Abstract
Despite the well-documented, broad global distribution of sperm whales (Physeter macrocephalus), their distributional patterns remain poorly known in Arctic regions, where year-round monitoring is challenging. Adult male sperm whales are known to migrate seasonally between nutrient-rich high latitude waters and low latitude breeding grounds. However, knowledge is limited regarding fine-scale distribution and seasonal presence at high latitudes. To investigate the acoustic occurrence of this vocally active species in the High Arctic of the Northeast Atlantic, this study combined automated and manual click detection methods to analyze passive acoustic data collected at eight locations around the Svalbard Archipelago, Norway, between 2012 and 2021. The results revealed the presence of sperm whales at six recording sites and demonstrated sperm whale “hotspots” in ice-free areas in eastern Fram Strait along the shelf break and close to the west coast of Spitsbergen from May–January, with some variation between years and locations. Although acoustic presence decreased with increasing latitude, even the northern-most location (81° N) recorded sperm whale vocal activity between August and January. This study provides a baseline for sperm whale acoustic presence in the High Arctic, which will be essential in the context of detecting future changes and also for predicting future distribution patterns in the rapidly changing Arctic marine environment.
Similar content being viewed by others
Introduction
The sperm whale (Physeter macrocephalus) is an extremely cosmopolitan species that ranges throughout the world’s oceans from the tropics to the poles1. It is estimated that the global population size for this species was as high as 1,900,000 individuals prior to whaling2. Although there are uncertainties in current sperm whale population sizes and trends, the global estimate is approximately 840,000 individuals2. The species is classified as “Vulnerable” on the IUCN Red List of Threatened Species1. Females and calves inhabit mid-latitude regions year-round, while mature males migrate between nutrient-rich high latitude waters in summer and lower latitude breeding grounds in winter. Females live in distinct social clans with other females and their calves3. Male sperm whales, on the other hand, are often found alone or in loose, all-male aggregations of 10–30 individuals, often spread over a large area, with individuals some kilometers apart. However, these large clusters are uncommon, and males appear to be more solitary and less social than females4. It is believed that the oldest and largest males are found at the highest latitudes5.
Even though sperm whales are known to be seasonally resident in the Arctic, their geographic distribution at a finer scale and the timing of their arrivals and departures from the north are not well documented5. Sea ice is known to limit their distribution; sperm whales typically avoid sea ice covered areas6,7. In the North Pacific, sperm whales are found in Alaskan waters8,9, in the Bering Sea, the Bering Strait and the southern Chukchi Sea, up to latitudes of about 68° N7,10. Further north, sea ice concentrations are relatively high year-round and there are no records of sperm whales. In contrast, in the North Atlantic, the sperm whales’ range extends much further north. Studies have reported sperm whales at latitudes of 75° N in Baffin Bay in the Northwest Atlantic6,11,12. In the Northeast Atlantic, the sperm whale’s range extends north to the Svalbard Archipelago, with observations as far as 81° N13,14,15,16,17. This is likely explained by the inflow of warm Atlantic Water in the West Spitsbergen Current, which keeps western and northern Svalbard waters relatively ice-free for most of the year18. Recent studies report a possible northward shift in sperm whale distribution with more frequent observations at higher latitudes around Svalbard as the northern sea ice edge has shifted northwards in recent years17,18,19. Sperm whales have also been reported in the relatively shallow waters of the Barents Sea, east of Svalbard, and strandings have occurred on the mainland in the western Russian Arctic20.
The High Arctic poses logistical challenges for observation-based data collection. Traditional visual survey methods are spatially and temporally limited in this vast region, with winter darkness precluding observations in winter months entirely. In the case of sperm whales, visual surveys, even under ideal conditions, can lead to underestimation of the species’ presence due to their long absences from the surface (while diving) and their low, dispersed blows when breathing at the surface can be overlooked easily15,21. Satellite tracking studies enable investigation of wide-ranging cetaceans22,23, but involve complicated and expensive logistics. One way to overcome some of these limitations is by using Passive Acoustic Monitoring (PAM), which consists of deployment of underwater recorders to capture sounds from the surrounding environment. PAM is a cost-effective, non-invasive method that is widely used for long-term studies to assess and monitor marine biodiversity, including species distribution and behavior of acoustically active species9,11,24. PAM also allows year-round data collection independent of sea or weather conditions in remote areas, like the High Arctic. However, PAM requires target species to be vocally active. Sperm whales produce stereotypic broadband frequency clicks during much of the time (up to 68% of the dive cycle) when they are diving, making them ideal subjects for PAM25,26,27,28. They produce several types of clicks including usual clicks and buzz clicks, which are highly directional broadband signals that serve as biosonar (echolocation) signals used for navigation and foraging25,28,29,30. Two other click types, codas and slow clicks, are used for socializing and general communication. Codas are mainly produced by females. These sounds are highly individualistic and might be used as individual identification codes29,31. Slow clicks, or ‘clangs’, which are usually produced by males30, are thought to serve for long range communication32. Propagation range estimates for sperm whale clicks vary between 6 and 80 km in various studies depending on location, season and click type30,33,34.
Several PAM-based studies have been conducted on sperm whale populations in recent years in different parts of the world, e.g., in the Antarctic35, off South Africa34, in the Bering Sea7, western North Atlantic36, Canadian Atlantic Arctic6 and off Japan37. However, no year-round, multi-year PAM studies have been published on sperm whale spatial and temporal vocal presence in the Northeast Atlantic High Arctic. This study provides a first multi-year assessment of acoustic presence of sperm whales around the Svalbard Archipelago, Norway. Passive acoustic data from eight different locations (Fig. 1) across a 10-year cumulative time span were analyzed using a combination of customized automated and manual detection methods to obtain a spatial and temporal baseline for sperm whale acoustic presence in this region. In addition, the relationship to environmental factors such as sea ice cover and biological productivity were explored to gain further insight into the potential drivers of sperm whale distribution in the study area.
Map of mooring locations around Svalbard. Depth is given in meters. WFS Western Fram Strait, EFS Eastern Fram Strait, KF Kongsfjorden, IF Isfjorden, RF Rijpfjorden, ATW Atwain, ESV-1 Eastern Svalbard 1, ESV-2 Eastern Svalbard 2. Map created in R using terra (package version 1.7.-65; available at https://cran.r-project.org/web/packages/terra/index.html), ggplot2 (Version 3.4.2.; available at https://cran.r-project.org/web/packages/ggplot2/index.html) and tidyterra (Version 0.5.2.; available at https://cran.r-project.org/web/packages/tidyterra/index.html).
Results
Sperm whale detections and detector performance
Sperm whale vocal presence was successfully identified in 2763 h out of a total of approximately 190,000 h, at six of the eight study locations (Fig. 2, Appendix Table A1). Most detections occurred at the Eastern Fram Strait and Isfjorden moorings, with 2125 of 17,150 h (12.3% of recordings) and 471 of 20,649 h (2.3%) containing vocal activity at these sites, respectively. At Atwain, sperm whales were detected at a low rate, with sperm whale clicks in only 142 of 32,351 h (0.4% of recordings), but they were consistently present across four deployment years. At Kongsfjorden, Rijpfjorden and in Eastern Svalbard 2, vocal activity was detected in very few recordings (< 0.1% of recordings in all cases). No sperm whales were detected at the Western Fram Strait or at the Eastern Svalbard 1 mooring during any of the deployment periods.
The automated detector was useful for identifying sperm whale presence in the vast amount of data collected by the PAM array. Periods containing ‘strong’ sperm whale vocalizations were particularly well detected (89% and 96% of hours with presence successfully detected in the detector validation datasets). For examples of detected click types and detector performance details, see Appendix Tables B1 and B2. The main sources of false positive events were identified as sea ice associated sounds (Appendix Figure C1) and self-noise from cable-strumming by the instrument or other components on the mooring (Appendix Figures C2 and C3). Occasional false positive detections were also triggered by walruses (Odobenus rosmarus) (Appendix Figures C4 and C5) or other odontocete sounds, such as narwhal (Monodon monoceros) clicks (Appendix Figure C6). The prevalence of false positive triggers varied across locations and years.
Temporal presence
At the six locations that had sperm whale detections, only Eastern Fram Strait, Isfjorden and Atwain demonstrated consistent, multi-year presence across the deployment periods. Sperm whales were detected at these locations from May until January, but predominantly between September and December. The highest presence was detected at Eastern Fram Strait (Fig. 3b), where sperm whales were detected during 18 and 30 days per month in September 2016 and October 2018, respectively. The earliest spring detections at Eastern Fram Strait occurred in May 2019 and the latest seasonal durations in the north extended until January 2017 and 2019. At Isfjorden (Fig. 3d), detections occurred from June to December, but most sperm whale vocalizations occurred from October to December. Sperm whale presence peaked in November of every deployment year at Isfjorden, with 14 days (2017 and 2018) and 7 days (2019). At Atwain (Fig. 3a), sperm whale presence was detected between August and January, with a maximum of 9 days a month(October 2013). Sperm whale presence at Kongsfjorden, Rijpfjorden and Eastern Svalbard 2 was limited to only a few days over the whole study period: 2 days (Nov 2015) at Rijpfjorden, 5 days (Nov–Dec 2013 and 2017) at Kongsfjorden, and 1 day (Oct 2021) at Eastern Svalbard 2 (Fig. 3c,e,f). In February, March and April, no sperm whales were detected at any of the mooring locations in any of the study years.
Only Eastern Fram Strait and Isfjorden had enough sperm whale detections to explore statistical relationships with biological covariates, ice cover and SST (Fig. 4). The probability of sperm whale occurrence was found to increase significantly with daily mean zooplankton concentration (OR 9.36, CI95% 2.42–36.17, p < 0.01) at Eastern Fram Strait, while there were no significant effects of sea ice, sea surface temperature or other biological variables. In Isfjorden, no significant effects were found for any of the environmental variables (see Appendix Figure A1). Comparing all locations, mean sea ice cover was significantly lower at times and locations where sperm whales were detected (Z = − 20.57, p < 0.005). For the rest of the locations, relationships to biological variables and SST were only examined visually (Appendix Figures A2–A7). At these locations, sperm whale activity increased after peaks in biological activity and SST, with different time lags across recording sites. The locations with the highest sperm whale detection rates were characterized by the highest sea floor mean slope and terrain heterogeneity (Fig. 5).
Daily mean net primary production, zooplankton and chlorophyll a concentration, daily sea ice cover, daily mean sea surface temperature and daily sperm whale acoustic presence at Eastern Fram Strait (top) and Isfjorden (bottom). Orange arrow marks the time point when the mooring was moved. Shaded (grey) areas indicate no data available.
Mean depth, slope, and TRI (Terrain Ruggedness Index) at all locations (50 km radius around mooring). In cases where moorings were moved, multiple data points exist per location. WFS Western Fram Strait, EFS Eastern Fram Strait, KF Kongsfjorden, IF Isfjorden, RF Rijpfjorden, ATW Atwain, ESV-1 Eastern Svalbard 1, ESV-2 Eastern Svalbard 2.
Diurnal variation in detections during three different Arctic light regimes (seasons) were visually compared at Eastern Fram Strait, Isfjorden and Atwain. Most detections occurred during seasons with light and dark hours or continuous darkness (Polar Night). During the day-night season, most detections occurred during daylight at Eastern Fram Strait and during twilight at Atwain (Fig. 6). During the Polar Night, most detections occurred between 18:00 and 24:00 at Atwain (Fig. 7). At Isfjorden, all detections occurred during the Polar Night.
Occurrence of vocalization types
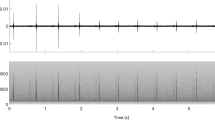
Usual clicks were present in nearly all recordings with sperm whale detections (Appendix Figure A7). Recordings containing buzz clicks were found in similar proportions at Isfjorden and Eastern Fram Strait (15.3% and 12.85% of the total number of recordings containing sperm whale detections at each location, respectively) but much less frequently at Atwain (2.82%). Slow clicks were present most commonly in Isfjorden and least commonly at Eastern Fram Strait. Overlapping vocalizations from more than one individual occurred in more than half of the recordings with sperm whale presence. For examples of detected clicks, see spectrograms in Appendix Figures C7–C15.
Discussion
This is the first multi-year, multi-location study of sperm whale acoustic presence in the Svalbard area. The results provide new insights into the spatial and temporal trends of sperm whale vocal presence in the High Arctic. Presence was detected at six of eight locations with two “hotspots” off Western Spitsbergen (Eastern Fram Strait and Isfjorden). Although acoustic presence decreased with increasing latitude, seasonal multi-year presence was detected even at the northernmost location in our study (Atwain—81° N), which is the northernmost record for sperm whale acoustic presence. While the automated detector was useful for screening large amounts of data, manual validation of the detector results was needed (i.e., removal of all false positive detections from the results) because of differences in false positive detection rates across locations (Appendix Table B2). Previous studies have already described the complexities of High Arctic soundscapes, characterized by a large variety of sounds of abiotic and biotic origins that exhibit strong seasonal variation38,39,40,41. Prevalent sounds such as sea ice or wind, as well as vocally active marine mammals, set challenges for signal detection because the likelihood of both signal masking and false detections is higher compared to many lower latitude soundscapes. The variations in detector performance across the study locations further reflect the high variability in soundscapes even within the High Arctic, highlighting the need for soundscape-specific detectors as well as the need for more annotated location-specific training data.
The Eastern Fram Strait location had the highest sperm whale occurrence in our study. While a visual survey from 1995 to 2001 did not report sperm whale sightings in the Eastern Fram Strait42, more recent studies from the same area do report their presence14,16,17,19. At Isfjorden, the overall occurrence rate, while much lower than at Eastern Fram Strait, was still higher than expected. Previous studies have reported some sightings around the mouth of this fjord16,17,19, but in general sperm whales are not common in the area. Sperm whales are known to avoid ice-covered areas6,11,35 and these two locations had the lowest overall ice cover of the eight sites during the deployment periods (Fig. 4). The Isfjorden mooring was moved after two deployment years, shifting to a site deeper into the fjord with a higher ice coverage surrounding the location. Sperm whale presence decreased following the move, compared to the first two years. Consequently, sea ice cover likely explains the total absence of sperm whale detections at the Western Fram Strait recording site (Appendix Figure A7) as the site was covered with very close drift ice most months each study year. The Western Fram Strait is a known habitat for ice-associated species such as narwhal43 and bowhead whales (Balaena mysticetus)44 and the former species shares a squid diet with sperm whales45 so lack of prey in the area is an unlikely explanation of their absence.
Similarly, the Eastern Svalbard locations had no detections, except for a single day at the Eastern Svalbard 2. These sites were also ice covered during most of the data collection periods (Appendix Figures A2 and A3). Although Rijpfjorden had longer open water periods (Appendix Figure A6), sperm whale presence was also limited to a couple of days during two recording years. This may be explained by the local bathymetry and oceanography. Sperm whales are known to forage in deep waters near shelf-edges17,46,47 rather than shallow fjord areas. The Rijpfjorden mooring is farther away from the shelf-edge and even though sperm whales sometimes do forage in shallower waters48,49,50, prey availability might be limited in this fjord, considering the extensive ice cover and short growing season. However, this mooring is located relatively close (< 10 km) to the mouth of the fjord, so detected vocal activity at this site might have arisen from individuals outside the fjord.
The Eastern Fram Strait moorings are located right at a shelf edge near the deepest part of Fram Strait, with the greatest sea floor depth, slope, and heterogeneity (Fig. 5). This region of the central Fram Strait is also where warm Atlantic Water transported northward by the Western Spitzbergen Current, divides into two currents. One of these currents continues northward and the other travels to the west creating turbulence51 and upwelling. Sperm whale presence has been shown to be correlated with increased circulation and turbulence52,53. These conditions typically promote upwelling and species richness and hence potential prey availability, which likely explains the highest occurrence in our study of sperm whales in Eastern Fram Strait. Similarly, the low yet consistent, multi-year presence at Atwain may be explained by both suitable bathymetry (shelf-edge) and the influence of the Western Spitsbergen Current, which keeps the sea ice cover relatively low. However, sea ice extent at Atwain varied significantly interannually54. During the data collection periods in this study, ice cover was generally low, but between 2014 and 2015 the area was covered in very close drift ice practically year-round, but unfortunately no acoustic data was collected from that period (Appendix Figure A4). Previously, single instances of acoustic sperm whale presence have been reported from Atwain15, but a consistent seasonal presence was not expected based on previous observational studies and habitat suitability models17. These contrasting results highlight the importance of combining both visual and acoustic methods55, especially for deep divers like sperm whales in areas that are difficult to access. The Isfjorden mooring, although on the shelf and in shallower water, is also exposed to an in-and-out circulation flow of Atlantic Water onto the shelf via the Isfjorden Trough, a trench deeper than 200 m, that creates a hybrid area between shelf and shelf edge waters56,57. Despite low ice cover and some Atlantic Water influence, sperm whale occurrence was scarce and inconsistent in Kongsfjorden. A recent study reported an increase in sightings near the mouth of Kongsfjorden, so more acoustic detections could have been expected19. The location of the hydrophone approx. 15 km into the fjord rather than at the mouth might reduce the reception of acoustic signals coming from outside of the fjord. Nonetheless, the present results are similar to Llobet et al.58 that found sperm whale vocal presence only on 1 day a year in this fjord. It is important to note, that the Eastern Fram Strait hydrophones were deployed significantly deeper (over 800 m) than the other sites (around 200 m) which might also contribute to the increased presence at Eastern Fram Strait, given that sperm whales are deep divers.
The timing of sperm whale migrations to the high latitude feeding grounds is not known with any degree of precision, but on-going tagging studies show quite asynchronous migration times for males (unpublished NPI/UiT data) with departures in the autumn or early winter being the norm. Sperm whale diet is believed to mainly consist of cephalopods and fish, but there is regional variation. Boreoatlantic armhook squid (Gonatus fabricii) has been identified as an important prey species in the North Sea59 and Northern Norway60 and this potential prey species does range up to northern Svalbard (Atwain). High concentrations have been reported around the Eastern Fram Strait site61 which is also a suspected spawning site for these squid62. MacKenzie et al.63 concluded based on their stable isotope analyses that sperm whales likely consume deep-water species such as squid. Fish species such as the lumpsucker (Cyclopterus lumpus) and Atlantic cod (Gadus morhua) have also been found to be part of sperm whale diet in the North Atlantic60,64 and these fish species are also found in the Eastern Fram Strait and off the west coast of Spitsbergen65,66. At Eastern Fram Strait, sperm whale presence peaked shortly after the peak in zooplankton concentration. This may indicate that sperm whale prey likely benefitted from the zooplankton blooms. Atlantic Water circulation via the Isfjorden Trough is strongest in the winter56,57, which might increase prey availability in the area at that time and explain the peak in sperm whale presence late in the year. Randelhoff et al.67 demonstrated that “potentially upwelling-favorable” winds north of Svalbard occur from October to April, which could explain sperm whale detections peaking late in the year at Atwain, while there were none in the summer or earlier in the autumn, despite practically ice-free conditions. Sperm whales in the Northern Hemisphere are known to breed between January and August68, which may explain their absence or very low presence in high latitude regions during spring and early summer.
In terms of diel patterns, in the day-night season at both Atwain and Eastern Fram Strait, most detections occurred during twilight (dusk or dawn) and daylight, and a low number of detections occurred during the night. It may be that sperm whales adjust their foraging behavior to diel movements of potential prey species. For example, the Boreoatlantic armhook squid performs diurnal vertical migrations69. It has been shown that sperm whales adjust their foraging strategy to optimize the relationship between prey energetic value and the foraging energy cost at different depths or prey layers48. It may be that the most energetically valuable prey is found in deeper waters and less scattered in the water column vertically during the day, allowing for more efficient foraging. During the Polar Night, there is a weak signal for increased occurrence of sperm whales from 18:00 to 01:00 at Atwain. However, more data would be needed to confirm these suggested patterns. Previous studies have reported contrasting diel patterns or an absence of diel patterns in sperm whale presence globally35,37,70.
Usual clicks, used for navigation and foraging, were the most common call type recorded. Despite a positive bias towards detection of usual clicks over other click types in this study, this result is expected given that the sperm whales are in these high latitude areas for foraging purposes. Buzz clicks, associated with foraging and prey capture attempts71, were detected at similar rates at Isfjorden and Eastern Fram Strait but at a lower rate than the other click types. However, the occurrence of buzz clicks is likely underestimated due to the limited detectability of these highly directional clicks with remote hydrophones30. Male sperm whales are considered to be increasingly solitary with age and it is older males that travel furthest north5. However, given that slow clicks are thought to serve a social, communication function35,36, the prevalence of slow clicks at the study locations even at the northernmost mooring (81° N) suggests that male sperm whales at high latitudes may be more social than previously thought. Indeed, the proximity of males during tagging efforts in the west coast shelf off Spitsbergen suggests that loose social groups do exist, with males clearly being within acoustic range of each other, and not spread randomly (K.M. Kovacs, C. Lydersen, personal observations). This is further supported by observations of overlapping click trains from more than one individual in at least half of the recordings. It is unknown whether food densities alone dictate these groupings, or whether males are social with one another. The relative occurrence of slow clicks in Eastern Fram Strait compared to Isfjorden and Atwain was low, which might indicate that Eastern Fram Strait is a feeding hotspot, and less time is allocated on non-foraging activities, like socializing or communication.
Conclusion
This study provides a multi-year, year-round baseline for sperm whale acoustic presence around the Svalbard Archipelago. Sperm whale presence was detected at most recording sites with the greatest presence at deep, shelf edge areas with low sea ice cover. The highest occurrence rates were found off western Svalbard, where sperm whale vocal activity was detected almost year-round. Even the northernmost location at 81° N demonstrated a consistent, reoccurring acoustic presence. In addition, the occurrence of slow clicks and overlapping vocalizations from multiple individuals supports the idea of loose aggregations of sperm whales rather than solitary behavior in the High Arctic. While some seasonality of detections was demonstrated, future studies should focus more on the drivers of the timing of sperm whale presence. Prey availability might play a role in the timing of their winter departures, but mating or other explanations are also plausible. With the increasing loss of sea ice in the High Arctic, the already documented northward shift of the sperm whales’ geographical range in the Svalbard area is likely to continue as new areas become accessible. The present study provides a baseline for future PAM-monitoring of acoustic presence of sperm whales around Svalbard. Research based on long-term monitoring will be especially crucial to predict cetacean community changes and to advise management authorities given the rapidly changing environment.
Methods
Data collection and deployment locations
This study used underwater passive acoustic data collected at eight different locations around the Svalbard Archipelago (Fig. 1). At each location, an autonomous underwater acoustic recorder was installed on an oceanographic mooring. These moorings were deployed and maintained as part of several long-term monitoring programs72,73,74,75,76,77,78,79. Eastern Fram Strait (EFS) moorings were maintained by the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (Bremerhaven, Germany); at these sites SonoVault recorders (SonoVault, develogic GmbH; RESON TC4037-3 hydrophone with a sensitivity of – 193 dB and a frequency response from 8 Hz to 48 kHz) were used. These moorings were located approximately 25 km apart, but for the data analysis they were considered as one location. At the rest of the moorings, AURALs (AURAL M2, Multi-Électronique Inc.; HTI-96-MIN hydrophone with receiving sensitivity of − 164 ± 1 dB re1 V μPa − 1 and frequency response from 2 Hz to 30 kHz) were used by the Norwegian Polar Institute (Tromsø, Norway). The Atwain mooring was moved after the first two recording years, in 2015, to shallower water, approximately 50 km away from the original location. The Isfjorden mooring was also moved after the first two years, to a site approximately 25 km away from the original location, deeper into the fjord. Despite small interannual differences in exact mooring locations and depths within recording sites, the years for each site were grouped for the analyses, given that this study aimed to investigate distribution of sperm whales first and foremost on a broad scale around Svalbard. The acoustic data reported in this study spans a 10-year period from 2012 to 2021; data was collected for at least two recording periods at each location. Sampling rate, duty cycle and recording period varied slightly between locations and years. The recorders at EFS recorded acoustic data continuously80. For this study, the data was subsampled by taking only the first 10 min of every hour to correspond with the duty cycle-based sampling schemes of the data from the other mooring sites. Due to a recorder software issue at Eastern Svalbard 1, about 70% of the data between February and July 2020 was lost at that location (Appendix Figure A2). Full deployment details and coordinates for each location and year are presented in Table 1.
Detection of sperm whale acoustic presence
Click detection and classification
A combination of automated and manual methods was used to detect sperm whale presence in the acoustic recordings (Appendix Figure B1). First, an automated detector was used to identify recordings containing potential sperm whale vocalizations. Note, that recording durations vary across sites and/or deployment years, see Table 1 for duty cycles. The automated detector setup combined two tools—an open-source bioacoustics software called PAMGuard (Version 2.02.02; available at http://www.pamguard.org)81 in combination with the R statistical program package (Version 4.2.). The first part consisted of processing sound recordings in PAMGuard using frequency band filters and the click detector module including click classifiers. First, a high pass frequency band filter (12th order Butterworth at 1 kHz) was applied to reduce energy at low frequencies, which are typically outside the sperm whale clicks main frequency range. In addition, EFS recordings were filtered (low pass 12th order Butterworth at 16 kHz) to match the frequency range of the other locations’ recordings. Then, a trigger frequency filter for the click detector module was set at 2 kHz threshold (high pass 6th order Butterworth) because the main energy in sperm whale clicks is typically above this threshold. The PAMGuard Basic Click Detector parameters were kept at default values (Appendix Table B3), including the signal-to-noise ratio (10 dB) because they captured sperm whale clicks effectively during initial exploratory analyses. Clicks detected in the Basic Click Detector were then passed through a set of classifiers. PAMGuard click classification works in a hierarchical manner where classes are tested for a click detection starting from the top of the classifiers list until a match is found. Therefore, the first classifiers on the list were defined to capture and discard as many false detections (i.e., sounds from ice, other odontocetes, walruses, and mooring self-noise such as cable strumming) as possible by setting limits for frequency content (peak, width, energy control bands) and signal length. Then two additional classifiers were defined to capture potential sperm whale clicks, one for peak frequency above 4 kHz (“sperm whale high”) and the other one between 1.5 and 6 kHz for longer duration clicks (“sperm whale low”). The detector was not designed to distinguish between click types per se, but the division into two sperm whale classifiers helped to reduce false detections from other low frequency signals such as walrus knocks. For exact classifier settings, see Appendix Table B4. The output from PAMGuard (i.e., detections classified as potential sperm whale clicks) went through a final sorting step to further reduce false detections using a custom-made script in R. Sperm whale clicks are known to occur in sequences of a certain Inter-Click-Interval (ICI)30. Therefore, single clicks or tight click clusters (i.e., click sequences of very low ICI) were unlikely to originate from sperm whales. Consequently, in the last step, click detections were accepted only if they appeared in sufficient numbers within a time window. If 5–50 clicks of the first classifier class (“sperm whale high”) appeared within a 10 s window (corresponding to an ICI between 0.2 and 2 s) or between 3 and 15 detections within a 30 s window (corresponding to an ICI between 2 and 10 s) in the second class (“sperm whale low”), they were accepted as potential sperm whale clicks. The recordings in which these conditions were fulfilled were labeled with “presence”. Note that the detector aimed to identify the presence of sperm whale clicks in the recordings but not to quantify them.
In a post-processing step, all recordings labeled with “presence” were manually (i.e., visually and aurally) assessed by a trained human analyst to confirm sperm whale click presence using Adobe Audition software (Version 22.2.0.61). Recordings that did not contain sperm whale clicks were removed from the results. Sperm whale presence was reported as occurrence rate (i.e. proportion of the total number of recordings per location and year that contained sperm whale vocalizations) as well as daily presence (given as number of hours per day), and hourly presence (indicated by 1 for acoustic presence or 0 for acoustic absence).The presence or absence of different click types was scored in each recording along with the presence (or absence) of overlapping click sequences produced by more than one individual. The presence of click types was reported as the proportion of recordings per each location to contain each click type.
Detector training and validation
The dataset used for detector training consisted of 40 recordings (of variable durations, see duty cycles in Table 1). After manually scanning through hundreds of recordings from the available data, 20 recordings that contained sperm whale clicks were selected and another 20 recordings that contained click-like sounds were chosen that would likely cause false positive detections. The recordings containing sperm whale clicks were additionally labelled with ‘strong’ or ‘faint’ to describe the quality of the clicks present. ‘Faint’ clicks had either a low signal-to-noise ratio, energy on a very narrow frequency range or they were too few (< 3 or < 5 per time-window depending on classifier class) to form a click train. These clicks likely resulted from the vocalizing individual being far away from the hydrophone or ‘off-axis’ (i.e., the animal not oriented towards the hydrophone) which can result in signal transmission loss and/or distortion. ‘Strong’ clicks contained energy on a broader frequency range and were more numerous, likely produced by individuals close to the hydrophone and/or heading to its direction (‘on-axis’). For examples of both categories, see Appendix Figures C7–C15. Systematic trials were performed with the training and test set where the click classifier parameters and the properties of the final sorting step in R were varied for each trial. The detector performance of sperm whale click presence was rated on the ability to label recordings correctly with 1 or 0, presence or absence of sperm whale clicks, respectively. Recall (= the proportion of recordings containing sperm whale clicks successfully detected) and precision (= the proportion of the recordings labelled “presence” actually containing sperm whale vocalizations) were used to decide on the final parameters for detector classifiers (Appendix Figure B2).
A randomly sampled ‘test set’ (1913 recordings or 1% of the data from each site) was used to further evaluate the detector performance, especially to assess the false positive rate (the proportion of recordings without sperm whale clicks labelled as “presence”) which reflected the manual inspection workload ahead. Due to the rare occurrence of sperm whales in this ‘test set’ (32 of 1913 recordings), an additional ‘validation set’ with a higher proportion of recordings containing sperm whale clicks was evaluated for recall. Recordings from two full months with some known sperm whale presence (October 2013 from Atwain and September 2016 from EFS, with a total of 623 of 1464 recordings containing sperm whale presence) were manually inspected and compared to detector results.
Environmental covariates and statistical analyses
Environmental data were retrieved from reanalysis datasets using EU Copernicus Marine Service Information: chlorophyll mass concentration and net primary production of biomass were obtained in 0.25° × 0.25° spatial resolution82; zooplankton mass content expressed as carbon in sea water were obtained in 0.083° × 0.083° spatial resolution83; and sea surface temperature was obtained in 0.05° × 0.05° spatial resolution84. All variables were retrieved as daily means for all locations and recording years in R (packages ncdf485 and raster86). Note that chlorophyll and net primary production data was not available from May 2020 onwards.
Previous studies report varying detection ranges for sperm whale clicks, with most estimates being around 50 km30,37,38. Based on this general estimate, all daily environmental data were averaged over a 50 km radius around each hydrophone mooring site using the coordinates for each deployment period. Daily sea ice data was retrieved from the Norwegian Meteorological Institute87 for each recording site. Daily ice cover for each location was computed using the same principles as in Llobet et al.88. Around each mooring location, 1000 random points were created within a 50 km radius. Each point was then associated with an ice cover category (< 10%, 10–40%, 40–70%, 70–90%, 90–100%, 100%) for each day and the proportion of points in each category was calculated. For the statistical models, a single variable was used to describe the sea ice cover, calculated as the proportion of the 50 km area that is covered with sea ice (any category > 10%). Sea floor elevation data came from the International Bathymetric Chart of the Arctic Ocean89 in 200 mm × 200 mm grid cell spacing. Sea floor depth, slope and terrain heterogeneity (Terrain Ruggedness Index) were computed for each mooring location and averaged over a 50 km radius area in R (package raster86). The daily acoustic presence data was examined using a generalized linear model with a binomial distribution and logit-link function (package glmmTMB in R) to evaluate potential effects of daily concentrations of chlorophyll a, zooplankton, net primary production, sea surface temperature and sea ice extent on daily sperm whale presence. To correct for temporal autocorrelation, an ar1-correlation structure was included in the models. Additionally, the sea ice coverage was compared between locations and times with sperm whale acoustic presence and absence using a non-parametric Wilcoxon test. All environmental covariates and ice cover were plotted against sperm whale presence for visual examination for each location.
Light regime and diel variations
Potential diel patterns in acoustic presence were also explored visually. Because of the very high latitude location of the recording sites, where sun exposure varies seasonally, detections were first divided into light regime categories by date: polar day (length of the day = 24 h, solar elevation is higher than 0° over the 24-h day), polar night (length of the day 0 h, solar elevation is lower than 0° over the 24-h day) and day-night season (length of day > 0 and < 24 h). The hourly distribution of acoustic presence in these three diel categories were compared. Then, in the day-night season category, every detection was associated with a sun exposure category: day (solar elevation higher than 0° at the start of the hour), twilight (solar elevation between 0° and − 24°) or night (solar elevation below − 24°).
Data availability
Acoustic spectrograms depicting sperm whale vocalizations can be found in the Supplementary Material, accompanied by recordings (.wav-files) for each spectrogram. The sperm whale presence data and environmental data used in analyses and figures are deposited in the Norwegian Polar Data Centre: doi: https://doi.org/10.21334/npolar.2023.1cc4e28b. The acoustic data from the Eastern Fram Strait recorder (2016–2017 deployment period) is available at doi:https://doi.org/10.1594/PANGAEA.945404 (Thomisch et al.80) via the data repository PANGAEA.
References
Taylor, B. L. et al. Physeter macrocephalus (amended version of 2008 assessment). IUCN Red List Threatened Species 2019, e.T41755A160983555. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T41755A160983555.en (2019).
Whitehead, H. & Shin, M. Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci. Rep. 12, 19468 (2022).
Engelhaupt, D. et al. Female philopatry in coastal basins and male dispersion across the North Atlantic in a highly mobile marine species, the sperm whale (Physeter macrocephalus). Mol. Ecol. 18, 4193–4205 (2009).
Lettevall, E. et al. Social structure and residency in aggregations of male sperm whales. Can. J. Zool. 80, 1189–1196 (2002).
Whitehead, H. Sperm whale: Physeter microcephalus. In Encyclopedia of Marine Mammals (eds Würsig, B. et al.) 919–925 (Elsevier Inc, 2018).
Posdaljian, N. et al. Changes in sea ice and range expansion of sperm whales in the eclipse sound region of Baffin Bay, Canada. Glob. Change Biol. 28, 3860–3870 (2022).
Seger, K. D. & Miksis-Olds, J. L. A decade of marine mammal acoustical presence and habitat preference in the Bering Sea. Polar Biol. 43, 1549–1569 (2020).
Mellinger, D. K., Stafford, K. M. & Fox, C. G. Seasonal occurrence of sperm whale (Physeter macrocephalus) in the Gulf of Alaska, 1999–2001. Mar. Mammal Sci. 20, 48–62 (2004).
Rice, A. et al. Cetacean occurrence in the Gulf of Alaska from long-term passive acoustic monitoring. Mar. Biol. 168, 72 (2021).
Barlow, J. & Taylor, B. L. Estimates of sperm whale abundance in the northeastern temperate Pacific from a combined acoustic and visual survey. Mar. Mammal Sci. 21, 429–445 (2005).
Frouin-Mouy, H., Kowarski, K., Martin, B. & Bröker, K. Seasonal trends in acoustic detection of marine mammals in Baffin Bay and Melville Bay, Northwest Greenland. Arctic 70, 59–76 (2017).
Davidson, E. R., Ferguson, S. H., Higdon, J. W. & Treble, M. A. Opportunistic sightings from fisheries surveys inform habitat suitability for northern bottlenose whales Hyperoodon ampullatus and sperm whales Physeter macrocephalus in Baffin Bay and Davis Strait, Canadian Arctic. Mar. Ecol. Prog. Ser. 723, 57–71 (2023).
Christensen, I., Haug, T. & Oien, N. Seasonal distribution, exploitation and present abundance of stocks of large baleen whales (Mysticeti) and sperm whales (Physeter macrocephalus) in Norwegian and adjacent waters. ICES J. mar. Sci. 49, 341–355 (1992).
Klinck, H. et al. Seasonal presence of cetaceans and ambient noise levels in polar waters of the North Atlantic. J. Acoust. Soc. Am. 132, 176–181 (2012).
Kovacs, K.M., Lydersen, C., Stafford, K. & Wiig, Ø. Passive tools for monitoring endangered species in Svalbard: Monitoring the distribution and relative abundance of Red Listed whales (Project 12/29). Svalbards Miljøvernfond. https://www.miljovernfondet.no/wp-content/uploads/2020/02/12-29-passive-tools-for-monitoring-endangered-spesies-in-svalbard.pdf (2015).
Leonard, D. & Øien, N. Estimated abundances of Cetacean species in the Northeast Atlantic from Norwegian Shipboard surveys conducted in 2014–2018. NAMMCO Sci. Publ. https://doi.org/10.7557/3.4694 (2020).
Storrie, L., Lydersen, C., Andersen, M., Wynn, R. B. & Kovacs, K. M. Determining the species assemblage and habitat use of cetaceans in the Svalbard Archipelago, based on observations from 2002 to 2014. Polar Res. 37, 2684 (2018).
Walczowski, W. & Piechura, J. Influence of the West Spitsbergen Current on the local climate. Int. J. Climatol. 31, 1088–1093 (2011).
Bengtsson, O., Lydersen, C. & Kovacs, K. M. Cetacean spatial trends from 2005 to 2019 in Svalbard, Norway. Polar Res. 41, 7773 (2022).
Popov, I. & Eichhorn, G. Occurrence of sperm whale (Physeter macrocephalus) in the Russian Arctic. Polar Res. 39, 4583 (2020).
Barlow, J., & Sexton, S. The effect of diving and searching behavior on the probability of detecting track-line groups, go, of long-diving whales during linetransect surveys. NOAA National Marine Fisheries Service, Southwest Fisheries Center Administrative Report, LJ-96-14 (1996).
Lefort, K. J., Hussey, N. E., Jones, J. M., Johnson, K. F. & Ferguson, S. H. Satellite-tracked sperm whale migrates from the Canadian Arctic to the subtropical western North Atlantic. Mar. Mamm. Sci. 8, 1242–1248 (2022).
Winsor, M. H., Irvine, L. M. & Mate, B. R. Analysis of the spatial distribution of satellite-tagged sperm whales (Physeter macrocephalus) in close proximity to seismic surveys in the Gulf of Mexico. Aquat. Mam. 43(4), 439–446 (2017).
Stanistreet, J. E. et al. Changes in the acoustic activity of beaked whales and sperm whales recorded during a naval training exercise off eastern Canada. Sci. Rep. 12, 1973 (2022).
Miller, P. J. O., Johnson, M. P. & Tyack, P. L. Sperm whale behaviour indicates the use of echolocation click buzzes “creaks” in prey capture. Proc. R. Soc. Lond. B. 271, 2239–2247 (2004).
Watwood, S. L., Miller, P. J. O., Johnson, M., Madsen, P. T. & Tyack, P. L. Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus). J. Anim. Ecol. 75, 814–825 (2006).
Madsen, P. T. et al. Sperm whale sound production studied with ultrasound time/depth-recording tags. J. Exp. Biol. 205, 9–47 (2002).
Møhl, B., Wahlberg, M., Madsen, P. T., Miller, L. A. & Surlykke, A. Sperm whale clicks: Directionality and source level revisited. J. Acoust. Soc. Am. 107, 638–648 (2000).
Oliveira, C. et al. Sperm whale codas may encode individuality as well as clan identity. J. Acoust. Soc. Am. 139, 2860–2869 (2016).
Madsen, P. T., Wahlberg, M. & Møhl, B. Male sperm whale (Physeter macrocephalus) acoustics in a high-latitude habitat: Implications for echolocation and communication. Behav. Ecol. Sociobiol. 53, 31–41 (2002).
Rendell, L. E. & Whitehead, H. Vocal clans in sperm whales (Physeter macrocephalus). Proc. R. Soc. Lond. B. 270, 225–231 (2003).
Oliveira, C., Wahlberg, M., Johnson, M., Miller, P. J. O. & Madsen, P. T. The function of male sperm whale slow clicks in a high latitude habitat: Communication, echolocation, or prey debilitation?. J. Acoust. Soc. Am. 133, 3135–3144 (2013).
Barlow, J. & Taylor, B. L. Estimates of sperm whale abundance in the northeastern temperate Pacific from a combined acoustic and visual survey. Mar. Mamm. Sci. 21, 429–445 (2005).
Shabangu, F. W. & Andrew, R. K. Clicking throughout the year: Sperm whale clicks in relation to environmental conditions off the west coast of South Africa. Endang. Species Res. 43, 475–494 (2020).
Miller, B. S. & Miller, E. J. The seasonal occupancy and diel behaviour of Antarctic sperm whales revealed by acoustic monitoring. Sci. Rep. 8, 5429 (2018).
Stanistreet, J. E. et al. Spatial and seasonal patterns in acoustic detections of sperm whales Physeter macrocephalus along the continental slope in the western North Atlantic Ocean. Endang. Species Res. 35, 1–13 (2018).
Otsuki, M., Akamatsu, T., Nobetsu, T. & Mitani, Y. Seasonal and diel changes in cetacean vocalizations monitored by passive acoustic methods in Nemuro Strait adjacent to the Shiretoko World Natural Heritage Site. Mar. Mamm. Sci. 37, 1330–1340 (2021).
Kinda, G., Simard, Y., Gervaise, C., Mars, J. I. & Fortier, L. Under-ice ambient noise in Eastern Beaufort Sea, Canadian Arctic, and its relation to environmental forcing. J. Acoust. Soc. Am. 134, 77–87 (2013).
Ladegaard, M. et al. Soundscape and ambient noise levels of the Arctic waters around Greenland. Sci. Rep. 11, 23360 (2021).
Sanjana, M. C., Latha, G., Thirunavukkarasu, A. & Venkatesan, R. Ambient noise field and propagation in an Arctic fjord Kongsfjorden, Svalbard. Polar Sci. 17, 40–49 (2018).
Menze, S., Zitterbart, D. P., van Opzeeland, I. & Boebel, O. The influence of sea ice, wind speed and marine mammals on Southern Ocean ambient sound. R. Soc. Open Sci. 4, 160370 (2017).
Øien, N. Distribution and abundance of large whales in Norwegian and adjacent waters based on ship surveys 1995–2001. NAMMCO Sci. Publ. 7, 31–47 (2009).
Ahonen, H., Stafford, K. M., Lydersen, C., de Steur, L. & Kovacs, K. M. A multi-year study of narwhal occurrence in the Western Fram Strait—detected via passive acoustic monitoring. Polar Res. 38, 3468 (2019).
Thomisch, K. et al. Acoustic presence and vocal repertoire of bowhead whales (Balaena mysticetus) in eastern and central Fram Strait. Front. Remote Sens. 3, 907105 (2022).
Skern-Mauritzen, M. et al. Marine mammal consumption and fisheries removals in the Nordic and Barents Sea. ICES J. Mar. Sci. 79, 1583–1603 (2022).
Kiszka, J., Macleod, K., Van Canneyt, O., Walker, D. & Ridoux, V. Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data. ICES J. Mar. Sci. 64, 1033–1043 (2007).
Rogan, E. et al. Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep-Sea Res. Part II(141), 8–19 (2017).
Fais, A. et al. Sperm whale echolocation behaviour reveals a directed, prior-based search strategy informed by prey distribution. Behav. Ecol. Sociobiol. 69, 663–674 (2015).
Isojunno, S. & Miller, P. J. O. Movement and biosonar behavior during prey encounters indicate that male sperm whales switch foraging strategy with depth. Front. Ecol. Evol. 6, 200 (2018).
Pedersen, G., Storheim, E., Doksæter, L. S., Godø, O. R. & Ødegaard, L. A. Concurrent passive and active acoustic observations of high-latitude shallow foraging sperm whales and mesopelagic prey layer. Proc. Mtgs. Acoust. 30, 010004 (2017).
Hattermann, T., Isachsen, P. E., von Appen, W.-J., Albretsen, J. & Sundfjord, A. Eddy-drive recirculation of Atlantic Water in Fram Strait. Geophys. Res. Lett. 43, 3406–3414 (2016).
Diogou, N. et al. Sperm whale (Physeter macrocephalus) acoustic ecology at Ocean Station PAPA in the Gulf of Alaska—Part 2: Oceanographic drivers of interannual variability. Deep-Sea Res. Part I. 150, 103044 (2019).
Wong, S. N. P. & Whitehead, H. Seasonal occurrence of sperm whales (Physeter macrocephalus) around Kelvin Seamount in the Sargasso Sea in relation to oceanographic processes. Deep-Sea Res. Part I(91), 10–16 (2014).
Lundesgaard, Ø., Sundfjord, A. & Renner, A. H. H. Drivers of interannual sea ice concentration variability in the Atlantic water inflow region North of Svalbard. JGR Oceans. 126, e2020JC016522 (2021).
El-Gabbas, A., Thomisch, K., Van Opzeeland, I., Burkhardt, E. & Boebel, O. Dynamic species distribution models of Antarctic blue whales in the Weddell Sea using visual sighting and passive acoustic monitoring data. Divers. Distrib. https://doi.org/10.1111/ddi.13790 (2023).
Nilsen, F., Skogseth, R., Vaardal-Lunde, J. & Inall, M. A simple shelf circulation model: Intrusion of Atlantic water on the West Spitsbergen Shelf. J. Phys. Oceanogr. 46, 1209–1230 (2016).
Menze, S. et al. Productive detours—Atlantic water inflow and acoustic backscatter in the major troughs along the Svalbard shelf. Prog. Oceanogr. 188, 102447 (2020).
Llobet, S. M., Ahonen, H., Lydersen, C. & Kovacs, K. M. The Arctic and the future Arctic? Soundscapes and marine mammal communities on the east and west sides of Svalbard characterized through acoustic data. Front. Mar. Sci. 10, 1208049 (2023).
Pierce, J. P., Ward, N., Brownlow, A. & Santos, B. S. Analysis of historical and recent diet and strandings of sperm whales (Physeter macrocephalus) in the North Sea. Lutra 61, 71–86 (2018).
Similä, T., Haug, T., Lindblom, L., Lockyer, C. & O’Callaghan, S. A. Stomach Contents of three sperm whales (Physeter macrocephalus) stranded on Andøya, Northern Norway. Aquat. Mamm. 48, 449–455 (2022).
Golikov, A. V., Sabirov, R. M. & Lubin, P. A. First assessment of biomass and abundance of cephalopods Rossia palpebrosa and Gonatus fabricii in the Barents Sea. J. Mar. Biol. Assoc. 97, 1605–1616 (2017).
Gardiner, K. & Dick, T. A. Arctic cephalopod distributions and their associated predators. Polar Res. 29, 209–227 (2010).
MacKenzie, K. M. et al. Niches of marine mammals in the European Arctic. Ecol. Indic. 136, 108661 (2022).
Martin, A. R. & Clarke, M. R. The diet of sperm whales (Physeter macrocephalus) captured between Iceland and Greenland. J. Mar. Biol. Assoc. 66, 779–790 (1986).
Eriksen, E., Durif, C. M. F. & Prozorkevich, D. Lumpfish (Cyclopterus lumpus) in the Barents Sea: Development of biomass and abundance indices, and spatial distribution. ICES J. Mar. Sci. 71, 2398–2402 (2014).
Ingvaldsen, R. B., Gjøsæter, H., Ona, E. & Michalsen, K. Atlantic cod (Gadus morhua) feeding over deep water in the High Arctic. Polar Biol. 40, 2105–2111 (2017).
Randelhoff, A. et al. Pan-Arctic ocean primary production constrained by turbulent nitrate fluxes. Front. Mar. Sci. 7, 150 (2020).
Rice, D. W. Sperm whale Physeter macrocephalus Linnaeus, 1758. In Handbook of Marine Mammals (eds Ridgway, S. H. & Harrison, R.) 177–233 (Academic Press, 1989).
Golikov, A. V. et al. Life history of the Arctic Squid Gonatus fabricii (Cephalopoda: Oegopsida) Reconstructed by analysis of individual ontogenetic stable isotopic trajectories. Animals 12, 3548 (2022).
Giorli, G. & Goetz, K. T. Foraging activity of sperm whales (Physeter macrocephalus) off the east coast of New Zealand. Sci. Rep. 9, 12182 (2019).
Fais, A., Johnson, M., Wilson, M., Aguilar Soto, N. & Madsen, P. T. Sperm whale predator-prey interactions involve chasing and buzzing, but no acoustic stunning. Sci. Rep. 6, 28562 (2016).
KROP: The Konsfjorden and Rijpfjorden Observatory Program. https://www.mare-incognitum.no/krop/.
Skogseth, R. & Ellingsen, P. G. Mooring Data from the Isfjorden Mouth—North (I-N) During 5 Oct 2017 to 24 Aug 2018 (Norwegian Polar Institute, 2019). https://doi.org/10.21334/npolar.2019.e9106051.
Skogseth, R. & PålGunnar, P. G. Mooring Data from the Isfjorden Mouth–North (I–N) During 29 Aug 2018 to 06 Sep 2019 (Norwegian Polar Institute, 2022). https://doi.org/10.21334/npolar.2022.e13b8ab0.
Skogseth, R. & Ellingsen, P. G. Mooring Data from Inside the Isfjorden Entrance-South (I-SE) During 08 Sep 2019 to 08 Jun 2020 (Norwegian Polar Institute, 2022). https://doi.org/10.21334/npolar.2022.249e9aa6.
Skogseth, R. et al. Variability and decadal trends in the Isfjorden (Svalbard) ocean climate and circulation—an indicator for climate change in the European Arctic. Prog. Oceanogr. 187, 102394. https://doi.org/10.1016/j.pocean.2020.102394 (2020).
A-TWAIN (Long-term variability and trends in the Atlantic Water inflow region) program. https://www.npolar.no/en/projects/a-twain/.
The Nansen Legacy. https://arvenetternansen.com/nansen-legacy-oceanographic-moorings/.
Soltwedel, T., Schauer, U., Boebel, O., Nöthig, E. M., Bracher, A. , Metfies, K., Schewe, I. , Klages, M., & Boetius, A. FRAM—FRontiers in Arctic marine Monitoring: Permanent Observations in a Gateway to the Arctic Ocean , OCEANS-Bergen, 2013 MTS/IEEE. https://doi.org/10.1109/OCEANS-Bergen.2013.6608008 (2013).
Thomisch, K., Spiesecke, S. & Boebel, O. Passive acoustic monitoring data recorded by recorder SV1088 at mooring F5-17 in Fram Strait in 2016/2017. PANGAEA. https://doi.org/10.1594/PANGAEA.956286 (2023).
Gillespie, D. et al. PAMGUARD: Semiautomated, open-source software for real-time acoustic detection and localization of cetaceans. Proc. Inst. Acoust. 30, 54–62 (2008).
Global Ocean Biogeochemistry Hindcast. E.U. Copernicus Marine Service Information (CMEMS). Marine Data Store (MDS). https://doi.org/10.48670/moi-00019.
Global ocean low and mid trophic levels biomass content hindcast. E.U. Copernicus Marine Service Information (CMEMS). Marine Data Store (MDS). https://doi.org/10.48670/moi-00020.
Good, S. et al. The current configuration of the OSTIA system for operational production of foundation sea surface temperature and ice concentration analyses. Remote Sens. 12, 720. https://doi.org/10.3390/rs12040720 (2020).
Pierce, D. ncdf4: Interface to Unidata netCDF. R-package version 1.21. https://cran.r-project.org/package=ncdf4 (2023).
Hijmans, R. raster: Geographic Data Analysis and Modeling. R package version 3.6-26. https://cran.r-project.org/package=raster (2023).
Norwegian Meteorological Institute. https://cryo.met.no/.
Llobet, S. M. et al. Bearded seal (Erignathus barbatus) vocalizations across seasons and habitat types in Svalbard, Norway. Polar Biol. 44, 1273–1287 (2021).
Jakobsson, M. et al. The International Bathymetric Chart of the Arctic Ocean Version 4.0. Sci. Data 7, 176. https://doi.org/10.1038/s41597-020-0520-9 (2020).
Acknowledgements
This study was funded by the Fram Centre MIKON program, the Norwegian Research Council (ICE-whales Grant no. 244488/E10 and NRC-ARK project no. 313678), Svalbard Environmental Protection Fund, Svalbard Science Forum, Arctic 2030 (Moby Dick), the Helmholtz strategic infrastructure FRAM and the Norwegian Polar Institute. Mooring services on the east side of Svalbard were operated under the NRC Arven etter Nansen project infrastructure, the Kongsfjorden mooring was serviced by SIOS and ATWAIN was supported by the Sea ice in the Arctic Ocean, technology and governance, Fram Centre Flagship Program (Project No. 66050). This project was also made possible by the European Union Erasmus traineeship Grant award to Viivi Pöyhönen (MSc student at Lund University). Many thanks to the logistics departments and crews of the Norwegian Polar Institute, the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, the Arctic University of Norway (UiT) and the University Centre in Svalbard (UNIS) for their contributions to the deployment and recovery of the passive acoustic recording devices. The authors also thank Andrew Lowther and Ulf Lindstrøm for their help with statistical analyses. Thanks also go to Marie-Anne Blanchet, Samuel Martinez Llobet and Sebastian Menze for advice regarding environmental data and to Sofia Aniceto, Luca Tassara, Brian Miller and Claudia Oliveira for advice regarding detector methodology and sperm whale vocalizations.
Funding
Open access funding provided by Norwegian Polar Institute.
Author information
Authors and Affiliations
Contributions
Data collection was facilitated by K.M.K., C.L., H.A. and K.T., H.A conceptualized the study and H.A., K.T., K.M.K. and C.L. supervised the project. V.P. developed the methodology and processed and analyzed the data with guidance from H.A. and K.T. V.P. drafted the manuscript and all authors participated in the interpretation of results and manuscript writing. All authors have reviewed and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pöyhönen, V., Thomisch, K., Kovacs, K.M. et al. High Arctic “hotspots” for sperm whales (Physeter macrocephalus) off western and northern Svalbard, Norway, revealed by multi-year Passive Acoustic Monitoring (PAM). Sci Rep 14, 5825 (2024). https://doi.org/10.1038/s41598-024-56287-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56287-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.