Abstract
To present our experience with laparoscopic ureteroneocystostomy with bladder flap (LUCBF) for treating benign ureteral stenosis and evaluate its feasibility and efficacy. The clinical data of 27 patients with benign ureteral stenosis who underwent LUCBF were retrospectively analyzed. After identification and excision of the ureteral stenosis segment, the healthy ureteral stump was dissected and incised longitudinally. A U-shaped or spiral bladder flap was harvested from the anterolateral bladder wall for ureteroplasty. All patients underwent LUCBF successfully, including 14 patients were combined with psoas hitch technique, between 90 and 220 min (median, 155 min). The median length of ureteral defect was 6 cm (range, 5–17 cm). The median blood loss was 40 ml (20–150 ml). The median indwelling time of double-J stent was 8 weeks (range, 4–8 weeks). Five patients (10.6%) suffered postoperative complications during the follow-up period (range, 12–48 months), including fever, hematuria, urinary tract infection and recurrent stenosis. The success rate was 96.3% (26/27). Patients with long ureter defects had longer operative time and more blood loss than short ureter defects. LUCBF was a safe and feasible technique for benign ureteral stenosis. Long ureter defect was related to longer operative time and more blood loss.
Similar content being viewed by others
Introduction
Iatrogenic injury is the most common causative factor of ureteral stenosis, and other factors include ureteral calculi, radiological damage, and ureteral mass1. The distal ureter is most susceptible to damage, followed by the middle and proximal segments2. The choice of therapeutic strategy for ureteral stenosis is based on the stenosis site, length of the ureter lesion, patient status, and surgeon preference.
Endoscopic procedures are more commonly used for short ureteral strictures; however, for long and complex stenosis, ureteral reconstruction surgery is strongly recommended3. For distal ureter stenosis, effective definitive reconstructive methods, including ureteroneocystostomy, psoas hitch (PH) technique and Boari bladder flap, are performed4. For the stenosis located at the middle or proximal ureter, transureteroureterostomy or pyeloplasty is applied for short stenosis with a good success rate. However, this approach is inappropriate for patients with ureteral avulsion or long stenosis at middle or proximal ureter. Boari flap constriction, bowel interposition or renal autotransplantation are suggested5.
In our study, we presented our experience with laparoscopic ureteroneocystostomy with bladder flap (LUCBF) for ureteral stenosis caused by benign ureteral lesions, and summarized the long-term outcomes.
Materials and methods
Data collection
The medical records of patients with unilateral ureteral stenosis caused by benign ureteral lesions who underwent LUCBF at the First Affiliated Hospital of Gannan Medical University between July 2019 and July 2022 were retrospectively reviewed. The exclusion criteria were as follows: (a) combined with urinary carcinoma; (b) open reconstructive surgery; (c) previous urinary diversion surgery, including cutaneous ureterostomy, ileal conduit, or neobladder after radical cystectomy. Finally, 27 patients were included, including 14 patients underwent LUCBF combined with PH technique. The surgeries were performed by a single senior surgeon with rich experience in urologic reconstruction and expertise in laparoscopic manipulations.
Preoperatively, various radiological examinations encompassing urinary ultrasound, intravenous urography (IVU), retrograde pyelogram (RGP), computed tomography (CT) urography or magnetic resonance urography (MRU) were used to assess the degree of hydronephrosis, the location and the length of ureteral stenosis. Routine urinalysis and urine culture were performed and appropriate antibiotics were administered preoperatively. Routine blood and serum tests were performed. Preoperative demographic characteristics including gender, age, body mass index, preoperative symptoms, operative side, location of the ureteral lesion, etiology of ureteral stenosis, and hydronephrosis were obtained from the medical records.
Ethics approval
Ethical approval for the study protocol was obtained from the Ethical Committee of the First Affiliated Hospital of Gannan Medical University (proof number: 2023032702), and the study was performed in accordance with the Declaration of Helsinki (as revised in 2013).
Informed consent
Written informed consent was obtained from all participants included in the study.
Surgical techniques
All patients were explicated with all alternative therapeutic strategies, and informed consent were obtained before the operation. After general anesthesia, the patient was placed in the supine position with the affected side was elevated at 30°-45°. A 20 Fr three-way Foley urethral catheter was inserted before the pneumoperitoneum was established. A 10 mm trocar was placed near the umbilicus for camera access, a 5 mm trocar was placed below the umbilicus along the midclavicular line on the contralateral affected side, and a 10 mm trocar was placed in the lower abdomen. For upper ureter stenosis, another 10 mm trocar was located on the affected side.
The colon was freed along the Toldt line to expose the retroperitoneal space. The ureter was identified at the crossing point with the ipsilateral common iliac artery. For ureter lesion was located above the iliac artery, the ipsilateral renal pelvis and renal lower pole were first separated as anatomical markers to identify the proximal ureter. The ureter was then freed anterogradely until reaching the ureteral stenosis segment. Careful ureterolysis was needed to avoid the devascularization of ureter and to protect healthy ureteral tissue. The ureter proximal to the stenosis segment was transected and dissected with scissors until the healthy pink ureteral mucosa was exposed. The posterior wall of the ureter was incised longitudinally to 2.0 cm to prepare for anastomosis.
The bladder was visually distended after it was filled with 300 mL of normal saline (0.9%). The anterior peritoneum overlying the bladder and the anterior and ipsilateral walls of the bladder were adequately mobilized. The length of the ureteral defect was measured intraoperatively from the distal end of the healthy ureter to the bladder. A U-shaped or spiral bladder flap with a width of 4–5 cm at the base and 2–3 cm at the tip was designed on the anterolateral bladder wall. The length of the flap was designed to be 2.0 cm longer than the ureteral defect. The length/width ratio was 3–4:1. The base of the flap was located at the dome and the tip was near the bladder neck. For long ureteral defect, especially for proximal or middle ureteral stenosis, the spiral bladder flap was harvested. The flap was harvested from the anterior wall of the contralateral bladder to the lateral wall, and then to the parietal wall, ending at the ipsilateral posterior wall. The base of the flap extended up to 6–8 cm and the PH technique was used, the flap was sutured to the psoas fascia. The entire thickness of the detrusor muscle of the posterior bladder flap was fixed to the psoas muscle.
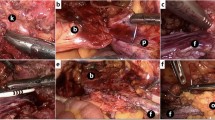
Tension-free anastomosis of the ureter posterior wall and apex of the flap was performed using a full-thickness style with 4–0 absorbable sutures in an interrupted fashion, and the suture needles were 1/2 circular, tapered and 17 mm long. After a 7 Fr/26 cm double J stent was inserted across the ureter-flap anastomosis site, a complete circumferential anastomosis was performed between the lateral edge of the flap and the ureteral margin using discontinuous sutures. The residual flap was tubularized with continuous 4–0 absorbable sutures. The bladder incision was closed with continuous 2–0 or 3–0 absorbable sutures, and both the suture needles were 1/2 circular, tapered and 26 mm long. Normal saline was reinjected into the bladder to check for anastomotic leakage. In patients with ureterovaginal fistulas, fistula dissection and vaginal stratified suturing were performed. Adjacent well-vascularized pedicled omentum was harvested to wrap around the ureterovesical anastomosis and tubularized flap in a tension-free condition, also for vaginal anastomosis if present. A 20 Fr drainage tube was inserted and the previous urethral catheter continued to indwell in bladder. The details of the surgical procedure are shown in Fig. 1a–i.
Surgical procedures. (a) separated the ureter; (b) posterior wall of ureter was incised longitudinally; (c) bladder flap was harvested; (d) anastomosis of ureter posterior wall and apex of flap; (e) a 7 Fr/26 cm double J stent was inserted; (f) tubularized flap; (g) bladder incision was closed; (h) pedicled omentum wrapped around anastomosis; (i) trocars placement.
Postoperative management and follow-up
The drainage tube was removed when the total volume was ≤ 20 ml/d. The urethral catheter was removed at 14 days after surgery. The double J stent was removed at 4–8 weeks after surgery. During the follow-up period, patients were recommended to be reassessed at 3, 6, and 12 months postoperatively and then annually. The protocols included clinical examination, laboratory examination and appropriate imaging studies including ultrasonography at each return visit, IVU or CT or MRU were performed at 3, 6, 12 months after surgery and yearly thereafter. Surgical success was defined as improvement or stabilization of hydronephrosis, relief of symptoms, and no recurrence of ureter stenosis.
Statistical analysis
Statistical analysis was executed using SPSS 21.0 Statistics (IBM, Armonk, NY). The median and range were used for the quantitative variables, and the Mann–Whitney U test was used to compare groups. Qualitative variables were expressed as percentages (%) or numbers(n), and differences between groups were analyzed using the Chi-squared test or Fisher's exact test. A two-tailed P value less than 0.05 was considered as an indicator of statistical significance.
Results
The demographic characteristics and preoperative data are summarized in Table 1. A total of 27 patients—10 male patients and 17 female patients —had a median age of 46 years (range, 21–81 years). Of these patients, 16 patients had a left-sided ureter defect and 11 patients had a defect on the right side. Flank and/or abdominal pain were the most common symptoms, followed by no clinical symptoms, vaginal leakage, fever, or hematuria. Nine patients suffered stenosis secondary to ureteral holmium laser lithotripsy, and six patients were diagnosed with ureterovaginal fistula after hysterectomy. There were two patients with previous ureterovesical reimplantation and one patient with previous ureteroureterostomy who experienced recurrence of ureteral stenosis. Ureteral injury occurred during ureteroscopy in three patients, two of whom had ureteral avulsion. Non-iatrogenic causes included ureteral polyps in three patients and impacted calculi in one patient. Preoperatively, six patients underwent preoperative ureteral stent placement, and two patients underwent percutaneous nephrostomy.
All surgical procedures were successfully completed without intraoperative complications, and none were converted to open surgery or other reconstructive treatments. All patients underwent LUCBF, including 14 patients who underwent surgery in combination with the PH technique during surgery. The median length of the ureter defects was 6 cm (range, 5–17 cm) and the duration of surgery ranged from 90 to 220 min (median, 155 min). The median blood loss was 40 ml (20–150 ml) and none of the patients required blood transfusion.
The drainage tube was removed at 2–4 days (median, 3 days) postoperatively, and the median postoperative hospitalization was 7 days (range, 5–9 days). All patients were discharged with a urethral catheter and the catheter was removed at 14 days after surgery. The median indwelling time of the double-J stent was 8 weeks (range, 4–8 weeks). The clinical outcomes are presented in Table 2.
The postoperative complications were classified by the Clavien-Dindo grade system6. A total of five patients suffered postoperative complications. Two patients suffered from fever (grade I) and were treated with antipyretics. Hematuria (grade II) was observed in one patient who had long-segment ureter defect and was cured with hemostatic. Urinary tract infection required antibiotics (Grade II) was seen in one patient. In one patient with proximal ureteral avulsion, renal colic and nausea occurred after removing the double-J tube at 8 weeks postoperatively, then ureteroscopy was performed, anastomotic edema and short stenosis (Grade III) were seen. Two 7 F double-J stents were inserted and successfully removed 6 months later.
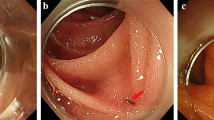
All 27 patients underwent physical, laboratory and radiological examinations at 3, 6 and 12 months after surgery (Fig. 2a,b). The median follow-up time was 24 months (range, 12–48 months). Except for the patient described above, no other patient experienced recurrence of ureteral stenosis during this period. Improvement of hydronephrosis were seen in 25 (92.6%) patients, the remaining two patients had hydronephrosis levels comparable to preoperative status, but radiological results indicated good ureteral patency. Preoperative flank or abdominal pain was relieved and other preoperative symptoms disappeared in all patients.
Patients were divided into two groups according to ureteral lesion location, including the distal ureter group (Group A) and the proximal or middle ureter group (Group B). Group B had longer ureteral defect length than that of Group A. The age, gender and operative side of the two groups were comparable and without significant differences. Group B had a longer operative time (177.5 min vs. 145.0 min; P = 0.002) and more blood loss (50 ml vs. 40 ml; P = 0.018) than Group A. No significant difference was observed in postoperative hospitalization and total complication rates between the two groups. The final success rate was 100% in both groups (Table 3).
Discussion
The ureter is well protected in the retroperitoneal space and is not susceptible to damage. Iatrogenic factor is the most common etiology of ureteral injury, and gynecological surgeries account for the largest proportion7, followed by urological procedures8. However, with the increasing popularity of endoscopic procedure and laparoscopic surgery, the proportional distribution of iatrogenic factors has changed9. In our study, urological surgery was the most common cause, followed by gynecological surgery. The majority of urological etiology was ureteral holmium laser lithotripsy, and laparoscopic hysterectomy was the most common gynecological etiology. Our result was similar to the previous study9.
The time to diagnosis of ureteral injury is an important factor for therapeutic options. However, 50–70% of ureteral injuries failed to be clarified intraoperatively in time10. Extensive ureteral perforation or avulsion can be detected during endoscopic or laparoscopic surgery, and immediate surgical repair is available2, however, minor perforation, mucosal abrasion and non-transversal injury are often ignored or undiscovered. Symptoms resulting from ureteral injuries include flank or abdominal pain, fever, hematuria, and urinary leakage11. The occurrence of these manifestations indicates the possibility of ureteral injury. Delayed diagnosis of ureteral injury may lead to ureteral stenosis or atresia, ureteric dilatation, hydronephrosis, urinary tract infection, and even progressive impairment of renal function12, which may need further intervention.
Currently, the ideal time for reconstructive surgery remains controversial13. Ambani et al. compared early (≤ 7 days) repair with delayed (> 7 days) repair of the injured ureter, they found no difference in outcomes between the two groups13. El-Abd et al. summarized their experience with immediate and late management of ureteral injuries, they found better long-term outcomes in the immediate management group14. A retrospective study of 12 patients with ureteric injuries during gynecologic surgery conducted by Han et al. showed that early recognition and management could prevent further morbidity15. Lee et al. demonstrated that ureteral rest, defined as no hardware crossing the ureter stenosis for ≥ 4 weeks, was associated with a higher reconstruction success rate and less blood loss16. In our study, patients underwent reconstructive surgery once the diagnosis was clear, rather than upon further waiting. For patients who were inappropriate for immediate surgery, a percutaneous nephrostomy tube was highly recommended. In addition to draining renal urine and protecting renal function, the more important role of the nephrostomy tube was to maintain ureteral rest and facilitate the identification of the stenotic segment during surgery.
In addition to the etiology and time of diagnosis, treatment protocols are related to other factors such as the characteristics of injury, the location of injured ureter, the patient status, and surgeon experience17. Compared to the proximal and middle ureter, the distal ureter is more susceptible to damage1,15,17. The distal ureter was also the most common injury site in our study. Endoscopic surgery is suggested for patients with short ureteral stenosis (< 2 cm)3. For long segment of ureteral stenosis, formal ureteral reconstructive procedures remain the gold standard treatment3,18. Ureteroneocystostomy is suitable for distal ureteral defect up to 4–5 cm in length. Psoas bladder hitch surgery could repair 5–8 cm gap above the ureteric orifice19. Boari bladder flap is usually used for bridging long defect up to 15cm20,21. Bai et al.reported their laparoscopic reconstructive experience with bladder muscle flap for treating full-length ureteral defect, and the longest length was 21 cm22. In our study, the median defect length was 6 cm, ranging from 5 to 17 cm. The bladder flap technique has been successfully utilized to bridge long-length ureteral defect above the ureteral orifice. However, for relatively longer ureteral defect, we combined bladder flap with PH technique to reduce anastomotic tension and stabilize the bladder.
Compared with open surgery, laparoscopic reconstructive technique superior in terms of less blood loss, fewer complications, and better cosmetic outcomes1,23. Numerous studies have demonstrated satisfactory outcomes and acceptable complications of laparoscopic bladder flap surgery 21,22,24,25. Laparoscopy has specificities of magnifying effect and extremely clear vision, which could avoid excessive dissection of the healthy ureter, achieve effect of mucosa -mucosa anastomosis and maximize protection of the ureteral blood supply. However, long ureteric defect still presents a challenge for urologists, so senior surgeons with rich experience in reconstructive surgery and laparoscopic skills are recommended. In our study, all reconstructive surgeries were successfully completed via laparoscopic approaches, and none were converted to open surgery or underwent other reconstructive surgeries. We compared the clinical outcomes of patients with different ureteral defect lengths. Long ureteral defect was associated with longer operative time and more blood loss, which was related to harvesting longer flap and consuming more time during the ureteroplasty process. However, the postoperative hospitalization, total complications rate and total success rate between short and long defect groups had no significant difference. Therefore, LUCBF was effective for long segmental defects, but it was best for experienced surgeons to perform this procedure.
The principles of laparoscopic reconstructive surgery are consistent with those of open approaches and include tension-free, watertight and mucosa–mucosa anastomosis, gentle manipulation of the ureter, maximal protection of ureteric vascularization, and reinforcement of the blood supply1,12. Based on these principles, several technical difficulties must be considered. First, the hard and cicatricial tissue surrounding the ureter, which we call "ureteral armor", must be completely excised until the pink healthy ureter proximal to the stenotic segment appeared, which was a key step in minimizing the possibility of recurrent stenosis. Residual scar tissue may interfere with incision healing and eventually lead to anastomotic leakage or poor healing. On the other hand, excessive ureteral dissection must be avoided and care should be taken to protect the intrinsic ureteral blood supply4. Then, the healthy ureter was freed for at least 2 cm long and cut longitudinally at the posterior wall, which was prepared for anastomosis. Second, several studies have reported the application of oblique22, spiraled26, trapezoidal or S-shaped1 bladder flaps in the reconstruction of ureteric defects. The different shapes were designed based on preoperative bladder capacity and morphology, with the aim of achieving tension-free anastomosis and maintaining adequate blood supply. Since the blood supply decreased from the base to the apex of the flap, we harvested flap with sufficiently broad base that was long enough for tension-free anastomosis. The length of the flap must be 2 cm longer than the ureteral defect, so that it could achieve the vertex of the incised ureteral posterior wall can be reached for tension-free anastomosis. The width of the bladder flap was 4–5 cm at the base and 2–3 cm at the tip, and the length/width ratio was 3–4:1, which was similar to the principles described in previous studies1,21. Third, the midpoint of the flap apex was anastomosed to the vertex of the incised ureteral posterior wall, and interrupted side-to-side anastomosis was used for both the edges of the bladder flap and the ureter. A trumpet-shaped anastomosis was formed to ensure the intraluminal patency. Finally, the ureter-flap anastomosis, tubularized flap and bladder incision were enclosed by pedicled omentum for extra blood supply. The omental wrapping had the functions of revascularization, tissue regeneration, promotion of wound healing and anti-infection27, which was especially suitable for reconstructive surgery.
The recurrence of stenosis is a serious postoperative complication, and most cases occur within the first year after surgery28; therefore, it is suggested that all patients be followed for at least 12 months. Currently, no standard protocols for postoperative surveillance exist. Ghosh et al. formulated a strict radiographic surveillance system, including the application of IVU at 3 months postoperatively, and ultrasonography was performed 6-monthly for 2 years28. However, we paid more attention to the follow-up protocols, and postoperative imaging was utilized at every 3 months within the first year. During the follow-up period, only one patient suffered stenosis recurrence and this patient was cured with prolonged double-J stent placement. None of the remaining patients in our study had recurrent stenosis or increased hydronephrosis.
The indications for bladder flap in our study included ureteral stenosis or avulsion, ureterogenital fistula, and ureteral polyp. In addition to benign ureter lesions, bladder flap could also be performed for treating transitional cell carcinoma29. With the popularity of minimally invasive techniques, LUCBF has been performed by several centers for various indications, as depicted in Table 44,21,22,25,30. Our outcomes were equivalent to the results of these studies.
Our study had several limitations. The main limitation was that this was a retrospective study in a single center, selective bias could not be avoided. Second, a control group was lacking and the total sample was small. For further study, a multicenter prospective study with larger sample size is recommended.
Conclusion
LUCBF is a safe and feasible technique for treating benign ureteral stenosis. Long ureter defect was related to longer operative time and more blood loss.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Ding, G. et al. Experience managing distal ureteral strictures with Boari flap-psoas hitch and comparison of open and laparoscopic procedures. Transl. Androl. Urol. 10(1), 56–65 (2021).
Ficarra, V. et al. A contemporary case series of complex surgical repair of surgical/endoscopic injuries to the abdominal ureter. Eur. Urol. Focus 7(6), 1476–1484 (2021).
Lucas, J. W. et al. Endoscopic management of ureteral strictures: Aan update. Curr. Urol. Rep. 19(4), 24 (2018).
Wu, Y. et al. Terminal augmented ureteroplasty with bladder onlay flap technique for recurrent distal ureteral stricture after ureteroneocystostomy: An initial case series. Transl. Androl. Urol. 10(8), 3332–3339 (2021).
Li, X. et al. The surgical outcomes of reconstruction for the treatment of ureteral stricture after holmium laser lithotripsy: The comprehensive experiences. Asian J. Surg. 45(12), 2713–2718 (2022).
Dindo, D. et al. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240(2), 205–213 (2004).
Smith, A. P. et al. Iatrogenic ureteral injury after gynecological surgery. Can. Urol. Assoc. J. 13(6), S51–S55 (2019).
Bole, R. et al. Malpractice litigation in iatrogenic ureteral injury: A legal database review. Urology 146, 19–24 (2020).
Ding, G. et al. Etiology and ureteral reconstruction strategy for iatrogenic ureteral injuries: A retrospective single-center experience. Urol. Int. 105(5–6), 470–476 (2021).
Cardenas-Trowers, O. et al. Cadaveric surgical demonstration of middle to distal ureteral injury repairs in urologic and gynecologic surgeries. Int. Urogynecol. J. 31(9), 1969–1971 (2020).
Siddighi, S. et al. Perioperative serum creatinine changes and ureteral injury. Int. Urol. Nephrol. 49(11), 1915–1919 (2017).
Wang, J. et al. The application of the “omental wrapping” technique with autologous onlay flap/graft ureteroplasty for the management of long ureteral strictures. Transl. Androl. Urol. 10(7), 2871–2878 (2021).
Ambani, S. N. et al. Does early ureteroneocystostomy after iatrogenic ureteral injury jeopardize outcome?. Urology 136, 245–250 (2020).
El Abd, A. S. et al. Immediate and late management of iatrogenic ureteric injuries: 28 years of experience. Arab. J. Urol. 13(4), 250–257 (2015).
Han, C. M. et al. Outcome of laparoscopic repair of ureteral injury: Follow-up of twelve cases. J. Minim. Invasive Gynecol. 19(1), 68–75 (2012).
Lee, Z. et al. Ureteral rest is associated with improved outcomes in patients undergoing robotic ureteral reconstruction of proximal and middle ureteral strictures. Urology 152, 160–166 (2021).
Durmaz, H. External-internal ureteral catheterization technique in treatment of ureteral injuries. Turk. J. Med. Sci. 49(4), 1132–1137 (2019).
Yang, K. et al. Lingual mucosa graft ureteroplasty for ureteral stricture: A narrative review of the current literature. Ann. Palliat. Med. 10(4), 4840–4845 (2021).
Stein, R. et al. Psoas hitch and Boari flap ureteroneocystostomy. BJU Int. 112(1), 137–155 (2013).
Stolzenburg, J. U. et al. Robot-assisted technique for Boari flap ureteric reimplantation: Replicating the techniques of open surgery in robotics. BJU Int. 118(3), 482–484 (2016).
Zhang, G. et al. Laparoscopic ureteral reimplantation with a Boari flap for long-segment ureteric avulsion or ureteric strictures: Our experience. Int. Urol. Nephrol. 54(8), 1865–1870 (2022).
Bai, Y. et al. Reconstruction of full-length ureter defects by laparoscopic bladder flap forming. Sci. Rep. 11(1), 3970 (2021).
Kim, T. N. et al. Three different laparoscopic techniques for the management of iatrogenic ureteral injury: A multi-institutional study with medium-term outcomes. Asian J. Surg. 44(7), 964–968 (2021).
Ito, W. E. et al. Laparoscopic Boari flap for treatment of benign midureter stricture. Int. Braz. J. Urol. 46(3), 483–484 (2020).
Singh, M. et al. Laparoscopic ureteroneocystostomy for mid and lower ureteric strictures: Experience from a tertiary center. Urol. Ann. 10(3), 243–248 (2018).
Li, Y. et al. Reconstructing full-length ureteral defects using a spiral bladder muscle flap with vascular pedicles. Urology 83, 1199–1204 (2014).
Mazzaferro, D. et al. The omental free flap-a review of usage and physiology. J. Reconstr. Microsurg. 34(3), 151–169 (2018).
Ghosh, B. et al. Managing mid and lower ureteral benign strictures: The laparoscopic way. J. Laparoendosc. Adv. Surg. Tech. A 28(1), 25–32 (2018).
White, C. et al. Ureteral reimplantation, psoas hitch, and boari flap. J. Endourol. 34(S1), S25–S30 (2020).
Castillo, O. A. et al. Laparoscopic ureteral replacement by Boari flap: Multi-institutional experience in 30 cases. Actas Urol. Esp. 37(10), 658–662 (2013).
Acknowledgements
We would like to thank the Health Commission of Jiangxi Province, the Department of Science and Technology in Jiangxi Province, and the Science and Technology Bureau in Ganzhou.
Funding
Funding was provided by the General Project from the Health Commission of Jiangxi Province, China (No.202210891 to Zhaolin Zhang), the Key Research and Development Plan Key Project from Department of Science and Technology in Jiangxi Province, China (No. 20212BBG71013 to Xiaofeng Zou), Key Research and Development Plan General Project from Department of Science and Technology in Jiangxi Province, China (No. 20202BBG73021 to Guoxi Zhang), the Science and Technology Innovation Talent Project from Science and Technology Bureau in Ganzhou, China (No. 2022CXRC9593 to Guoxi Zhang).
Author information
Authors and Affiliations
Contributions
Study conception: Z.Z., Y.W. and H.X.; Study design: Z.Z., Z.H. and H.X.; Manuscript draft: Z.Z. and R.H.; Funding acquisition: Z.Z., X.Z. and G.Z.; Critical revision: X.Z., G.Z., Y.Y., G.W. and H.X.; Data collection: R.H., L.L., Q.Z. and Y.W.; Data analysis: R.H., T.X. and Z.H.; Operation: Y.Y.; Software: T.X. and L.L. All authors contributed to the article. All authors reviewed the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Huang, R., Xie, T. et al. Laparoscopic ureteroneocystostomy with bladder flap for benign ureteral stenosis: our initial experience. Sci Rep 14, 2041 (2024). https://doi.org/10.1038/s41598-024-52497-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52497-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.