Abstract
Despite that surgical resection is widely regarded as the most effective approach to the treatment of liver cancer, its safety and efficacy upon centrally located hepatocellular carcinoma (HCC) remain unsatisfactory. In consequence, seeking an integrated treatment, like combined with adjuvant radiotherapy, to enhance the prognosis of patients is of critical importance. By recruiting patients undergoing surgical resection for centrally located HCC ranging from June 2015 to 2020, they were divided into liver resection combined with adjuvant radiotherapy (LR + RT) and mere liver resection (LR) groups. The calculation of propensity score and model of Cox proportional hazards regression were utilized. 193 patients were recruited in aggregation, containing 88 ones undergoing LR + RT, while 105 handled with LR. RT was verified to be an independent factor of prognosis for relapse (HR 0.60). In propensity-score analyses, significant association existed between adjuvant radiotherapy and better disease-free survival (DFS) (Matched, HR 0.60; Adjustment of propensity score, HR 0.60; Inverse probability weighting, HR 0.63). The difference of DFS was apparent within two groups (p value = 0.022), and RT significantly down-regulated early relapse (p value < 0.05) in subgroup analysis. The calculation of E-value revealed robustness of unmeasured confounding. The combination of liver surgical resection with RT is safe and effective towards patients with centrally located HCC, which would notably enhance the prognosis and decrease the early relapse of HCC.
Similar content being viewed by others
Introduction
As the globally fourth-leading factor of cancer-associated death, hepatocellular carcinoma (HCC) takes up almost 90% of primary liver cancer (PLC)1, which is a fatal illness accompanied by severe morbidity, negative prognosis, along with a series of clinical complications2. HCC leads to 4.7% of newly-confirmed malignant diseases and 8.3% of tumor-related deaths globally3. The strategies towards HCC are diverse, ranging from radical resection, local ablation, transcatheter arterial chemoembolization (TACE), liver transplantation, and systemic therapies, among which resection is mostly utilized and commonly regarded as most effective4.
Centrally located HCC, which commonly exists in divergence of portal vein, junction of primary hepatic vein, inferior vena cava or less than 1 cm (cm) from posterior inferior vena cava trunk, mostly lied in Couinaud segment I, IV, V, VIII, or among convergence of core sections 5,6. Since centrally located HCC is neighbouring to dominant blood vessels and bile ducts, its clinical therapy is rather challenging and draws much attention globally, with the rate of relapse rising up to 90%, while the 5-year DFS only around 15–30%7. The probability of relapse would significantly escalate when margins of resection are narrower (< 1 cm) or even null, facilitating the diffusion of microscopic residual lesions via intrahepatic vessels8. Under this condition, it has been a critical and topical issue to conduct researches over combined therapies on the basis of surgical resection.
At present, surgical resection is considered as the core choice for resectable tumor mass of HCC patients, with adjuvant therapies performed based on pathological examinations postoperative. In preceding researches, the radiotherapy (RT) has been proved to be a safe and effective type of adjuvant therapy for centrally located HCC. Recently, it has been accessible for patients to receive accurate radiotherapy thanks to technical advances, like the appearance of intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT) and three-dimensional conformal radiotherapy (3D-CRT)9,10,11. In this way, we explored the effect of adjuvant radiotherapy accompanied by surgical resection in order to solve the problem above.
As far as I know, there was no real-world research analyzing possible prognostic benefits of adjuvant radiotherapy following surgical resection for centrally located HCC before, and this is the aim for the study to bridge the gap.
Materials and methods
Selection of patients
Data for patients who underwent resection for HCC ranging from June 2015 to 2020 were gathered. People were recruited based on the including and excluding standards below. The content of inclusion criteria were as follows: (1) no less than 18 years old; (2) without extrahepatic metastasis; (3) centrally located tumor adhered to or within < 1 cm from the portal vein, hepatic vein, primary hepatic branch of the biliary system or retrohepatic inferior vena cava verified via imaging before surgery or intraoperative macroscopic test; (4) integral clinical and pathological information; (5) Child‒Pugh class A; and (6) ECOG 0 or 1. The excluded criteria were presented below: (1) non-HCC confirmed by pathological examination postoperative; (2) radiotherapy before tumor resection. Recruitment of all patients was decided by a multidisciplinary team (MDT) containing surgeons, physicians, radiologists, pathologists, etc., who were all covered in the determination regarding the treatment of patients.
Treatment
Surgical treatment
We held a discussion in the form of MDT for every patient before surgery. The standardization of team. Initially, by performing exploratory laparotomy on the abdomen and pelvis, the condition of extrahepatic metastasis was examined, during which intraoperative ultrasound would be used to check the liver cancer if necessary. The region of resection was comprehensively decided by the general tumor and cirrhosis of liver. All patients underwent radical resection of HCC (R0 resection) to ensure that the postoperative pathological margin was tumor negative. During the operation, selective and dynamic region-specific vascular occlusion (SDRVO) technique was utilized to ascertain accurate liver resection individually5. Possible choices for HCC surgical resection contained anatomic hepatectomy and nonanatomical hepatectomy. The tumor was peeled off from the surface of biliary tract or large blood vessels via Cavitron Ultrasonic Surgical Aspirator (CUSA) without incision margins to refrain from cutting off primary vessels, when the tumor mass was adhered to critical tracts.
Adjuvant radiotherapy
Postoperative IMRT was applied to every patient in the LR + RT group, which was arranged and performed as depicted before11. In brief, tumor bed along with a 1 cm margin was considered as the clinical target volume (CTV). However, it was decided with a 1.5 cm margin surrounding the tumor bed when the tumor mass located next to main vessels. In the left–right and anterior–posterior directions, a 0.5-cm margin around the CTV was contained in the planning target volume (PTV), while in the cranial-caudal direction, a 1.0-cm margin was included. According to the PTV, the dose of RT was prescribed. Mainly dependent on dose constraints of specific organs, the prescribed dose for 95% of the PTV was arranged as 50–60 Gy among 25–30 fractions across 5–6 weeks.
Follow-up
Recurrence was defined in this way: fast in and out features revealed in the imaging of hepatocellular nodular (≥ 2 cm) or HCC verified via histological/cytological examinations. AFP levels, liver and kidney function examinations, regular blood check, abdominal magnetic resonance imaging (MRI), computed tomography (CT) scans and chest X-ray were routinely checked. Since operation, patients were rechecked each 3 months in the first 2 years, each 4–6 months before 5 years, followed by each 6–12 months since that. Generally, subjects would be evaluated if they undergo physical discomfort among follow-up, which was continued up to May 2022 for all of the subjects. This research got approval from the Ethics Committee of Cancer Hospital of Chinese Academy of Medical Science. Without interrupting process of diagnosis and therapy, this was a nonintervention cohort study, whose results would be published via statistical data of analysis and exclude information that could identify patients. On the basis of the Helsinki Declaration, we did not reveal related data of patients, and all participants were willing to participate in this study and gave their informed consent.
Treatment of recurrence
According to features of the tumor, liver function, overall status and decision from the patient, as well as suggestions from MDT, strategies including reoperation, hepatectomy, radiofrequency ablation (RFA), TACE, systemic therapy like immunotherapy or molecular targeted therapy were employed to deal with HCC relapse.
Definition and analysis
The period from the data of operation to the time of relapse was referred to as disease-free survival (DFS). Aside from RT, we selected variables with possible influences on survival rate to calculate a propensity score12. We utilized the Clavien grading system to assess complications among hospitalizations, which were as follows: Grade I: intervention by drugs, endoscopy, radiotherapy and operation was not needed since operation, but physiotherapy, diuretics, electrolytes, antiemetics, antipyretics and antipyretics were permitted; Grade II: medicine except ones included in Grade I were required, containing parenteral nutrition and blood transfusion; Grade III: intervention by endoscopy, radiotherapy or operation was in need; Grade IV: life-threatening syndromes, like central nervous system (CNS) complications (cerebral hemorrhage and subarachnoid hemorrhage), and entry into the ICU were in requirement; and then Grade V: dead among hospitalization. We regarded occurrence of grade I or II syndromes to be mild level, while grade III, IV, and V to be severe. Clinical target volume (CTV) is the range of tumor focus and its possible infiltration; Planning target volume (PTV) include the CTV and the error range caused by positioning or movement, posture repeatability, and target volume movement.
Statistical methods
Analysis was performed by steps below: (1) compare baseline data of two groups via standardized mean difference (SMD); (2) priori verification of confounders which might confound results (based on relations of confounders with results of interest or modifications in effect estimation of over 10%); (3) utilize Cox proportional-hazards regression models to evaluate correlation among exposed elements and prognosis, which includes crude and multi-variable calculation; (4) employment of three matching methods, covering Propensity-score Match (PSM), Covariate adjustment for Propensity Score (CAPS) and Inverse Probability Weighting (IPTW) to reduce the difference among groups and control confounding; (5) apply Kaplan‒Meier method to evaluate DFS, and log-rank test to calculate variance between groups; (6) research clinical benefits of radiotherapy upon early stage of relapse via subgroup analysis; (7) explore the potential of unsurveyed confounding among cohorts by counting E-value13. E-values quantify necessary magnitude of unsurveyed confounders which might deny calculated correlation between RT and DFS.
The 1:1 matching algorithm was utilized to produce matched cohorts between groups with a caliper of 0.02 set towards the scale of propensity score. And there was no replacement for 1:1 sampling. We compared all characteristics of patients which were contained in producing and distributing propensity scores via standardized mean difference (SMD), before and after matching propensity scores. We considered the threshold no more than 0.1 to be acceptable14.
Descriptive analyses were used to describe the demographic and clinical characteristics of participants. Categorical variables were described by frequency and percentage. Continuous variables that follow a normal distribution are described by the mean and standard deviation, while continuous variables that do not follow a normal distribution are described by the median and interquartile ranges. The analysis of statistics was conducted by R software, version 4.2.0 (http://www.R-project.org) and IBM SPSS Statistics 23.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Cancer Hospital of Chinese Academy of Medical Science. All methods were carried out according to the Helsinki Declaration. The relevant data for the patients remained confidential, and all participants were willing to participate in this study and gave their informed consent.
Results
Patients
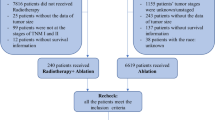
We recruited 211 patients in total based on the inclusion criteria; and 18 of them were excluded due to the exclusion criteria. Then, 193 patients were finally chose in all. Based on whether RT was employed, we allocated the patients into two groups, surgical resection along with adjuvant radiotherapy (LR + RT, 88 patients) or mere liver resection (LR, 105 patients) groups. The rates for 1-, 3-, and 5-year DFS were 98%, 85% and 74% for patients in LR + RT cohort, while 76%, 55%, and 44% respectively in the LR group. The flow chat for screening of patients is given in Fig. 1. In Table 1, we shown baseline demographics and clinicopathological characteristics of patients. Before matching, the variance between groups was notable (SMD > 0.1) according to the standardized mean difference.
Cox regression
As it is revealed through the forest plot in Fig. 2 via crude analysis, significant correlation was existent between LR + RT and enhanced DFS (HR 0.67, 95% CI [0.45, 0.98], Table 2, Fig. 2). Afterwards, confounding elements were filtered out based on the strategies above, containing ALT, MVI, number and size of tumor. We covered the aforementioned confounding elements in the multiple regression equation for adjustment. And RT was disclosed to be an independent factor of prognosis for centrally located HCC via multivariate Cox analysis (HR 0.60, 95% CI [0.40, 0.88], Table 2, Fig. 2).
Propensity score analyses
Initially, we utilized propensity-score matching (PSM) method to decrease the discrepancy among groups due to imbalanced baseline information. We covered baseline information and confounders, including sex (Male or Female), age (≤ 60 or > 60), smoking (Yes or NO), alcohol consumption (Yes or NO), ALT value, AST value, TBIL value, HBV (Yes or NO), HCV (Yes or NO), tumor number (Single or Multiple), tumor size, and MVI (Yes or NO) as matching factors. 70 patients in total were successfully matched. As revealed in Table 1, there was no significant difference (SMD < 0.1) in baseline characteristics of patients among groups after matching. However, the association between LR + RT and prolonged DFS was significant after PSM (HR 0.60, 95% CI [0.38, 0.49], Table 2). Then, covariate adjustment applying propensity score (CAPS)15 was utilized to regulate confounding. After CAPS, the association between LR + RT and prolonged DFS was notable (HR 0.60, 95% CI [0.38, 0.49], Table 2). At last, IPTW was adopted to count the stabilized inverse-probability weight by analyzing predicted probabilities by previous propensity-score model16. After IPTW, we revealed the existence of significant correlation between LR + RT and extended DFS (HR 0.63, 95% CI [0.40, 0.98], Table 2). Therefore, through statistical method, notably prolonged DFS in the LR + RT group was statistically verified compared with the LR group on the basis of real-world data.
Survival analysis
Before matching analysis, the Kaplan‒Meier curve was given in Fig. 3 with significance between groups (p value = 0.034). Afterwards, by matching 70 patients at 1:1, there was no apparent difference on baseline data. And Fig. 4 presented the Kaplan‒Meier curves of DFS after matching analysis, in which DFS was enhanced and remarkably distinct in the LR + RT group compared with that of the LR group (p value = 0.022).
Recurrence pattern and subgroup analysis
After matching analysis, we encompassed 140 patients in all in the research; 77 of them developed relapse post-operation, involving 33 in the LR + RT group, while 44 in the LR group respectively. Moreover, the occurrence of intrahepatic relapse and extrahepatic metastasis was separately 31 and 2 for LR + RT group, while 41 and 3 for LR group. As the primary determinant of prognosis post hepatectomy, high rate of relapse would be separated into early and late stage of recurrence17,18, among which the clinical prognosis of patients experiencing early relapse has been widely reported to be worse than those with late stage of relapse in large quantities of researches19,20,21. Currently, 12 or 24 months are widely regarded to be proper time points for early stage of relapse by most scholars17,22,23,24. In this way, we performed subgroup calculation to further explore the influence of RT upon early stage relapse, disclosing that RT would decrease the occurrence of relapse whatever the time point for early relapse was set up at 12 or 24 months (p value = 0.001, p value = 0.002, respectively. Table 3).
Sensitivity analysis
An E-value was produced to evaluate the sensitivity of unsurveyed confounding. The initial results were robust unless there existed unsurveyed confounder, whose HR was higher than 2.20. And in this study, the analysis of E-value indicated robustness to unsurveyed confounder.
Complications and safety
When it comes to safety, all of 70 patients across both groups succeeded in undergoing operation and and all underwent R0 resection. There were no significant differences upon intraoperative bleeding and duration of operation in two cohorts (p value = 0.614, p value = 0.125, separately). Moreover, people across two groups acquired proper operative strategies without occurrence of perioperative death. Mild and severe complications after matching analysis were not significantly variant (66 mild complications and 4 severe complications in both groups, p value > 0.05).
Discussion
In the conventional concept of centrally located HCC, it is regarded as the tumor mass located in IV, V and VIII Couinaud segments of the liver25. Since this concept fails to emphasize the association between tumor and peripheral structures, including primary bile duct and blood vessel, it is of poor guidance for the surgery. We put forward a revised version of definition, in which central located HCC is referred to liver tumor adhered to or within 1 cm from the hepatic vein, portal vein, primary hepatic branches of biliary system or retrohepatic inferior vena cava verified by imaging before surgery, macroscopic examination intraoperative, or pathological results post operation, commonly lied in Couinaud segment I, IV, V, and VIII, or located at the convergence of core sections6,26. Owing to its extreme proximity from the vein, the centrally located HCC is characterized by demanding surgery, poor rates of radical resection, diverse postoperative complications and high rates of relapse. Generally, understanding and exploration towards its therapeutic strategies symbolize the gradual progress of clinical medicine treatment towards liver cancer. It is rather challenging to figure out proper methods to safely get rid of centrally located HCC and upregulate OS of HCC patients post operation. To solve this problem, our surgical group has carried on long-term relevant researches upon combined therapy towards centrally located HCC on the basis of resection9,11,26,27,28.
This is the first real-world research analyzing promising advantages of liver resection integrated with adjuvant radiotherapy towards centrally located HCC, whose consequences reveal that comprehensive therapy possesses remarkable effects and notably upgrades clinical prognosis.
As a critical type of therapeutic choice, radiotherapy has been broadly utilized upon clinical remedy for various types of malignant tumor. Since the rapid technical advances of accurate radiotherapy which enable the arrival of high-dose radiation rays to the targeting region, radioactive injuries towards the surrounding normal liver tissues and other organs are effectively cut down. According to the demonstration from other related researches, clinical prognosis of HCC patients might be enhanced post radiotherapy. And corresponding to our phase II clinical trial and other research studies9,10,11,26,29,30, adjuvant radiotherapy is efficient, well tolerated, and potential for patients with centrally located HCC. Compared with previous researches, this research is superior from the following aspects. Initially, we made sure that all of patients that we recruited had access to clinical treatment and management of high quality from the same team of doctors in the hospital. Second, the statistical means we employed to cut down selection bias of patients added to the authenticity and reliability of our results. Moreover, the comparably long time of follow-up facilitated the reflection of long-term prognosis and functioned better upon clinical guidance.
Based on the consensus of guideline, a conserved minimized surrogate threshold effect of HR ≤ 0.6 towards DFS would be capable of suggesting notable rising upon overall survival (OS)31. And since our outcomes met this standard (Table 2), it is reasonable to assume the capacity of radiotherapy to prolong OS of patients with centrally located HCC post operation. In multivariate analysis, RT was confirmed to be an independent prognostic factor, while multiple liver tumor and MVI were confirmed to be independent risk factors. Most studies believe that MVI is a high-risk factor for intrahepatic metastasis and postoperative recurrence20,22, which will eventually lead to the occurrence of multiple tumors in the liver, which is consistent with our results. In addition, research has shown that AFP value is a risk factor for the OS or DFS of patients with HCC after surgery32. For these high-risk patients, in addition to postoperative RT, a previous study showed that liver transplantation is also one of the effective treatment methods32. Clinically, the appropriate treatment should be selected according to the actual situation of patients.
In terms of the extended survival time, there exists several possible reasons. To begin with, RT decreases the potential harm caused by narrow margin resection, which has been verified that, resection with wide-margin results in prolonged OS compared with that of narrow-margin ones (< 1 cm)33,34. Furthermore, it is interesting to note that RT effectively decrease the rate of relapse in the early stage, which might partly due to its destruction towards minimal residual disease (MRD)35,36. Decline of relapse rate facilitates the enhanced OS. Nevertheless, limitations still exist in this research, for which our study was still a retrospective research, and further randomized controlled studies are still in need to verify our consequences.
Conclusion
Combination of surgical resection with adjuvant radiotherapy is safe and effective for remedy of patients with centrally located HCC, which would clearly enhance clinical outcomes and decline the occurrence of relapse in the early stage.
Data availability
All data related to this study are included in this paper. Details are available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- RT:
-
Adjuvant radiotherapy
- LR:
-
Liver resection
- SMD:
-
Standardized mean difference
- PLC:
-
Primary liver cancer
- TACE:
-
Transcatheter arterial chemoembolization
- RT:
-
Radiotherapy
- SDRVO:
-
Selective and dynamic region-specific vascular occlusion
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- PSM:
-
Propensity-score match
- CAPS:
-
Covariate adjustment for Propensity Score
- IPTW:
-
Inverse probability weighting
References
Nagtegaal, I. D. et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 76(2), 182–188 (2020).
Zhou, J. et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 7(3), 235–260 (2018).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer J. Clin. 71(3), 209–249 (2021).
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67(1), 358–380 (2018).
Yu, W. et al. R1 hepatectomy with exposure of tumor surface for centrally located hepatocellular carcinoma. World J. Surg. 38(7), 1777–1785 (2014).
Wu, F. et al. Phase 2 evaluation of neoadjuvant intensity-modulated radiotherapy in centrally located hepatocellular carcinoma: A nonrandomized controlled trial. JAMA Surg. 157(12), 1089–1096 (2022).
Cheng, C. H. et al. Surgical resection of centrally located large hepatocellular carcinoma. Chang Gung Med. J. 35(2), 178–191 (2012).
Shi, M. et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial. Ann. Surg. 245(1), 36–43 (2007).
Rong, W. et al. Adjuvant radiotherapy in central hepatocellular carcinoma after narrow-margin hepatectomy: A 10-year real-world evidence. Chin. J. Cancer Res. 32(5), 645–653 (2020).
Wang, W. H. et al. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int. 35(12), 2603–2610 (2015).
Chen, B. et al. Phase 2 study of adjuvant radiotherapy following narrow-margin hepatectomy in patients with HCC. Hepatology 74(5), 2595–2604 (2021).
Brookhart, M. A. et al. Variable selection for propensity score models. Am. J. Epidemiol. 163(12), 1149–1156 (2006).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321(6), 602–603 (2019).
Reitz, K. M. et al. Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg. 155(6), e200416 (2020).
Kurth, T. et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am. J. Epidemiol. 163(3), 262–270 (2006).
Robins, J. M., Hernán, M. A. & Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 11(5), 550–560 (2000).
Jung, S. M. et al. Characteristics of early recurrence after curative liver resection for solitary hepatocellular carcinoma. J. Gastrointest. Surg. 23(2), 304–311 (2019).
Portolani, N. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann. Surg. 243(2), 229–235 (2006).
Yamamoto, Y. et al. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J. Gastroenterol. 21(4), 1207–1215 (2015).
Chan, A. et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 69(6), 1284–1293 (2018).
Xing, H. et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 22(5), 677-689 (2020).
Xu, X. F. et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: A multicenter study from China. JAMA Surg. 154(3), 209–217 (2019).
Poon, R. T. et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 89(3), 500–507 (2000).
Singal, A. G. et al. Direct-acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology 156(6), 1683–1692 (2019).
Wu, C. C. et al. Mesohepatectomy for centrally located hepatocellular carcinoma: An appraisal of a rare procedure. J. Am. Coll. Surg. 188(5), 508–515 (1999).
Yu, W. et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (< 1 cm): A prospective randomized study. J. Am. Coll. Surg. 218(3), 381–392 (2014).
Wu, Y. L. et al. Long-term outcome of centrally located hepatocellular carcinomas treated by radical resection combined with intraoperative electron radiotherapy (IOERT). Front. Oncol. 12, 773301 (2022).
Rong, W. et al. Serum metabolic effects of corn oligopeptides with 7-day supplementation on early post-surgery primary liver cancer patients: A double-blind randomized controlled trial. Hepatobiliary Surg. Nutr. 11(6), 834–847 (2022).
Wang, L. et al. Postoperative adjuvant treatment strategy for hepatocellular carcinoma with microvascular invasion: A non-randomized interventional clinical study. BMC Cancer 20(1), 614 (2020).
Wang, L. et al. Optimal postoperative adjuvant treatment strategy for HBV-related hepatocellular carcinoma with microvascular invasion: A propensity score analysis. OncoTargets Ther. 12, 1237–1247 (2019).
Llovet, J. M. & Lencioni, R. mRECIST for HCC: Performance and novel refinements. J. Hepatol. 72(2), 288–306 (2020).
Mazzotta, A. D. et al. Number of hepatocellular carcinoma nodules in patients listed for liver transplantation within alpha-fetoprotein score: a new prognostic risk factor. Transpl. Int. 34(5), 954-963 (2021).
Aoki, T. et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br. J. Surg. 107(1), 113–120 (2020).
Zhong, F. P., Zhang, Y. J., Liu, Y. & Zou, S. B. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 96(37), e8043 (2017).
Luskin, M. R., Murakami, M. A., Manalis, S. R. & Weinstock, D. M. Targeting minimal residual disease: A path to cure. Nat. Rev. Cancer. 18(4), 255–263 (2018).
Garcia-Murillas, I. et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 7(302), 302ra133 (2015).
Funding
This study was supported by Beijing Hope Run Special Fund of Cancer Foundation of China (LC2020L05).
Author information
Authors and Affiliations
Contributions
WJ and RW conceived of and designed the study. TC, HN, LY and WH gathered data, analyzed the data, wrote the first manuscript draft, and provided the literature search. WJ, WF, WL, WZ, ZK and CB verified the data and revised the manuscript. All authors were involved in patient enrollment and care during the study. All authors participated in its writing and approved for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors who contributed to the study agree to its publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tao, C., Hu, N., Liu, Y. et al. Long-term outcome of adjuvant radiotherapy upon postoperative relapse of centrally located hepatocellular carcinoma: a real-world study. Sci Rep 14, 8506 (2024). https://doi.org/10.1038/s41598-024-59180-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59180-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.