Abstract
Metal–organic frameworks (MOFs) are vital in modern material science, offering unique properties for gas storage, catalysis, and drug delivery due to their highly porous and customizable structures. Chemical graph theory emerges as a critical tool, providing a mathematical model to represent the molecular structure of these frameworks. Topological indices/molecular descriptors are mathematical formulations applied to molecular models, enabling the analysis of physicochemical properties and circumventing costly lab experiments. These descriptors are crucial for quantitative structure-property and structure-activity relationship studies in mathematical chemistry. In this paper, we study the different molecular descriptors of tetracyanobenzene metal–organic framework. We also give numerical comparison of computed molecular descriptors.
Similar content being viewed by others
Introduction
Metal–organic Frameworks, generally called MOFs, constitute a class of compounds that have captivated the research community due to their unique characteristics and applications in various fields1,2,3. As a hybrid of inorganic and organic materials, MOFs are characterized with the aid of their crystalline structures, which are composed of metal ions or clusters coordinated to natural ligands. This combination outcomes in a highly porous fabric with a giant internal surface area, often as compared to the size of a football field within a gram of material. As of now, the Cambridge Structural Database (CSD) has recorded a total of 10,636 synthesized metal–organic Framework (MOF) crystals, along with approximately 114,373 structures that resemble MOFs4. Previous initiatives have involved building extensive databases of MOFs and implementing automated, high-throughput processes like molecular simulation and machine learning to forecast the properties of MOFs. These MOF databases comprise actual synthesized and theoretical, or hypothesized, MOF structures5.
The defining properties of MOFs is their molecular structures, that’s modular and particularly tunable6. At the center, those frameworks encompass metallic nodes, which may be single ions, clusters of ions, or even nanoparticles. These nodes are connected with the aid of natural molecules, regularly referred to as linkers, that are normally composed of carbon, hydrogen, oxygen, and from time-to-time nitrogen7. The choice of metal and linker sorts gives upward thrust to a numerous range of MOFs, every with its unique set of properties. These structures aren’t simplest noteworthy for their porosity, however, also for their normal, regularly symmetrical geometries, which can be leveraged in diverse applications.
The synthesis of MOFs entails the self-meeting of steel ions and organic linkers beneath precise conditions. This technique may be stimulated by using factors like temperature, solvent, and pH. Despite the advancements in synthesizing MOFs, challenges remain, in particular in controlling the structure and capability of these frameworks8. The crystallization technique main to the formation of MOFs is complicated and now not completely understood, making it tough to predict the outcomes of synthesis with truth9.
The MOFs have various uses and applications. One of their most important application is in fuel storage and separation. Due to their excessive porosity, MOFs can keep huge volumes of gases like hydrogen or methane, making them promising materials for clean electricity packages. In carbon capture, MOFs are explored for their capacity to selectively absorb carbon dioxide from commercial emissions, a critical step in fighting climate change10,11,12.
In catalysis, MOFs function as catalysts or catalysts supports. Their well-defined structure offers specific environments for chemical reactions, which can be fantastic in developing more efficient and selective catalysts13. MOFs also are being researched for their uses in drug delivery. Their porous nature permits them to encapsulate pharmaceuticals, doubtlessly permitting targeted delivery and controlled release of drugs14,15.
Moreover, MOFs has various applications in sensing and detection. Their systems can be designed to change within the presence of precise molecules, making them beneficial in detecting pollution, pollutants, or even biomarkers for sicknesses. In electrochemical power garage, MOFs are being investigated to be used in batteries and supercapacitors. Their structure can contribute to the efficiency and ability of those electricity storage devices16.
Chemical structures are every so often considered as complex and vast. They can be challenging to analyze in their natural forms. However, mathematical analysis, particularly graph theory, has been instrumental in simplifying and interpreting these structures in different scientific fields. In graph theory, atoms are represented as vertices and the connections between them as edges, transforming complex chemical or molecular structures into more comprehensible forms.
A topological index is used in chemical graph theory to describe a molecular structure. A topological descriptor reflects important structural characteristics of molecules. Most importantly, it is a structural invariant, meaning its value does not change regardless of how the chemical graph is labeled or depicted. These descriptors have found extensive use in correlating and predicting various chemical properties17,18. Topological indices are increasingly used in the development of molecules with pharmacological benefits. See references19,20,21,22 for further information.
Gutman and Trinajstc introduced the degree-based topological descriptors, known as the first and second Zagreb indices23. Since then, various such descriptors have been defined. In 1975, Randić proposed the Randić index \(R_{-1/2}\)24. This concept was later expanded by Bollobás and Erdős25, who generalized the Randić index for any real number \(\alpha\). Further contributions include the atom bond connectivity index by Estrada et al.26, and the geometric arithmetic index GA, introduced by Vukicevic et al.27. In 2008, Doslic defined the first and second Zagreb coindices28. Additionally, Zhou and Trinajsti’c presented the general sum-connectivity index in29, refining their earlier sum-connectivity index described in30. For recent work, we refer to see31,32,33,34.
Consider a graph \({\chi }\) with a vertex set \(V\left( {\chi }\right)\) comprising \({n}_{\chi }\) vertices and an edge set \(E\left( {\chi }\right)\) containing \({m}_{\chi }\) edges. The degree of a vertex \({\lambda _u}\) is the count of edges incident to it. The edges of \({\chi }\) can be categorized based on the degrees of their end vertices, denoted as \(\left( {\lambda }_u,{\lambda }_v\right)\), where \({\lambda }_u\) and \({\lambda }_v\) are the degrees of the end vertices u and v, respectively.
Introduced by Gutman and Trinajstic in 197223, and Das and Gutman35 the first and second Zagreb indices respectively of \({\chi }\) are given by:
In 2008, Doślić defined the first and second Zagreb coindices36:
In 2016, Gutman et al. established a relationship to measure Zagreb coindices for a graph \({\chi }\) with \(n_{\chi }\) vertices and \(m_{\chi }\) edges37:
In 2013, Shirdel et al. introduced the Hyper-Zagreb index38:
Ghorbani and Azimi in 201239, and Ranjini et al. in 201340, defined the first and second multiple Zagreb indices and the redefined first, second, and third Zagreb indices, respectively:
Gutman et al. in 2014 introduced the reduced second Zagreb index41:
In 2010, Ghorbani and Hosseinzadeh introduced a third version of the Zagreb index42:
Lastly, the generalized Zagreb index, as defined by Azari and Iranmanesh43:
An essential aspect of this study is to address the problem of predicting the physical properties of TCNB MOFs through topological analysis. This analysis is pivotal in understanding the fundamental properties of these frameworks and plays a crucial role in the design and synthesis of similar compounds. By leveraging the calculated topological indices, we aim to create a more efficient pathway for synthesizing MOFs with desired properties. Additionally, this study enriches scientific databases with detailed topological data, facilitating further research in the field. The topological indices calculated in this study are instrumental in predicting various physical properties of TCNB MOFs, such as thermal stability, porosity, and electrical conductivity. These indices serve as a quantitative measure that can guide the synthesis of new MOFs with tailored properties. Furthermore, including these topological data in scientific databases enhances the scope of computational research, offering a valuable resource for researchers exploring the vast potential of MOFs.
Tetracyanobenzene metal–organic framework (TCNB MOFs)
Tetracyanobenzene metal–organic framework (TCNB MOFs) is a type of porous material, synthesized by reacting a metal salt with TCNB in a solvent, where metal ions coordinate with the cyano groups on TCNB molecules to form an interconnected network. Characterized by high porosity, thermal stability, and electrical conductivity, these MOFs
are versatile in applications like gas storage, catalysis, and energy storage. The TCNB framework, a two-dimensional network involving transition metal ions (TM) like Ti, V, Cr, Fe, Co, Ni, Cu, and Zn with tetracyanobenzene, exhibits a unique structure that varies from metallic to half-metallic. Metals such as Ni, Fe, Zn, and Co result in fully metallic structures, while Ti, V, Cr, and Mn create half-metallic MOFs, showing a gap in one spin direction. The arrangement in the TCNB network, especially with Ti, V, Cr, and Co, displays a screening effect, where the properties of these metals are significantly influenced by spin-polarized electrons from the surrounding organic ligands, unlike in \(TM-Pc\). This effect is absent in \(Ni-TCNB\) and \(Zn-TCNB\) structures44,45,46.
Results and discussion
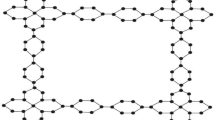
The chemical structure of TCNB(m, n), depicted in Fig. 1, exhibits a horizontal expansion of \({m}\ge 1\) and a vertical expansion of \({n}\ge 1\). This structure comprises a total of \(25{m}+25{n}+32{m}{n}+17\) atoms and \(32{m}+32{n}+44{m}{n}+20\) bonds. It features four distinct atom types, categorized by degrees ranging from 1 to 4, with further specifics provided in Table 1. Additionally, the structure includes five different bond types, classified based on the degrees of both end atoms, as elaborated in Table 2.
First Zagreb Index of TCNB(m, n) We will compute the first Zagreb index of TCNB(m, n), by using the formula:
Tetracyanobenzene (\(TCNB\left( m,n\right)\)) structure consist of five types of edge partitions, given in Table 2. By using these values, we have
Second Zagreb Index of TCNB(m, n) Second Zagreb index of \(TCNB\left( m,n\right)\) is
First and Second Zagreb Coindices of TCNB(m, n) First Zagreb coindex of TCNB(m, n) is
Second Zagreb Coindex of TCNB(m, n) is
Hyper Zagreb Index of TCNB(m, n) Now, we will compute the hyper Zagreb index of TCNB(m, n) by using the equation below and partition given in Table 2.
First and Second Multiplicative Zagreb Indices of TCNB(m, n) Edge types for tetracyanobenzene transition metal organic network are defined in Table 2. Again using these values, we calculated the first multiplicative Zagreb index of TCNB(m, n) is:
Second multiplicative Zagreb indices of TCNB(m, n) is:
The first, second, and third redefined Zagreb indices of TCNB(m, n)
The Reduced Second Zagreb index of TCNB(m, n)
The Third Zagreb index of TCNB(m, n)
The Generalized Zagreb index of TCNB(m, n)
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Comparative discussion and conclusion
In this study, we comprehensively analyzed various degree and neighborhood-based topological indices of the tetracyanobenzene transition metal organic network, \(TCNB (m, n)\). This investigation, focusing on analytical results, highlighted the intricate relationships between these indices and the structural properties of the TCNB network, thereby revealing their potential applications in diverse scientific and industrial fields.
The correlations observed between the topological indices and the physical properties of TCNB MOFs, such as thermal stability, porosity, and electrical conductivity, underscore the utility of topological analysis in predicting MOF behavior and properties. This is pivotal for synthesizing new materials with desired characteristics and contributes significantly to the field of material science, particularly in designing MOFs for specific applications like gas storage, catalysis, or drug delivery. Employing mathematical methods, especially those from chemical graph theory, has allowed for a more efficient and accurate prediction of MOFs’ physical properties. By simplifying complex molecular structures into understandable forms, these methods have proven effective in providing a quantitative framework for interpreting MOFs’ structural attributes. The application of topological indices in MOF analysis presents promising research, opening possibilities for developing more efficient methods for predicting material properties and designing new materials with customized functionalities. Future research could extend this approach to other types of MOFs, further enhancing the predictive models for material properties and contributing to the advancement of computational research in this domain.
In our comprehensive analysis of the Tetracyanobenzene metal–organic Frameworks (TCNB MOFs), symbolized as \(TCNB (m, n)\), we observed consistent trends across various topological indices, as detailed in Tables 3, 4, 5, 6, 7, and 8, and shown in Figs. 2, 3, 4, 5, 6 and 7. For the first Zagreb index (\(M_1\)), values began at 704.0 for \(TCNB (1,1)\) and increased progressively, reaching 9596.0 for \(TCNB (5,6)\) as shown in Table 3. This pattern was mirrored in the second Zagreb index (\(M_2\)), which started at 1228.0 for \(TCNB (1,1)\) and escalated to 13864.0 for \(TCNB (5,6)\), also documented in Table 3. Interestingly, the first Zagreb index values were consistently lower than the second Zagreb index but higher compared to the third Zagreb index (\(M_3\)), which started at -64.0 for \(TCNB (1,1)\) and decreased further to -788.0 for \(TCNB (5,6)\), as depicted in Table 3.
Similarly, for the Zagreb coindices (\({\overline{M}}_1\) and \({\overline{M}}_2\)), there was a notable increase in values as the size of \(m, n\) increased, as can be seen in Table 4. For instance, \({\overline{M}}_1\) for \(TCNB (1,1)\) was 24640.0, escalating significantly to 4325300.0 for \(TCNB (5,6)\). This trend of increasing values was consistent across all topological indices studied, including the Hyper Zagreb Index (\(HM\)) and Reduced Zagreb Indices (\(ReZG_1, ReZG_2, ReZG_3\)) as presented in Tables 5 and 7, as well as the Multiple Zagreb Indices (\(PM_1, PM_2\)) and Generalized Zagreb Indices (\(M_{1,1}, M_{1,2}, M_{2,2}\)) shown in Tables 6 and 8. Each of these indices showed a clear pattern of escalation with the increase in the size of the MOF network, reflecting the growing complexity and interaction within the TCNB MOFs.
Data availability
The data that support the findings of this study are available in the manuscript.
References
Guo, X. et al. Fluorescence detection platform of metal–organic frameworks for biomarkers. Biointerfaces Colloids Surfaces B 229, 113455 (2023).
Luo, D. et al. Metal–organic frameworks (MOFs) as apt luminescent probes for the detection of biochemical analytes. J. Mater. Chem. B 11(29), 6802–6822 (2023).
Tan, G. et al. Detection mechanism and the outlook of metal–organic frameworks for the detection of hazardous substances in milk. Food Chem. 430, 136934 (2023).
Moghadam, P. Z. et al. Development of a Cambridge Structural Database subset: A collection of metal–organic frameworks for past, present, and future. Chem. Mater. 29(7), 2618–2625 (2017).
Anderson, R. & Gómez-Gualdrón, D. A. Increasing topological diversity during computational synthesis of porous crystals: How and why. CrystEngComm 21(10), 1653–1665 (2019).
Yaghi, O. M. Reticular chemistry in all dimensions. ACS Cent. Sci. 5(8), 1295–1300 (2019).
Yuan, S., Qin, J. S., Lollar, C. T. & Zhou, H. C. Stable metal–organic frameworks with group 4 metals: Current status and trends. ACS Cent. Sci. 4(4), 440–450 (2018).
Kong, X. J. & Li, J. R. An overview of metal–organic frameworks for green chemical engineering. Engineering 7(8), 1115–1139 (2021).
Stock, N. & Biswas, S. Synthesis of metal–organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 112(2), 933–969 (2012).
Hu, X. et al. Metal–organic framework-based catalysts for lithium-sulfur batteries. Coord. Chem. Rev. 475, 214879 (2023).
Andrade, L. S. et al. Metal–organic frameworks as catalysts and biocatalysts for methane oxidation: The current state of the art. Coord. Chem. Rev. 481, 215042 (2023).
Dong, A., Chen, D., Li, Q. & Qian, J. Metal–organic Frameworks for Greenhouse Gas Applications. Small 19(10), 2201550 (2023).
Goetjen, T. A. et al. Metal–organic framework (MOF) materials as polymerization catalysts: A review and recent advances. Chem. Commun. 56(72), 10409–10418 (2020).
Lazaro, I. A. & Forgan, R. S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 380, 230–259 (2019).
Mallakpour, S., Nikkhoo, E. & Hussain, C. M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 451, 214262 (2022).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341(6149), 1230444 (2013).
Basak, S. C., Magnuson, V. R., Niemi, G. J., Regal, R. R. & Veith, G. D. Topological indices: Their nature, mutual relatedness, and applications. Math. Model. 8, 300–305 (1987).
Zhang, Q., Xiao, K., Chen, M. & Xu, L. Calculation of topological indices from molecular structures and applications. J. Chemom. 32(11), e2928 (2018).
Malik, M. Y. H., Binyamin, M. A. & Hayat, S. Correlation ability of degree-based topological indices for physicochemical properties of polycyclic aromatic hydrocarbons with applications. Polycyclic Aromat. Compd. 42(9), 6267–6281 (2022).
Shafiei, F. Relationship between topological indices and thermodynamic properties and of the monocarboxylic acids applications in QSPR. Iran. J. Math. Chem. 6(1), 15–28 (2015).
Gutman, I., Monsalve, J. & Rada, J. A relation between a vertex-degree-based topological index and its energy. Linear Algebra Appl. 636, 134–142 (2022).
Diudea, M. V., Gutman, I. & Jantschi, L. Molecular Topology 332 (Nova Science Publishers, 2001).
Gutman, I. & Trinajstić, N. Graph theory and molecular orbitals. Total Φ-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 17(4), 535–538 (1972).
Randic, M. Characterization of molecular branching. J. Am. Chem. Soc. 97(23), 6609–6615 (1975).
Bollobás, B. & Erdös, P. Graphs of extremal weights. ARS Combin. 50, 225 (1998).
Estrada, E., Torres, L., Rodriguez, L. & Gutman, I. An atom-bond connectivity index: Modelling the enthalpy of formation of alkanes. (1998)
Vukičević, D. & Furtula, B. Topological index based on the ratios of geometrical and arithmetical means of end-vertex degrees of edges. J. Math. Chem. 46, 1369–1376 (2009).
Doslic, T. Vertex-weighted Wiener polynomials for composite graphs. ARS Math. Contemp. 1(1), 66–80 (2008).
Zhou, B. & Trinajstić, N. On general sum-connectivity index. J. Math. Chem. 47, 210–218 (2010).
Zhou, B. & Trinajstić, N. On a novel connectivity index. J. Math. Chem. 46, 1252–1270 (2009).
Ullah, A., Zaman, S., Hussain, A., Jabeen, A. & Belay, M. B. Derivation of mathematical closed form expressions for certain irregular topological indices of 2D nanotubes. Sci. Rep. 13(1), 11187 (2023).
Ravi, V. & Desikan, K. Curvilinear regression analysis of benzenoid hydrocarbons and computation of some reduced reverse degree based topological indices for hyaluronic acid-paclitaxel conjugates. Sci. Rep. 13(1), 3239 (2023).
Akers, C., Faulkner, T., Lin, S. & Rath, P. Reflected entropy in random tensor networks. Part II. A topological index from canonical purification. J. High Energy Phys. 2023(1), 1–74 (2023).
Ghani, M. U. et al. Characterizations of chemical networks entropies by K-Banhatii topological indices. Symmetry 15(1), 143 (2023).
Das, K. C. & Gutman, I. Some properties of the second Zagreb index. MATCH Commun. Math. Comput. Chem. 52(1), 103–112 (2004).
Došlić, T. Vertex-weighted Wiener polynomials for composite graphs. ARS Mathe. Contemp. 1, 66–80 (2008).
Gutman, I., Furtula, B., Kovijanić Vukićević, Ž & Popivoda, G. On Zagreb indices and coindices. MATCH Commun. Math. Comput. Chem. 74, 5–16 (2015).
Shirdel, G. H., Rezapour, H. & Sayadi, A. M. The hyper-Zagreb index of graph operations. Iran. J. Math. Chem. 4(2), 213–220 (2013).
Ghorbani, M. & Azimi, N. Note on multiple Zagreb indices. Iran. J. Math. Chem. 3(2), 137–143 (2012).
Ranjini, P. S., Lokesha, V. & Usha, A. Relation between phenylene and hexagonal squeeze using harmonic index. Int. J. Graph Theory 1(4), 116–121 (2013).
Gutman, I., Furtula, B. & Elphick, C. Three new/old vertex-degree-based topological indices. MATCH Commun. Math. Comput. Chem. 72, 617–632 (2014).
Ghorbani, M. & Hosseinzadeh, M. A. A note of Zagreb indices of nanostar dendrimers. Optoelectron. Adv. Mater. Rapid Commun. 4, 1877–1880 (2010).
Azari, M. & Iranmanesh, A. Generalized Zagreb index of graphs. Stud. Univ. Babes-Bolyai, Chem. 56(3), 59–71 (2011).
Schneider, C., Bodesheim, D., Keupp, J., Schmid, R. & Kieslich, G. Retrofitting metal–organic frameworks. Nat. Commun. 10(1), 4921 (2019).
Mabrouk, M., Hayn, R. & Chaabane, R. B. First-principle study of metal–organic frameworks of the 4D and 5D transition metal series with phthalocyanine and tetracyanobenzene. Superlattices Microstruct. 130, 122–126 (2019).
Mabrouk, M., Hayn, R., Denawi, H. & Chaabane, R. B. Possibility of a ferromagnetic and conducting metal–organic network. J. Magn. Magn. Mater. 453, 48–52 (2018).
Acknowledgements
The authors extend their appreciation to the Deputyship for Research \& Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023102. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
Author information
Authors and Affiliations
Contributions
Conceptualization, I.A., M.F.N. and M.A.K.; methodology, M.F.N and M.A.I.; validation, I.A., M.F.N., and M.A.K.; formal analysis, M.F.N. and M.A.K.; investigation, I.A., and M.A.K.; writing—original draft preparation, M.F.N. and M.A.K.; writing—review and editing, I.A. and M.A.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Dayel, I., Nadeem, M.F. & Khan, M.A. Topological analysis of tetracyanobenzene metal–organic framework. Sci Rep 14, 1789 (2024). https://doi.org/10.1038/s41598-024-52194-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52194-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.