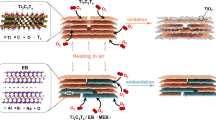
Abstract
In this study, we deposited Ti3C2Tx-modified, rare-earth-doped PbO2 on the surface of a carbon fabric via electrodeposition. The surface morphology and electronic structure of the electrode were characterized with SEM, XRD and XPS. The layered Ti3C2Tx did not change the structure of β-PbO2, and at the same time, it improved the crystallinity of the material and reduced the grains of PbO2. Electrochemical experiments showed that the addition of Ti3C2Tx increased the electrochemical activity of the electrode and produced more H2O2, which contributed to the degradation of pollutants. The efficiency of sulfamethoxazole (SMX) degradation reached 95% after 120 min at pH 3 with a current density of 50 mA/cm2. Moreover, the electrode has good cycling performance, and the degradation efficiency was still 80% after 120 min after 10 cycles of recycling. Based on the intermediates identified by HPLC‒MS, a mechanism for SMX degradation was proposed. Our results will provide a new idea for the development of efficient electrocatalytic degradation of antibiotics.
Similar content being viewed by others
Introduction
Antibiotics are primarily used to treat diseases associated with infections in humans and animals1,2,3,4. Current treatment methods do not remove antibiotics effectively and pose significant risks to aquatic ecosystems and human health. Sulfonamides are common antibiotics used in aquatic environments5. However, the conventional activated sludge method does not degrade sulfonamide wastewater sufficiently, and the effluent quality generally fails to meet water quality standards6. Therefore, a water treatment technology that removes trace amounts of sulfamethoxazole (SMX) from wastewater is needed.
Electrochemical oxidation is a simple and effective method for the treatment of various organic pollutants in liquid media7,8. Rare earth doped PbO2 is a promising cathode material for electrochemical oxidations of organic pollutants. Doping with rare earth elements enabled Fenton-like reactions with H2O2 and Ce4+/Ce3+9,10 or Eu3+/Eu2+11. The rare earth elements increased the mass transfer rate of O2, such as Ce, which generally exists in the form of CeO2 particles. After the conversion of Ce4+ to Ce3+, the electrode generated oxygen vacancies and adsorbed O2 to generate H2O2. However, rare earth-modified PbO2 electrodes are generally hydrophobic and unstable12. In addition, hydrophobic electrodes have weak electron transfer capabilities for O2 reduction, which affect the efficiencies of H2O2 generation. This all affects the activities of rare-earth doped PbO2 electrodes, and further modification is needed for improved electrochemical activity.
Ti3C2Tx, a new 2D material, was first discovered in 201113. Compared with other two-dimensional materials, Ti3C2Tx has been widely used in Electrocatalytic applications due to its thin atomic layer, high electrical conductivity, high hydrophilicity, many active sites, and good mechanical properties.14,15,16,17,18,19. Ti3C2Tx increases the efficiencies of oxidation reactions and the electrochemical oxidation activity, which is essential for the production of H2O220,21. Additionally, Ti3C2Tx increases the efficiency of H2O2 decomposition: (1) Ti3+ and Ti2+ lose electrons to form Ti4+ and produce oxidized material22; (2) Most of the functional groups are hydrophilic, which facilitates the activation of H2O2. Doping with Ti3C2Tx increases the conductivity of the electrode, thus reducing energy consumption20. In addition, the surface functional groups of 2D Ti3C2Tx adsorb organic pollutants through electrostatic interactions, hydrogen bonding, surface complexation, and π-π interactions23,24. This increases the rate of antibiotic diffusion. Therefore, Ti3C2Tx is an ideal material for electrode modification.
Therefore, we propose an innovative approach to fabricate an electrode material with a composite-structured electrode by compositing Ti3C2Tx with a rare-earth-modified PbO2 electrode. Based on an earlier report, we believe that by introducing Ti3C2Tx, the ability of the PbO2 electrode to generate H2O2 can be effectively enhanced, thereby improving the electrode's activity for electrocatalytic degradation of antibiotics. In this study, we used carbon fabric (CF) as the substrate. The Eu-doped PbO2-CeO2-Ti3C2@CF anode material was prepared by electrochemical deposition. Ti3C2 was introduced into an electrochemical conservation system. The results showed that the concentration of hydrogen peroxide generated with the Ti3C2 anode was three times that of the anode without Ti3C2. Under certain conditions, the efficiency of sulfamethoxazole degradation reached 91% in 60 min, and the anode was recycled at least ten times.
Methods
Chemicals and materials
MAX (Ti3AlC2) powder (90%) was purchased from Xfnano Materials Tech Co., Ltd.; hydrofluoric acid (HF, 40%) and methanol (MeOH, 99.7%) was purchased from Sinopharm Chemical Reagent Co., Ltd.; dimethyl sulfoxide (DMSO, 99.7%), Pb(NO)3 (99%), and HNO3 (65–68%) were purchased from Lingfeng Chemical Reagent Co.; Eu(NO)3 (99.99%), CeO2 (99.95%), SMX (98%) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.; and CF was purchased from Suzhou Zhengtairong New Material Co., Ltd.
Preparation of Ti3C2Tx
Multilayered Ti3C2 was prepared by HF etching25. Then, 200 mg of Ti3C2 was added to 20 mL of DMSO and stirred for 24 h at room temperature. Ti3C2 was separated by centrifugation and dried under vacuum at 90 °C for 24 h to obtain DMSO-intercalated Ti3C2. After drying, 50 mL of deionized water was added and sonicated for 2 h to obtain an aqueous Ti3C2Tx suspension.
Preparation of Eu-doped PbO2-CeO2-Ti3C2@CF
The CF was cut into 2 × 2 cm pieces and cleaned by ultrasonication with nitric acid, ethanol and deionized water in turn for 15 min. First, 0.02 mol of Pb(NO3)3, 0.04 mol of Eu(NO3)3, 0.01 mol of HNO3 and 400 mg of CeO2 were added to 50 ml of the previously prepared Ti3C2Tx suspension, and deionized water was added to raise the total volume of the electrolyte 100 ml. The electrolyte was ultrasonically mixed for 30 min and then electrodeposited at a current intensity of 10 mA/cm2 for 40 min to prepare Eu-doped PbO2-CeO2-Ti3C2@CF. Different electrode materials were prepared by changing the amounts of Pb(NO3)3, Eu(NO3)3, CeO2 and Ti3C2Tx suspensions.
Characterization
The surface structures of the electrodes were characterized with a Hitachi SU-70 scanning electron microscope (SEM), and the elemental distribution of the electrodes was characterized with a Bruker super-X energy dispersive X-ray spectrometer (EDS). X-ray diffraction (XRD) was performed with a Rigaku SmartLab SE diffractometer to analyze the crystal structures of the electrodes. X-ray photoelectron spectroscopy (XPS) was performed with a Thermo Scientific K-Alpha instrument and monochromatic Al Kα radiation to analyze the elemental composition, chemical state and molecular structure of the electrode surface.
Cyclic voltammetry (CV) and linear scanning voltammetry (LSV) were performed with an electrochemical workstation and a three-electrode system (CHI 760E, Shanghai Chenhua Instruments Co., Ltd., China). Eu-PbO2-CeO2@CF or Eu-PbO2-CeO2-Ti3C2@CF were used as the working electrodes, Pt sheets as the counter electrodes, and saturated mercuric glycol electrodes as the reference electrodes. The electrode was tested in a 0.05 M sodium sulfate solution at pH 3. CV was performed in the voltage range − 0.8 to 1 V with a sweep rate of 10 mV/s. LSV was performed in the voltage range − 1.2 to 0 V with a sweep rate of 10 mV/s.
Electrolysis
Electrolysis was conducted with a galvanostat to investigate the electrochemical performance of Eu-PbO2-CeO2-Ti3C2@CF. The antibiotics were electrolyzed in a 100 mL electrolytic cell with 0.05 M Na2SO4, the electrode prepared above served as the cathode and a platinum sheet as the anode, and the solution was electrolyzed in a 100 mL electrolytic cell with 0.05 Na2SO4 used as the electrolyte, the prepared electrode was used as the cathode, and the Pt sheet as the anode. A magnetic stirrer was used to stir the solution during the electrolysis. The effects of different experimental conditions (initial concentration, pH, and current intensity) on the degradation of SMX were explored. After every 20 min, a 3 ml water sample was taken, the SMX solution was scanned with an ultraviolet and visible (UV‒Vis) spectrophotometer (Thermo Fisher Evolution 201) over the wavelength range 200–450 nm, and the concentration of SMX was determined from the peak at 262 nm. The concentration of H2O2 was analyzed by the potassium oxalate titanium method. Analyzing TOC in electrolytes by Shimadzu TOV-V CPH. Pb ion concentrations were measured by inductively coupled plasma (ICP, Agilent 720ES).
High-performance liquid chromatography‒mass spectrometry (HPLC‒MS)
Under the optimal reaction conditions, the solutions reacted for different periods were taken to determine the intermediate products. The method used an Agilent 1290-6465Q-TOF HPLC‒MS system for the analyses. A C18 chromatographic column was used with a column temperature of 40 °C, a mobile phase of 40% acetonitrile and 60% pure water, including 1% HPLC grade acetic acid, a flow rate of 1 mL/min and a detection wavelength of 269 nm with an injection volume of 20 μL, and a full-scan acquisition. The mass spectra of the compounds were acquired in positive ion mode for m/z between 100 and 400.
Results and discussion
The surface morphologies of the Eu-PbO2-CeO2@CF (Fig. 1a,b) and Eu-PbO2-CeO2-Ti3C2@CF (Fig. 1c,d) electrodes were characterized with SEM. The Eu-PbO2-CeO2@CF particles were mainly pyramidal with rough surfaces and obvious height differences. With the introduction of Ti3C2, the grain sizes decreased obviously, and the surface was smoother and contained some circular polygonal nanocrystals. In addition, there were sheet-like structures on the surface of the Eu-PbO2-CeO2-Ti3C2@CF electrode, which were speculated to be Ti3C2 nanosheets. Compared to PbO2 electrodes (Fig. S1a), this electrode with a complex surface morphology and small crystal sizes had a large electrochemically active surface area. The compositions of the Eu-PbO2-CeO2@CF (Table S1) and Eu-PbO2-CeO2-Ti3C2@CF (Fig. 1e) electrodes were characterized with EDS, and the Eu-PbO2-CeO2@CF electrode was composed of Eu, Pb, Ce, O and C, confirming the presence of Eu and CeO2. The EDS analysis of Eu-PbO2-CeO2-Ti3C2@CF showed that, in addition to the above 5 elements, Ti was also observed, and the content was 2.05 at.%, which also confirmed the presence of Ti3C2.
The structures of the CF, Eu-PbO2-CeO2-Ti3C2@CF and Eu-PbO2-CeO2-Ti3C2@CF electrode were explored with XRD (Fig. 2). As seen from the graphs, the high intensities and narrow shapes of the diffraction peaks for both electrodes indicated that the electrodes were highly crystalline, suggesting that the modification with Ti3C2 did not affect the cleanliness of the PbO2 substrate. Additionally, the XRD diffraction peaks were compared with those on standard PDF cards (α-PbO2 and β-PbO2). Most of the diffraction peaks for the two electrodes matched the diffraction peaks of β-PbO2, and a few matched the diffraction peaks of α-PbO2, indicating that the main component of the electrodes prepared by this method was β-PbO2. The UV–Vis spectra (Fig. S2) also confirmed that the introduction of Ti3C2 did not change the structure of the electrode. In addition, based on the XRD spectra (Fig. S1b), the proportion of β-PbO2 in the Eu-PbO2-CeO2@CF electrode was much larger than that in PbO2-CeO2@CF. α-PbO2 had a higher electrochemical activity and lower stability than β-PbO2. The grain sizes of both were calculated separately by Scheller's formula, and the grain size of added Ti3C2 was 10.5 nm, which was much smaller than the size (28.5 nm) of unadded Ti3C2. The previous SEM image (Fig. 1a) also showed that the surface structure of Eu-PbO2-CeO2@CF was less dense and easily peeled off during the electrochemical process. In contrast, after the addition of Ti3C2Tx, the basic structure was still β-PbO2, but the surface morphology was denser (Fig. 1c) with better stability and less likely to be peeled off.
The chemical and electronic states of Eu-PbO2-CeO2-Ti3C2@CF were analyzed by XPS (Fig. 3). During preparation of the Ti3C2 nanosheets, many functional groups were generated on the surface, such as OH–, O2–, and F–13. Their presence was verified by XPS (Fig. 3a). Figure 3b shows the C 1s spectrum of Eu-PbO2-CeO2-Ti3C2@CF. C-Ti bonds were present in the samples, which indicated that the Ti3C2 nanosheets retained their original properties after electrodeposition. The XPS spectrum of the Eu-PbO2-CeO2@CF electrode did not show the same peaks (Fig. S3a). Figure 3c shows the XPS spectrum of the O 1s core layer. The O 1s spectrum had three peaks at 528.8 eV, 531.9 eV and 532.3 eV associated with the Pb–O, surface O-C and O = C groups, respectively. The typical Pb 4f XPS peaks (Fig. 3d) at 137.6 eV for Eu-PbO2-CeO2-Ti3C2@CF were assigned to Pb4+. The peak at 138.5 eV is the formation of a small amount of PbCO3 on the surface as a result of the binding of PbO2 to CO2 in the air. Figure 3e,f shows the Eu 3d and Ce 3d XRD data. Ce 3d has a clear spin–orbit splitting peak and the Ce 3d5/2 peak at the rightmost 882.18 eV confirms the presence of CeO2 nanoparticles.
The H2O2 generated in the electrochemical oxidation system is critical for the degradation of SMX. Therefore, the concentration of H2O2 was studied spectrophotometrically with potassium titanyl oxalate (Fig. 4a). The concentration of H2O2 increased sharply in the first 20 min, reaching a peak at 40 min. The activity of the electrode in generating H2O2 increased with the addition of Ti3C2. The electrode prepared by adding 200 mg of Ti3C2 to the electrolyte produced three times more H2O2 than the electrode without Ti3C2 and 1.5 times more H2O2 than the electrode with 100 mg of Ti3C2. The CV and LSV curves of the Eu-PbO2-CeO2@CF (black) and Eu-PbO2-CeO2-Ti3C2@CF (red) electrodes were then generated separately. As shown in Fig. 4b, the redox peak current and peak area for Eu-PbO2-CeO2-Ti3C2@CF were significantly greater than those for Eu-PbO2-CeO2@CF. Figure 4c shows that the PbO2-CeO2-Ti3C2@CF cathode had a stronger current response when a strong reduction peak corresponding to the ORR process was observed, as the Ti3C2 material significantly improved the electron transfer and catalytic activity of the ORR. Thus, when assembled into an electrochemical oxidation system, the PbO2-CeO2-Ti3C2@CF cathodes enabled in situ generation of -OH and contaminant degradation.
To determine the optimal ratio of Pb(NO3)3, Eu(NO3)3 and CeO2, the efficiencies of SMX degradation at these electrodes were studied by varying the concentrations in the electrolyte and under established conditions (an initial SMX concentration of 30 mg/L, an initial pH of 3 and an applied current density of 50 mA/cm2) (Fig. S4). The electrolytic efficiencies of the prepared electrodes were highest when the concentrations of Pb(NO3)3, Eu(NO3)3 and CeO2 were 2 mol/L, 4 mol/L and 2 g/L, respectively. The rate of SMX degradation reached 85% after 2 h.
Based on these results, 2 g/L Ti3C2Tx was added to the electrolyte, and the efficiency of SMX degradation was investigated under the same conditions (Fig. 5a). For electrodes with different Pb and Eu ratios, the addition of Ti3C2Tx improved the rate of SMX degradation. For the electrode with a Pb: Eu ratio of 2:4, the degradation efficiency increased by 10% with the addition of Ti3C2Tx and reached 95% at 120 min. Then the TOC in the electrolyte was detected by a TOC analyzer, and it was 4.04 mg/L, with a removal rate of 71.6%. According to the relevant literature26,27,28,29, the concentration of SMX currently has been much smaller than the minimum inhibitory concentration. During degradation, the rate of the electrode reaction was calculated as:
where C0 (mg/L) and Ct (mg/L) are the SMT concentrations at time 0 and t min, respectively (Fig. 5b). All four electrodes fit the first order kinetic model. With the addition of Ti3C2Tx, the electrolytic (Pb:Eu = 2:4) degradation rate constantly increased from 0.0157 ± 0.00052 to 0.0240 ± 0.0011. Moreover, the degradation rate of the electrode without Ti3C2Tx was slower in the first 20 min compared to that of the electrode with Ti3C2Tx. This was mainly due to the reduction rate of O2 on the hydrophobic surface and the lower efficiency of H2O2 activity. The addition of Ti3C2Tx increased the hydrophilic functional groups and improved the degradation activity. The electrode degradation activity of Eu-PbO2-CeO2-Ti3C2@CF was compared with that of electrodes reported in the literature (Table 1). Eu-PbO2-CeO2-Ti3C2@CF showed excellent activity at higher initial concentrations of SMX and without additional ventilation.
To demonstrate the high electrocatalytic activity of the electrode, the effects of different operating parameters (including the initial concentration, current density and pH) on the electrochemical oxidation efficiency were investigated (Fig. 6).
Figure 6a shows the effect of initial SMX concentrations on SMX degradation efficiency. The experimental conditions included a current density of 50 mA/cm2, a pH of 3 and SMX concentrations of 10, 30 and 50 mg/L. As the concentration of the pollutant decreased, the degradation efficiency of SMX increased, but the amount of degradation decreased. This was mainly because at low concentrations, electrocatalytic oxidation was faster than diffusion, thus allowing effective degradation of the organic matter. As the concentration of the pollutant increased, so did the amount of organic matter produced during degradation, including pollutants and intermediate products. In addition, the electrode produced a limited number of hydroxyl radicals. As the pollutant concentration increased, the number of hydroxyl radicals acting per unit of pollutant decreased, which made the degradation of SMX less effective30. Therefore, an SMX concentration of 30 mg/L was chosen for subsequent experiments.
The current density is a key factor in the electrochemical oxidation process because it regulates the generation of hydroxyl radicals31. Figure 6b shows the rate of SMX removal for different applied current densities. As the current density was increased from 10 to 50 mA/cm2, the efficiency of SMX removal increased from 58 to 95%. When the current density was increased from 30 to 50 mA/cm2, the increase in the degradation efficiency was smaller than before. The higher current density may have enhanced the reaction of oxygen on the anode surface, thus competing with the oxidation of organic matter on the electrodes surface and affecting the removal efficiency32. In addition, the diffusion rate of contaminants to the electrode is limited at the same concentration, limiting the degradation rate at high currents and decreasing the current efficiency. Therefore, after careful consideration, the SMX degradations were performed at a current density of 50 mA/cm2.
Figure 6c shows the effect of different pHs on the efficiency of SMX degradation33. The results show that the fastest SMX degradation rate was achieved at pH 3. This is because hydroxyl radicals are more favorable for SMX degradation under acidic conditions34. In addition, CO2 was the main product from electrochemical degradation of SMX, and under alkaline conditions, CO2 dissolved in the solution and inhibited pollutant oxidation. Based on the above analysis, pH 3 was used as the initial pH for the electrochemical degradation of SMX.
The cycling performance of the electrodes was investigated. The SMX cycled for 120 min under the optimal conditions for SMX degradation. After each run, the degradation rate of SMX decreased slightly and remained at 80% after 10 cycles (Fig. 7). In addition, the electrodes after electrochemical degradation were characterized by XRD (Fig. S5), it can be found that the structure as well as the morphology did not change significantly. In addition, the content of Pb ions in the electrolyte was detected by ICP-MS, and the concentration of Pb ion released from the Eu-PbO2-CeO2-Ti3C2@CF electrode was 3.68 μg/L, which is less than the World Health Organization's requirements for lead ion concentrations in drinking water and China's permitted effluent discharge standards for Pb in surface waters. Therefore, the electrochemical degradation of this electrode is safe and reliable for the environment and human health. Therefore, the Eu-PbO2-CeO2-Ti3C2@CF electrode had good stability.
To further investigate the mechanism of SMX degradation, methanol was used as a scavenger (Fig. S6) to investigate the role of ·OH. It can be found that the degradation efficiency of SMX decreased significantly after the addition of methanol, indicating that ·OH plays a major role in the degradation process.
To further investigate the degradation mechanism, The intermediates produced during SMX degradation were characterized by HPLC–MS (Table S2). Possible degradation pathways have been proposed in conjunction with the literature (Fig. 8).
In pathway I, the degradation pathway was hydroxylation of the arene ring. The aniline portion was attacked by the hydroxyl radical, resulting in the disappearance of the amine group and the formation of 25535.
In pathway II, the amino group on the arene ring in SMX was attacked by a hydroxyl radical to form NO2-SMX (283)36. Subsequently, the S‒N bond of NO2-SMX was cleaved to give 155 and 9937. In addition, ·OH react with the isoxazole ring to form 28838. Subsequently, the C-N bond breaks and 158 and 132 are formed. Then, 158 lost a -NH2 to form 141. Ions 132 and 99 coupled with N-centered radicals to form 22739. The remaining 99 was stripped of a methyl group to form 8540.
In pathway 3, the isoxazole ring in SMX opened to form 256, followed by C-N bond breakage to form 17441. Subsequent coupling was centered around the N atom to form 34042. All intermediates eventually degrade to water, carbon dioxide and inorganic ions.
Conclusion
In summary, Ti3C2Tx-modified rare earth element-doped PbO2 electrodes were prepared via electrodeposition and were fully characterized by SEM, XRD, and XPS. The results showed that Ti3C2Tx was doped into the PbO2 electrode and optimized the surface morphology as well as the electronic structure of the electrode. The optimal degradation conditions for the electrochemical degradation of SMX by electrodes were also investigated. The electrodes showed good stability and were recycled and reused at least 10 times. In addition, a degradation pathway was proposed based on an analysis of the HPLC–MS intermediates. All these results indicate that Ti3C2Tx is an ideal electrochemical oxidation modification material, which can effectively improve the electrochemical activity of the electrode. This work provides a new idea for electrode modification, which has a broad and great application prospect in treating difficult-to-degrade medical wastewater.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Zhao, Z. et al. Investigation of the degradation and dehalogenation properties of florfenicol by heterogeneous Fenton reaction activated with MIL-53(Al)-supported nano zero-valent iron. Chem. Eng. J. 453, 139420 (2023).
Wu, D. et al. Two-dimensional manganese-iron bimetallic MOF-74 for electro-Fenton degradation of sulfamethoxazole. Chemosphere 327, 138514 (2023).
Xu, W., Zheng, X., Shangguan, Z., Qu, J. & Zhang, W. A low-cost magnetic catalyst (MnFe2O4) for ciprofloxacin degradation via periodate activation: The synergistic effect of Mn and Fe. Chem. Eng. J. 464, 142562 (2023).
Shen, J. et al. Visible light-mediated ring opening and cyclization of aryl cyclopropanes: Efficient synthesis of pyrrolo[1,2- a ]quinoxalin-4(5 H )-ones with antineoplastic activity. Org. Chem. Front. 11, 1758–1764 (2024).
García-Espinoza, J. D. & Nacheva, P. M. Degradation of pharmaceutical compounds in water by oxygenated electrochemical oxidation: Parametric optimization, kinetic studies and toxicity assessment. Sci. Total. Environ. 691, 417–429 (2019).
Song, Y. et al. Photodegradation of sulfonamides by g-C3N4 under visible light irradiation: Effectiveness, mechanism and pathways. Appl. Catal. B-environ. 210, 88–96 (2017).
Qi, H., Shi, X., Liu, Z., Yan, Z. & Sun, Z. In situ etched graphite felt modified with CuFe2O4/Cu2O/Cu catalyst derived from CuFe PBA for the efficient removal of sulfamethoxazole through a heterogeneous electro-Fenton process. Appl. Catal. B-environ. 331, 122722 (2023).
Miao, L. et al. Norfloxacin degradation in synthetic human urine using nickel converter slag-laterite heterogeneous Electro-Fenton process. J. Water Process. Eng. 53, 103723 (2023).
Song, Y., Wei, G. & Xiong, R. Structure and properties of PbO2–CeO2 anodes on stainless steel. Electrochim. Acta. 52, 7022–7027 (2007).
Yao, Y. et al. Preparation and characterization of PbO2 -CeO2 nanocomposite electrode with high cerium content and its appplication in the electrocatalytic degradation of malachite green. J. Electrochem. Soc. 162, H693–H698 (2015).
Zhang, Z. et al. Electrochemical oxidation of hydroquinone using Eu-doped PbO2 electrodes: Electrode characterization, influencing factors and degradation pathways. J. Electroanal. Chem. 895, 115493 (2021).
Lyu, J. et al. Enhancement of the electrocatalytic oxidation of dyeing wastewater (reactive brilliant blue KN-R) over the Ce-modified Ti-PbO2 electrode with surface hydrophobicity. J. Solid State Electr. 23, 847–859 (2019).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Zhu, J. et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 352, 306–327 (2017).
Ma, X. et al. Degradable Ti3C2Tx MXene nanosheets containing a lignin polyurethane photothermal foam (LPUF) for rapid crude oil cleanup. ACS Appl. Nano Mater. 5, 2848–2858 (2022).
Li, W. et al. Peroxymonosulfate activation by oxygen vacancies-enriched MXene nano-Co3O4 co-catalyst for efficient degradation of refractory organic matter: Efficiency, mechanism, and stability. J. Hazard. Mater. 432, 128719 (2022).
Li, Y. et al. 1T-MoS2 nanopatch/Ti3C2 MXene/TiO2 nanosheet hybrids for efficient photocatalytic hydrogen evolution. Mater. Chem. Front. 3, 2673–2680 (2019).
Kannan, K. et al. Two dimensional MAX supported copper oxide/nickel Oxide/MAX as an efficient and novel photocatalyst for hydrogen evolution. Int. J. Hydrogen Energ. 48, 7273–7283 (2023).
Chanda, D. et al. Effect of the interfacial electronic coupling of nickel-iron sulfide nanosheets with layer Ti3C2 MXenes as efficient bifunctional electrocatalysts for anion-exchange membrane water electrolysis. Appl. Catal. B-Environ. 321, 122039 (2023).
Ma, Y., Lv, X., Xiong, D., Zhao, X. & Zhang, Z. Catalytic degradation of ranitidine using novel magnetic Ti3C2-based MXene nanosheets modified with nanoscale zero-valent iron particles. Appl. Catal. B-Environ. 284, 119720 (2021).
Xia, Y. et al. Fabrication of novel FeS2 NWs/Ti3C2 cathode for Photo-Electro-Fenton degradation of sulfamethazine. Chem. Eng. J. 426, 130719 (2021).
Yang, P. et al. Singlet oxygen-dominated activation of peroxymonosulfate by CuO/MXene nanocomposites for efficient decontamination of carbamazepine under high salinity conditions: Performance and singlet oxygen evolution mechanism. Sep. Purif. Technol. 285, 120288 (2022).
Sun, X., Yang, J., Su, D., Wang, C. & Wang, G. Highly efficient adsorption of bilirubin by Ti3C2Tx MXene. Chem-Asian J. 16, 1949–1955 (2021).
Ghani, A. A. et al. Adsorption and electrochemical regeneration of intercalated Ti3C2Tx MXene for the removal of ciprofloxacin from wastewater. Chem. Eng. J. 421, 127780 (2021).
Zhu, D. et al. One-step synthesis of PdCu@Ti3C2 with high catalytic activity in the Suzuki-Miyaura coupling reaction. Nanoscale Adv. 4, 3362–3369 (2022).
Baskin, H., Dogan, Y., Bahar, I. H. & Yulug, N. Effect of subminimal inhibitory concentrations of gentamicin, penicillin, trimethoprim-sulfamethoxazole on adherence of uropathogenic Escherichia coli strains. J. Chemother. 14, 161–165 (2002).
Ho, M.-C. et al. Antimicrobial susceptibility, inimum inhibitory concentrations, and clinical profiles of stenotrophomonas maltophilia endophthalmitis. Microorganisms 9, 1840 (2021).
Voigt, M., Bartels, I., Nickisch-Hartfiel, A. & Jaeger, M. Determination of minimum inhibitory concentration and half maximal inhibitory concentration of antibiotics and their degradation products to assess the eco-toxicological potential. Toxicol Environ. Chem. 101, 315–338 (2019).
Ho, M.M.-C. et al. Antibiotic Susceptibility and minimum inhibitory concentration for stenotrophomonas maltophilia ocular infections. Antibiotics 11, 1457 (2022).
Dolatabadi, M. et al. Simultaneous electrochemical degradation of pesticides from the aqueous environment using Ti/SnO2–Sb2O3/PbO2/Bi electrode; process modeling and mechanism insight. Chemosphere 311, 137001 (2023).
Chen, J., Xia, Y. & Dai, Q. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochim. Acta. 165, 277–287 (2015).
Phan Quang, H. H. et al. Advanced electro-Fenton degradation of a mixture of pharmaceutical and steel industrial wastewater by pallet-activated-carbon using three-dimensional electrode reactor. Chemosphere 297, 134074 (2022).
Dolatabadi, M., Świergosz, T., Wang, C. & Ahmadzadeh, S. Accelerated degradation of groundwater-containing malathion using persulfate activated magnetic Fe3O4/graphene oxide nanocomposite for advanced water treatment. Arab. J. Chem. 16, 104424 (2023).
Rosales, E., Pazos, M. & Sanromán, M. A. Advances in the electro-Fenton process for remediation of recalcitrant organic compounds. Chem. Eng. Technol. 35, 609–617 (2012).
Hai, H., Xing, X., Li, S., Xia, S. & Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 738, 139909 (2020).
Yu, Y., Ji, Y., Lu, J., Yin, X. & Zhou, Q. Degradation of sulfamethoxazole by Co3O4-palygorskite composites activated peroxymonosulfate oxidation. Chem. Eng. J. 406, 126759 (2021).
Wang, W. et al. Kapok fiber derived biochar as an efficient electro-catalyst for H2O2 in-situ generation in an electro-Fenton system for sulfamethoxazole degradation. J. Water Process. Eng. 50, 103311 (2022).
Hu, Z.-T. et al. Enhanced BiFeO3/Bi2Fe4O9/H2O2 heterogeneous system for sulfamethoxazole decontamination: system optimization and degradation pathways. J. Colloid Interf. Sci. 577, 54–65 (2020).
Du, J., Guo, W., Che, D. & Ren, N. Weak magnetic field for enhanced oxidation of sulfamethoxazole by Fe0/H2O2 and Fe0/persulfate: performance, mechanisms, and degradation pathways. Chem. Eng. J. 351, 532–539 (2018).
Zhao, G., Li, W., Zhang, H., Wang, W. & Ren, Y. Single atom Fe-dispersed graphitic carbon nitride (g-C3N4) as a highly efficient peroxymonosulfate photocatalytic activator for sulfamethoxazole degradation. Chem. Eng. J. 430, 132937 (2022).
Qi, H., Sun, X. & Sun, Z. Cu-doped Fe2O3 nanoparticles/etched graphite felt as bifunctional cathode for efficient degradation of sulfamethoxazole in the heterogeneous electro-Fenton process. Chem. Eng. J. 427, 131695 (2022).
Guo, R. et al. Efficient degradation of sulfamethoxazole by CoCu LDH composite membrane activating peroxymonosulfate with decreased metal ion leaching. Chem. Eng. J. 417, 127887 (2021).
Li, S., Lin, Y., Zhu, S. & Liu, G. Electrocatalytic degradation of sulfamethylthiadiazole by GAC@Ni/Fe three-dimensional particle electrode. Environ. Sci. Pollut. R. 29, 57112–57126 (2022).
Acknowledgements
We thank Sujuan Ding and Yichen Li from Zhejiang University for the SEM measurements. The authors would like to thank Zhiqi Liu and Qiannan Ma from Shiyanjia Lab (www.shiyanjia.com) for XRD, and XPS analyses.
Funding
Financial support was provided by the National Natural Science Foundation of China (No. 22302178) and Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ21E020003).
Author information
Authors and Affiliations
Contributions
C.S. and D.Z. conceived the project; D.Z. and Y.W. carried out most of the experiments and analyzed the data with the assistance of K.Z. and H.X. C.C. and J.Q. contributed to the discussions; D.Z. and C.S. co-wrote the paper with the inputs and suggestions from others.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, D., Wu, Y., Zheng, K. et al. Preparation of Ti3C2Tx modified rare earth doped PbO2 electrodes for efficient removal of sulfamethoxazole. Sci Rep 14, 8068 (2024). https://doi.org/10.1038/s41598-024-58893-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58893-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.