek, J. M. Biometry of the fetal heart between 10 and 17 weeks of gestation. Fetal Diagn. Ther. 15, 20–31 (2000).
Sirilert, S., Tongprasert, F., Srisupundit, K., Tongsong, T. & Luewan, S. Z score reference ranges of fetal cardiothoracic diameter ratio. J. Ultrasound Med. 38, 999–1007 (2019).
Leung, K. Y. et al. A new strategy for prenatal diagnosis of homozygous alpha(0)-thalassemia. Ultrasound Obstet. Gynecol. 28, 173–177 (2006).
Wanapirak, C. et al. Appearance of abnormal cardiothoracic ratio of fetuses with hemoglobin Bart’s disease: Life table analysis. Ultraschall Med. 38, 544–548 (2017).
Society for Maternal-Fetal Medicine et al. Society for maternal-fetal medicine (SMFM) clinical guideline #8: The fetus at risk for anemia–diagnosis and management. Am. J. Obstet. Gynecol. 212, 697–710 (2015).
Tongsong, T., Wanapirak, C., Sirichotiyakul, S., Tongprasert, F. & Srisupundit, K. Middle cerebral artery peak systolic velocity of healthy fetuses in the first half of pregnancy. J. Ultrasound Med. 26, 1013–1017 (2007).
Tongsong, T., Wanapirak, C. & Sirichotiyakul, S. Placental thickness at mid-pregnancy as a predictor of Hb Bart’s disease. Prenat. Diagn. 19, 1027–1030 (1999).
Ghosh, A., Tang, M. H., Lam, Y. H., Fung, E. & Chan, V. Ultrasound measurement of placental thickness to detect pregnancies affected by homozygous alpha-thalassaemia-1. Lancet 344, 988–989 (1994).
Kontomanolis, E. N. & Fasoulakis, Z. Hydrops fetalis and THE parvovirus B-19. Curr. Pediatr. Rev. 14, 239–252 (2018).
Chankhunaphas, W. et al. Comparison of the performances of middle cerebral artery peak systolic velocity and cardiothoracic diameter ratio in predicting fetal anemia: Using fetal hemoglobin Bart’s disease as a study model. Fetal Diagn. Ther. 48, 738–745 (2021).
Harn, A. M. P. et al. Effectiveness of ultrasound algorithm in prenatal diagnosis of hemoglobin Bart’s disease among pregnancies at risk. Int. J. Gynaecol. Obstet. 159, 451–456 (2022).
Srisupundit, K., Piyamongkol, W. & Tongsong, T. Identification of fetuses with hemoglobin Bart’s disease using middle cerebral artery peak systolic velocity. Ultrasound Obstet. Gynecol. 33, 694–697 (2009).
Tongprasert, F. et al. The best cutoff value of middle cerebral artery peak systolic velocity for the diagnosis of fetal homozygous alpha thalassemia-1 disease. Prenat. Diagn. 39, 232–237 (2019).
Luewan, S., Tongprasert, F., Piyamongkol, W., Wanapirak, C. & Tongsong, T. Fetal liver length measurement at mid-pregnancy among fetuses at risk as a predictor of hemoglobin Bart’s disease. J. Perinatol. 31, 157–160 (2011).
Mari, G. et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization: Collaborative group for doppler assessment of the blood velocity in anemic fetuses. N. Engl. J. Med. 342, 9–14 (2000).
Srisupundit, K., Piyamongkol, W. & Tongsong, T. Comparison of red blood cell hematology among normal, alpha-thalassemia-1 trait, and hemoglobin Bart’s fetuses at mid-pregnancy. Am. J. Hematol. 83, 908–910 (2008).
Lam, Y. H., Tang, M. H., Lee, C. P. & Tse, H. Y. Echogenic bowel in fetuses with homozygous alpha-thalassemia-1 in the first and second trimesters. Ultrasound Obstet. Gynecol. 14, 180–182 (1999).
Acknowledgements
The authors gratefully acknowledge the women who generously agreed to participate in this study and Ms. Julaporn Pooliam for her statistical analyses.
Funding
The Siriraj Research Development Fund, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, supported this study (Grant Number RO16531043).
Author information
Authors and Affiliations
Contributions
SA: conceptualization, methodology, investigation, formal analysis, and writing review and editing. PR: methodology, investigation, formal analysis, and writing—original draft. NS: methodology, investigation, and writing—original draft. PR: methodology and supervision, writing review and editing. SV: resources and investigation. All coauthors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anuwutnavin, S., Rangseechamrat, P., Sompagdee, N. et al. Comparing three cardiothoracic ratio measurement techniques and creating multivariable scoring system to predict Bart’s hydrops fetalis at 17–22 weeks’ gestation. Sci Rep 14, 8894 (2024). https://doi.org/10.1038/s41598-024-59719-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59719-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.