Abstract
To compare visual and anatomical outcomes between peeling and embedding of epiretinal proliferation in patients with full-thickness macular holes (FTMH) with epiretinal proliferation (EP), this retrospective cohort study classified patients into two groups based on whether EP was completely peeled (peeling group, n = 25 eyes), or embedded into the hole (embedding group, n = 31 eyes) during surgery. Preoperative characteristics and postoperative outcomes, including best-corrected visual acuity and the length of the disrupted external limiting membrane and ellipsoid zone, were compared. Preoperative features including visual acuity and hole size did not differ between the two groups. All studied eyes achieved closure of the macular hole postoperatively. Visual acuity significantly improved at 3, 6, and 12 months postoperatively in both groups. The visual acuity 1-month after surgery was better in the embedding group than that in the peeling group (0.28 ± 0.29 vs. 0.50 ± 0.42 logarithm of the minimum angle of resolution, P = 0.016), although the difference was not noted after 3 months postoperatively. The embedding group showed shorter disruption of the external limiting membrane than the peeling group postoperatively (62.6 ± 40.2 μm vs. 326.2 ± 463.9 μm at postoperative 12 months, P = 0.045). In conclusion, the embedding technique during surgical repair of a FTMH with EP facilitates recovery of the outer foveal layers and promotes earlier restoration of visual function.
Similar content being viewed by others
Introduction
Epiretinal proliferation (EP) is located at the edge of the lamellar macular hole (LMH) or full-thickness macular hole (FTMH) and manifests as a homogeneous, medium reflective material on top of the internal limiting membrane on Fourier-domain optical coherence tomography (OCT)1,2,3,4,5. It is also known as atypical epithelial tissue6,7,8. Previous histopathologic studies on EP have revealed that it is mainly composed of migrated Müller cells from the retina9,10. In addition, OCT studies have shown that FTMH with EP frequently accompanied complete posterior vitreous attachment or detachment11. The developmental mechanism of FTMH with EP is different from that of idiopathic FTMH, which is mainly caused by vitreofoveal traction12,13. To date, FTMH with EP is regarded as a manifestation of slowly progressive foveal degeneration that evolves from LMH with EP11,14,15,16.
Previous studies have shown that FTMH with EP had worse surgical outcomes than those without EP, including worse postoperative visual acuity and larger disruption of the external limiting membrane (ELM), ellipsoid, and interdigitation zones12,17. In contrast to FTMH without EP, which develops rather abruptly by vitreofoveal traction13, FTMH with EP are caused by relatively chronic and degenerative nature of disease progression and seems to be associated with its worse surgical outcomes11.
The main cellular constituent of EP originates from Müller cells9, an indispensable cellular component that maintains retinal architecture. This gave rise to the idea that embedding of EP in the hole during surgical repair would “return back” the escaped tissue18. The embedding technique on LMH with EP has been reported19,20. However, its benefit over total removal of EP in eyes with FTMH has not been studied. In this study, we compared the clinical outcomes of embedding and complete peeling of EP during surgical repair of FTMH with EP.
Methods
Setting
This was a retrospective cohort study of patients with FTMH with EP who underwent pars-plana vitrectomy and were followed up for at least 3 months after surgery. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Samsung Medical Center, Seoul, South Korea (IRB number 2022-11-069). The Institutional Review Board of Samsung Medical Center waived the need for informed consent owing to the retrospective design of this study.
Participants
The electronic medical records of patients who underwent surgical repair for FTMH with EP between December 2014 and 2021 at Samsung Medical Center were retrospectively reviewed. The presence of EP was defined when well-demarcated homogenous medium reflective material was substantially located on the epiretinal surface around the hole12 in the preoperative B-mode or en-face optical coherence tomography (OCT). Patients were classified into the peeling group (the whole extent of EP was peeled off) or the embedding group (the EP was peeled centripetally up to the hole margin and trimmed to an adequate size. Subsequently, the trimmed residual epiretinal tissue was inverted and embedded into the hole). To exclude myopic FTMH, eyes with axial length > 26.5 mm were excluded. Patients who had undergone FTMH repair surgery before and had a history of retinal detachment, advanced glaucoma, age-related macular degeneration more severe than intermediate stage, and diabetic retinopathy equal or severe than severe non-proliferative diabetic retinopathy were excluded. Patients with secondary FTMH, resulting from trauma, solar retinopathy, and idiopathic juxtafoveal telangiectasia, were excluded. Patients who were followed up for less than 3 months after surgery were also excluded.
Surgical technique
Phacoemulsification and posterior chamber intraocular lens implantation were performed if the patient had vision-affecting cataracts or if the age of patients was older than 60 years. Surgery was performed by one surgeon (S.W.K). The procedure consisted of 23- or 25-gauge sutureless vitrectomy (Constellation Vision System; Alcon Laboratories, Inc., Fort Worth, TX, USA), internal limiting membrane peeling with the assistance of 0.3 mg/mL indocyanine green dye staining and intraocular gas tamponade (air, sulfur hexafluoride, or perfluoropropane gas). In eyes without complete posterior vitreous detachment (PVD), PVD induction and partial posterior hyaloidectomy were performed21. In cases where epiretinal membrane was present, it was completely removed. Discriminating between EP and epiretinal membrane was straightforward as epiretinal membrane was located outside the extent of EP. Additionally, EP appeared softer and thicker and exhibited a yellow color attributed to xanthophyll pigmentation11. EP was completely peeled in all eyes that underwent surgery from 2014 to 2018 (peeling group) and embedded in all eyes (embedding group) from 2019. There was no substantial difference between the two groups regarding any aspects of surgical technique and postoperative care, except for peeling or embedding of EP. Figure 1 illustrates the embedding technique in current study. Double staining of indocyanine green was employed to securely isolate EP and to completely peel off the parafoveal internal limiting membrane. Staining was first performed to distinguish the EP from the internal limiting membrane. Then, the margin of EP was lifted and peeled centripetally from the internal limiting membrane. The lifted EP and internal limiting membrane were completely peeled off and removed in the peeling group. When peeling EP attached to the margin of the macular hole, force was not solely applied in one direction. Instead, it was delicately applied from various direction around the hole to minimize foveal damage caused by retinal traction. EP peeling was carried out utilizing Maxgrip® forceps (Alcon Laboratories, Inc., Fort Worth, TX, USA). In the embedding group, we had the lifted EP tissue attached to the hole margin, Indocyanine green dye was then used again to stain the unstained internal limiting membrane that was beneath the EP during the first staining. After the second indocyanine green dye staining, the curvilinear peeling of internal limiting membrane was completed. Utilizing a vitreous cutter (Ultravit® Vitrectomy Probe; Alcon Laboratories, Inc., Fort Worth, TX, USA), the lifted EP tissue was trimmed so as to be accommodated into the hole. Then the inverted flap of EP tissue was embedded into the base of the hole, taking extra caution to avoid compression of the retinal pigment epithelium at the hole bed. Fluid–air and air–gas exchange was performed gently to avoid dislocating the embedded EP. All patients were instructed to maintain a prone position for 24 h post-surgery, followed by either prone or seated with facedown positioning for the subsequent 7 days, irrespective of tamponade type.
Epiretinal proliferation embedding in surgery for macular hole with epiretinal proliferation. (A) After initial staining with indocyanine green dye, unstained epiretinal proliferation (represented by the yellow membrane) is differentiated from the stained internal limiting membrane (represented by the green membrane). (B) The epiretinal proliferation is lifted and peeled from the internal limiting membrane up to the hole margin, and then the second indocyanine green dye staining was performed to stain the unstained portion of internal limiting membrane that was beneath the epiretinal proliferation during the first stain. (C) After completion of curvilinear internal limiting membrane peeling, the lifted epiretinal proliferation was trimmed using a vitreous cutter (D) The trimmed epiretinal proliferation is inverted and embedded into the macular hole.
Examinations
Preoperative demographic data, best-corrected visual acuity (BCVA), slit-lamp biomicroscopic examination, axial length, FTMH size, and manifest refraction were assessed. The axial length was measured using swept-source OCT (Argos; Movu, Inc., CA, U.S.A). B-mode macular images using spectral-domain OCT (Spectralis™; Heidelberg Engineering GmbH, Heidelberg, Germany) of 8 mm horizontal and vertical images crossing the central fovea and nineteen 6 mm horizontal raster images encompassing the macular area were taken in all patients. En-face OCT images were obtained using either spectral-domain OCT with 20° × 15° macular volume scans or swept-source OCT (DRI OCT Triton; Topcon Corporation, Tokyo, Japan) in the 6 mm × 6 mm macular region. FTMH size in this study was defined as the mean of the minimal hole diameters in the horizontal and vertical sections of B-mode OCT images.
Patients were followed up at 1 week and 1, 3, 6, and 12 months postoperatively. BCVA was measured at each postoperative visit, and OCT images were taken at all follow-up visits except at the week 1 visit. The ELM and ellipsoid zone disruption were measured as the mean length of disruption of each layer from horizontal and vertical OCT scans crossing the foveal center. Preoperative and postoperative OCT parameters were measured manually by two observers (S.H and K.Y.S) using built-in OCT software, and the mean value was used.
Statistical analyses
Preoperative and postoperative data at 1, 3, 6, and 12 months were compared between the peeling group and the embedding group. BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) scale for statistical analysis. Wilcoxon rank-sum test was used to compare the continuous variables between the two groups. The chi-square test was used to compare categorical variables. Wilcoxon signed-rank test was used to compare postoperative BCVA with the preoperative BCVA in each group. Statistical analyses were performed with R software (version 4.2.1; R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). Statistical significance was set at P value less than 0.05.
Ethics declarations
This research followed the tenets of the Declaration of Helsinki and was approved by Institutional Review Board (IRB) of the Samsung Medical Center, Seoul, South Korea Informed consent was waived by the board as the research was retrospective, anonymous, and presented no threat to the rights and welfare of the research participants.
Results
Fifty-six eyes of 56 patients met the inclusion criteria. The mean age was 64.0 ± 9.4 years, and 29 (51.8%) patients were female. Of 56, 31 (55.4%) patients were in the embedding group and 25 (44.6%) were in the peeling group. Table 1 shows the baseline demographic and clinical characteristics of the two groups. The mean age and sex ratio did not different between the two groups. The mean preoperative BCVA was 0.41 ± 0.30 logMAR (20/50) in the embedding group and 0.47 ± 0.28 logMAR (20/63) in the peeling group (P = 0.339). The mean hole size and axial length did not differ between the two groups.
All 56 eyes achieved closure of FTMH after surgery. Compared with baseline, BCVA was significantly improved at 3, 6, and 12 months postoperatively in both groups. In the embedding group, preoperative BCVA was 0.41 ± 0.30 logMAR. After surgery, BCVAs at 1, 3, 6, and 12 months were 0.28 ± 0.29, 0.18 ± 0.17, 0.22 ± 0.22, and 0.15 ± 0.17 logMAR, respectively (P = 0.004, 0.001, 0.006, and 0.004, respectively). In contrast, the peeling group had a preoperative BCVA of 0.47 ± 0.28 logMAR. Postoperative BCVAs at 1, 3, 6, and 12 months were 0.50 ± 0.42, 0.26 ± 0.22, 0.21 ± 0.21, and 0.17 ± 0.17, respectively (P = 0.901, 0.001, 0.001, and 0.008, respectively). The mean postoperative BCVA 1 month after surgery was better in the embedding group than that in the peeling group (0.28 ± 0.29 logMAR (20/40) vs. 0.50 ± 0.42 logMAR (20/63), respectively, P = 0.016). Three months after surgery and afterwards, however, no difference was noted in the mean postoperative BCVAs between the two groups (Fig. 2). The visual change was also better in the embedding group than in the peeling group 1 month after surgery (− 0.11 ± 0.19 logMAR vs. 0.02 ± 0.28 logMAR, P = 0.037, respectively). However, there was no difference at 3, 6, and 12 months after surgery.
Comparison of pre- and postoperative BCVA between the embedding and peeling groups. After surgical repair, BCVA was significantly improved in both groups at 3, 6, and 12 months. However, after 1 month postoperation, only the embedding group showed improved BCVA and it was significantly better than that of the peeling group. BCVA best-corrected visual acuity, logMAR logarithm of the minimal angle of resolution, n number. *Statistically significant at P < 0.05.
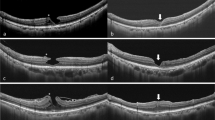
OCT examinations revealed that the mean ELM disruption length was significantly shorter in the embedding group at all postoperative follow-ups. The mean ellipsoid zone disruption length was shorter in the embedding group 1 month after surgery (465.7 ± 265.5 μm vs. 691.6 ± 424.8 μm, respectively, P = 0.045). However, this difference was not noted 3 months after surgery (Table 2). Figure 3 shows representative cases from the embedding group and the peeling group.
Representative cases from the embedding and peeling groups. (A–F) The embedding group. (A, C, E) Preoperative OCT images show full-thickness macular hole with epiretinal proliferation. (B, D, F) Postoperative 12-month OCT images show sealed macular hole, with defect at the external limiting membrane and ellipsoid zone in some cases (D, F). (G–L): The peeling group. (G, I, K) Preoperative OCT images. (H, J, L) Postoperative 12-month OCT images. The defects in the ellipsoid zone (H, J, L) and external limiting membrane (H, J) were presented. Note that the extent of ellipsoid zone is larger than that of the external limiting membrane (H, J). OCT, optical coherence tomography.
Discussion
The gender distribution of the study population is nearly 1:1. This finding aligns with the results observed in previous research related to EP associate with FTMH17. Moreover, it starkly demonstrates a distinct difference from the FTMH without EP, which exhibits a clear female dominance22,23. Several studies have compared the visual prognosis of FTMH with EP and those without EP and showed that patients with FTMH with EP had poorer postoperative visual acuity than FTMH patients without EP11,12. In addition, the pathophysiology of FTMH with EP has been reported to be different from that of FTMH without EP8,9. Therefore, it may not be appropriate to apply the same surgical technique to FTMH with EP as those without EP.
In this study, both the embedding and peeling groups achieved complete FTMH sealing and improved visual acuity after surgery. However, the embedding group showed better BCVA improvement than the peeling group at postoperative 1 month. In other words, the embedding technique promoted faster visual recovery after surgery than complete peeling of epiretinal proliferation. It remains unclear how the embedding technique expedited visual recovery. One possible explanation is that the concept of the embedding technique that brings back the dislocated glial tissue to its original position could contribute to early vision recovery by promoting restoration of the outer retinal layers. Another hypothesis is that embedding technique aids in preventing foveal tractional damage, which may result from the peeling or EP.
Postoperative OCT examination revealed that the ELM disruption length was significantly shorter in the embedding group than in the peeling group at all follow-ups. Furthermore, the embedding group had a shorter ellipsoid zone disruption length than the peeling group, although statistical significance was noted only 1 month postoperatively. The ELM is composed of a connection between the photoreceptor inner layer and Müller cell process24. Previous studies on EP showed that an increase in its extent correlates with the worsening of foveal cavitation8,14. Consequently, EP is considered to consisted of centrifugally dislocated Müller cells rather than Müller cells proliferation. Repositioning of EP to its original position might help restore ELM. The ELM and ellipsoid zone integrity are well-known predictors of the visual prognosis in idiopathic FTMH and LMH25,26,27,28,29,30. This study supports that, as with idiopathic FTMH and LMH, postoperative visual outcome in FTMH with EP was also associated with the integrity of the ELM and ellipsoid zone. In particular, ellipsoid zone integrity was closely related to postoperative visual changes in patients with FTMH with EP. A previous study showed that intact ELM is indispensable for ellipsoid zone recovery after FTMH repair surgery31. Therefore, it can be assumed that the restoration of ELM precedes the ellipsoid zone restoration, and the EP embedding technique might expedite both ELM and ellipsoid zone recovery.
Double staining with indocyanine green can be a useful method to visualize both EP and the internal limiting membrane underneath the epiretinal proliferation. That is, the double staining with indocyanine green helps us to differentiate and isolate EP from internal limiting membrane by first stain, and then to visualize internal limiting membrane beneath the EP by second stain after lifting up EP11.
Because EP is connected to the inner retina at the margin of the FTMH8, there is a risk that the retinal tissue at the margin of the hole is lifted up by the tractional force when EP is peeled in the peeling group. Previous research demonstrated that internal limiting membrane peeling during surgery for lamellar macular holes can lead to the development of FTMH postoperatively6,32. Given the strong attachment of EP to inner retina at the macular hole margin11, caution is required to minimize tractional during EP peeling. Therefore, in the peeling group, efforts were made to reduce tractional foveal damage by carefully pulling EP attached to the macular hole margin from multiple directions rather than one direction. In this process, Maxgrip® forceps offered an advantage over end-gripping forceps due to its broader grasping extent, thereby aiding in prevention of EP tearing. On the other hand, in the embedding group, caution is required because of the potential risk of unintentional trauma to the hole base, especially the retinal pigment epithelium, during the embedding process. Although the embedding technique expedites earlier postoperative visual recovery than the peeling group, both peeling and embedding procedures may impose an inherent risk of surgical trauma. To figure this out, the use of intraoperative OCT would be helpful.
This study had several limitations. First, this was a retrospective study with a small number of participants. Second, the results were obtained over a relatively short follow-up period. All patients were followed up until 3 months postoperatively, and 44 (78.6%) and 27 (48.2%) patients were followed up at 6 and 12 months, respectively. This may have limited the power of our statements. Beyond the surgical technique, the impact of additional pre- and post-operative structural features which were not evaluated in the current study may influence surgical outcomes17,33. An additional prospective comparative study with a larger sample size, considering various pre- and post-operative anatomical features is needed.
Despite these limitations, this study is worthwhile as the first report demonstrating the safeness and usefulness of the embedding technique to promote better restoration of the outer retinal layers and earlier visual recovery in FTMH with EP. Because faster recovery of vision after FTMH surgery can improve patients’ quality of life and allow early return to work or daily life, the benefit of the embedding technique in patients with FTMH with EP is warranted.
In conclusion, current study suggests that the embedding technique used during the surgery for FTMH with EP may promote early visual restoration and anatomical recovery of outer retina.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pang, C. E., Spaide, R. F. & Freund, K. B. Epiretinal proliferation seen in association with lamellar macular holes: A distinct clinical entity. Retina 34, 1513–1523. https://doi.org/10.1097/iae.0000000000000163 (2014).
Lai, T. T., Chen, S. N. & Yang, C. M. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: Clinical and surgical findings. Graefes Arch. Clin. Exp. Ophthalmol. 254, 629–638. https://doi.org/10.1007/s00417-015-3133-9 (2016).
Govetto, A. et al. Lamellar macular hole: Two distinct clinical entities?. Am. J. Ophthalmol. 164, 99–109. https://doi.org/10.1016/j.ajo.2016.02.008 (2016).
Hubschman, J. P. et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br. J. Ophthalmol. 104, 1741–1747. https://doi.org/10.1136/bjophthalmol-2019-315432 (2020).
Itoh, Y. et al. Prevalence and characteristics of hyporeflective preretinal tissue in vitreomacular interface disorders. Br. J. Ophthalmol. 100, 399–404. https://doi.org/10.1136/bjophthalmol-2015-306986 (2016).
Parolini, B., Schumann, R. G., Cereda, M. G., Haritoglou, C. & Pertile, G. Lamellar macular hole: A clinicopathologic correlation of surgically excised epiretinal membranes. Invest. Ophthalmol. Vis. Sci. 52, 9074–9083. https://doi.org/10.1167/iovs.11-8227 (2011).
Schumann, R. G. et al. Epiretinal membrane characteristics correlate with photoreceptor layer defects in lamellar macular holes and macular pseudoholes. Retina 35, 727–735. https://doi.org/10.1097/iae.0000000000000375 (2015).
Eun, J. S. et al. En-face imaging of atypical epiretinal tissue in lamellar macular hole. Retina 42, 298–305. https://doi.org/10.1097/iae.0000000000003303 (2022).
Pang, C. E. et al. Lamellar hole-associate epiretinal proliferation: A clinicopathologic correlation. Retina 36, 1408–1412. https://doi.org/10.1097/iae.0000000000001069 (2016).
Schumann, R. G. et al. Premacular cell proliferation profiles in tangential traction vitreo-maculopathies suggest a key role for hyalocytes. Ophthalmologica 242, 106–112. https://doi.org/10.1159/000495853 (2019).
Hwang, S. & Kang, S. W. The clinical and pathogenic significance of atypical epiretinal tissue in macular hole. Graefes Arch. Clin. Exp. Ophthalmol. 260, 2791–2798. https://doi.org/10.1007/s00417-022-05750-2 (2022).
Bae, K. et al. Atypical epiretinal tissue in full-thickness macular holes: pathogenic and prognostic significance. Br. J. Ophthalmol. 103, 251–256. https://doi.org/10.1136/bjophthalmol-2017-311810 (2019).
Duker, J. S. et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 120, 2611–2619. https://doi.org/10.1016/j.ophtha.2013.07.042 (2013).
Compera, D. et al. Progression of lamellar hole-associated epiretinal proliferation and retinal changes during long-term follow-up. Br. J. Ophthalmol. 102, 84–90. https://doi.org/10.1136/bjophthalmol-2016-310128 (2018).
Tsai, C. Y., Hsieh, Y. T. & Yang, C. M. Epiretinal membrane-induced full-thickness macular holes: The clinical features and surgical outcomes. Retina 36, 1679–1687. https://doi.org/10.1097/iae.0000000000000999 (2016).
Chehaibou, I. et al. Spontaneous conversion of lamellar macular holes to full-thickness macular holes: clinical features and surgical outcomes. Ophthalmol. Retina 5, 1009–1016. https://doi.org/10.1016/j.oret.2020.12.023 (2021).
Kim, E. L. et al. Characterization of epiretinal proliferation in full-thickness macular holes and effects on surgical outcomes. Ophthalmol. Retina 3, 694–702. https://doi.org/10.1016/j.oret.2019.03.022 (2019).
Shiraga, F. et al. Modified vitreous surgery for symptomatic lamellar macular hole with epiretinal membrane containing macular pigment. Retina 33, 1263–1269. https://doi.org/10.1097/IAE.0b013e31828bcb61 (2013).
Takahashi, K. et al. Results of lamellar macular hole-associated epiretinal proliferation embedding technique for the treatment of degenerative lamellar macular hole. Graefes Arch. Clin. Exp. Ophthalmol. 257, 2147–2154. https://doi.org/10.1007/s00417-019-04425-9 (2019).
Lai, T.-T., Hsieh, Y.-T., Lee, Y. & Yang, C.-M. Embedding and sparing of lamellar hole: Associated epiretinal proliferation in the treatment of lamellar macular holes. Eye 36, 1308–1313. https://doi.org/10.1038/s41433-021-01631-w (2022).
Kim, J. H., Kang, S. W., Kim, Y. T., Kim, S. J. & Chung, S. E. Partial posterior hyaloidectomy for macular disorders. Eye 27, 946–951. https://doi.org/10.1038/eye.2013.117 (2013).
Ali, F. S., Stein, J. D., Blachley, T. S., Ackley, S. & Stewart, J. M. Incidence of and risk factors for developing idiopathic macular hole among a diverse group of patients throughout the United States. JAMA Ophthalmol. 135, 299–305. https://doi.org/10.1001/jamaophthalmol.2016.5870 (2017).
McCannel, C. A., Ensminger, J. L., Diehl, N. N. & Hodge, D. N. Population-based incidence of macular holes. Ophthalmology 116, 1366–1369. https://doi.org/10.1016/j.ophtha.2009.01.052 (2009).
Gloesmann, M. et al. Histologic correlation of pig retina radial stratification with ultrahigh-resolution optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 44, 1696–1703. https://doi.org/10.1167/iovs.02-0654 (2003).
Houly, J. R., Veloso, C. E., Passos, E. & Nehemy, M. B. Quantitative analysis of external limiting membrane, ellipsoid zone and interdigitation zone defects in patients with macular holes. Graefes Arch. Clin. Exp. Ophthalmol. 255, 1297–1306. https://doi.org/10.1007/s00417-017-3636-7 (2017).
Lee, M.-W., Kim, T.-Y., Song, Y.-Y., Baek, S.-K. & Lee, Y.-H. Changes in each retinal layer and ellipsoid zone recovery after full-thickness macular hole surgery. Sci. Rep. 11, 11351. https://doi.org/10.1038/s41598-021-90955-4 (2021).
Choi, W. S., Merlau, D. J. & Chang, S. Vitrectomy for macular disordes associated with lamellar macular hole epiretinal proliferation. Retina 38, 664–669. https://doi.org/10.1097/iae.0000000000001591 (2018).
Cho, H. Y., Kim, Y. T. & Kang, S. W. Laser photocoagulation as adjuvant therapy to surgery for large macular holes. Korean J. Ophthalmol. 20, 93–98. https://doi.org/10.3341/kjo.2006.20.2.93 (2006).
Kim, J. M. et al. Epiretinal membrane: Prevalence and risk factors from the Korea national health and nutrition examination survey, 2008 through 2012. Korean J. Ophthalmol. 31, 514–523. https://doi.org/10.3341/kjo.2016.0098 (2017).
Caprani, S. M. et al. Macular hole surgery: The healing process of outer retinal layers to visual acuity recovery. Eur. J. Ophthalmol. 27, 235–239. https://doi.org/10.5301/ejo.5000905 (2017).
Landa, G., Gentile, R. C., Garcia, P. M., Muldoon, T. O. & Rosen, R. B. External limiting membrane and visual outcome in macular hole repair: Spectral domain OCT analysis. Eye 26, 61–69. https://doi.org/10.1038/eye.2011.237 (2012).
Witkin, A. J. et al. Redefining lamellar holes and the vitreomacular interface: An ultrahigh-resolution optical coherence tomography study. Ophthalmology 113, 388–397. https://doi.org/10.1016/j.ophtha.2005.10.047 (2006).
Kusuhara, S. & Negi, A. Predicting visual outcome following surgery for idiopathic macular holes. Ophthalmologica 231, 125–132. https://doi.org/10.1159/000355492 (2014).
Acknowledgements
The authors would like to thank Da Hyeun Lee, an audiovisual engineer at Samsung Medical Information & Medical Services, for designing Fig. 1 for this work.
Author information
Authors and Affiliations
Contributions
Concepts and design: Se Woong Kang, Jaehwan Choi. Acquisition, analysis, or interpretation of the data: Jaehwan Choi, Sungsoon Hwang, Ki Young Son. Drafting of the manuscript: Jaehwan Choi. Critical revision of the manuscript for important intellectual content: Se Woong Kang, San Jin Kim. Administration, technical, or material support: Se Woong Kang, Sungsoon Hwang. Supervision: Se Woong Kang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, J., Kim, S.J., Kang, S.W. et al. Outcomes of epiretinal proliferation embedding technique in the surgery for full-thickness macular hole. Sci Rep 14, 8170 (2024). https://doi.org/10.1038/s41598-024-58449-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58449-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.