Abstract
The current study reported a facile co-precipitation technique for synthesizing novel NiCo2S4/chitosan nanocomposite. The photocatalytic activity of the prepared nanocomposite was evaluated using congo red (CR) dye as a target pollutant. The central composite design was employed to examine the impact of different reaction conditions on CR dye degradation. This study selected the pH, photocatalyst loading, initial CR concentration and reaction time as reaction parameters, while the degradation efficiency (%) was selected as the response. A desirability factor of 1 suggested the adequacy of the model. Maximum degradation of 93.46% of 35 ppm dye solution was observed after 60 min of visible light irradiation. The response to surface methodology (RSM) is a helpful technique to predict the optimum reaction conditions of the photodegradation of CR dye. Moreover, NiCo2S4/Ch displayed high recyclability and reusability up to four consecutive cycles. The present study suggests that the prepared NiCo2S4/chitosan nanocomposite could prove to be a viable photocatalyst for the treatment of dye-contaminated wastewater.
Similar content being viewed by others
Introduction
Secure and pure water availability has become a serious concern because of the persistent and unacceptable discharge of organic contaminants into waterways from various sources1,2. These contaminants are regarded as highly toxic and persistent in the ecology, having an impact on both human health and the environment3,4. Numerous sectors, including textile, pharmaceutical, nourishment, beauty aids, plastic, rubber, and paper, use significant quantities of organic dyes to colour their goods5,6. When released into the ecosystem, these coloured compounds can harm aquatic life and human health and can be observed even at minimal concentrations7,8. Organic dyes such as Congo red (CR) have been recognized as potent human carcinogens by the IARC "International Agency for Research on Cancer" due to their ability to induce tumours in animals9. It contaminates water and soil, leading to harmful effects on aquatic organisms and other wildlife.
Many traditional techniques for cleaning wastewater, including biodegradation, electrochemical combustion, and chemical oxidation, are ineffective in removing organic pollutants9,10. A heterogeneous photocatalysis method using semiconductor heterojunctions as photocatalysts has been designed to efficiently utilize solar power as a visible source to degrade organic pollutants from water11. Recent research has demonstrated that photocatalysis methods are thought to be the most effective and practical methods for eliminating organic matter from wastewater due to their high rate of degradation, reduced generation of secondary pollutants, efficacy, and cost-effectiveness12,13. Photocatalysis is the term for the light-induced chemical reaction that generates electron and hole pairs when subjected to sufficiently strong light. Reactive oxygen species (ROS), such as superoxide (•O2) and hydroxyl (•OH) radicals, are generated when these photogenerated electron/hole pairs interact with oxygen and water. These ROS then attack the target pollutants, degrading them into less harmful intermediates and ultimately converting them into carbon dioxide and water14,15.
Metal sulfides have recently attracted much interest as possible heterogeneous catalysts for the photodegradation organic molecules. They have become an excellent replacement for conventional catalysts due to their increased surface area, porous structure, better charge transportation property, non-toxicity, and appropriate bandgap energy suitable for visible regions16. However, research into their extensive uses for removing contaminants from industrial effluent is still lacking. In addition, many currently available sulfide-based photocatalysts such as ZnS/ZnO17, CuS/CdS18, MoS219, etc., have poor effectiveness restrictions and are only helpful in situations where the amount of pollutants is very low. Due to their superior electrical and redox responses, mixed metal sulphides (MMSs) significantly outperform mono-metal sulphides in photocatalytic performance20. Nickel–cobalt sulfide (NiCo2S4), a significant ternary transition metal sulfide, has recently received great attention as a potential photocatalyst owing to its optimum bandgap, chemical and thermal resistivity, and quantum size impact21. NiCo2S4 has emerged as an effective photocatalyst for water treatment due to its narrow bandgap energy of 1.2–2.4 eV and excellent light harvesting properties22,23.
Furthermore, recent research using its high carrier density as a basis demonstrated that NiCo2S4 is undoubtedly a metal and not a semiconductor substance24. However, NiCo2S4 suffers from a limitation of firstly, faster charge recombination rate and, secondly, agglomeration of nanoparticles during synthesis25. Therefore, NiCo2S4 utilization as a useful catalyst material for visible-light-induced photocatalytic degradation is still challenging. Thus, the effectiveness of NiCo2S4 for the photodegradation of organic pollutants could be enhanced by forming a nanocomposite, which will prevent its agglomeration and slow down the charge recombination rate26.
Biopolymer-doped metal hydroxides, oxides, and sulfides proved to be potential photocatalysts for degrading dyes from water samples. The efficacy of photodegradation is significantly influenced by biopolymers27,28. One of the most prevalent polysaccharides, chitosan (CS) [poly-(1 4) 2-amino-2-deoxy-d-glucose], is a cationic biopolymer that is an unprocessed carbohydrate derived from plants and animals. Strong intra- and intermolecular hydrogen interactions are present in this semi-crystalline polymer. In addition to being steady and secure, it is also reactive, biocompatible, and compostable. It is possible to modify chitosan because it contains volatile amino- and hydroxyl groups29. Chitosan is widely used in numerous biological and chemical applications because of its biological traits, non-toxicity, ability to fight cancer, antioxidant properties, ability to heal wounds30, and its use in water purification31.
The present study illustrates the synthesis of novel NiCo2S4/chitosan (NiCo2S4/Ch) via a simple co-precipitation technique and its applicability as a photocatalyst for degrading CR from an aqueous stream. To our understanding, it's the first research on the degradation of CR using a NiCo2S4/chitosan nanocomposite. The prepared photocatalyst showed enhanced photodegradation of CR under visible light. The enhanced degradation efficiency was attributed to incorporating chitosan, as it helps delocalize photoinduced charges. The presence of reactive groups (NH2 and OH) on the chitosan surface could be effective in dye molecule adsorption, which enhances dye degradation by NiCo2S4 nanoparticles32. The effect of reaction parameters on the degradation of CR dye was accessed using response to surface methodology (RSM) with a central composite design (CCD).
Experimentation
Reagents
Chitosan [C18H35N3O13], Nickel (II) nitrate hexahydrate [Ni(NO3)2.6H2O], Cobalt (II) nitrate hexahydrate [Co(NO3)2.6H2O], Sodium sulfide hydrate [Na2S.9H2O], Acetic acid [CH3COOH], Congo red (CR), and distilled water.
The reagents were acquired from Sigma Aldrich and were of excellent analytical quality and used without extra purification. Distilled water was utilized during the experiment.
Synthesis of NiCo2S4/Ch nanocomposite
The NiCo2S4/chitosan was synthesized via a facile precipitation technique. Initially, 100 mg of chitosan and 5 mL acetic acid were mixed in a 200 mL beaker under constant stirring until a clear solution was obtained. Then, 30 mL of distilled water was added to the above solution and stirred for 15 min at 50 °C. Meanwhile, 2 mmol of [Ni(NO3)2.6H2O] and 4 mmol of [Co(NO3)2.6H2O] were dissolved in 50 mL of distilled water under constant stirring for 15 min. The salt solution was then added dropwise to the beaker containing chitosan, and the resultant mixture was stirred for 30 min at 50 °C. In another beaker, a solution of 8 mmol of [Na2S.9H2O] in 20 mL distilled water was made and added dropwise to the salt solution. A black-coloured precipitate was observed. The resultant mixture was stirred for another 2 h at the same temperature, and the solution was kept for ageing for 24 h. The final black precipitate was centrifuged, washed and kept for drying in an oven at 80 °C for 7 h. The exact process was carried out without chitosan to synthesize pure NiCo2S4 nanoparticles.
Characterization
The X-ray diffraction (XRD) spectrum of the samples was taken from an X'PERT powder X-ray diffractometer with Cu kα radiation. The X-ray photoelectron spectrum of NiCo2S4/chitosan was recorded using a Thermo Fisher Scientific Pvt. Ltd. ESCALAB Xi + X-ray photoelectron spectrometer (XPS). The shape and particle size of the sample were analyzed through a JEOL JEM-2100 PlusElectron high-resolution transmission electron microscope (HRTEM). The morphology and the energy dispersive X-ray analysis (EDAX) of NiCo2S4/Ch were accessed using a Carl ZEISS SIGMA field emission scanning electron microscope (FESEM). The liquid chromatography-mass spectroscopy (LCMS) spectrum was recorded using a Xevo XS QTof mass spectrometer. Cary 5000 UV–Vis-NIR spectrophotometer was used to record the UV-DRS spectra. The Fourier Transform Infrared (FTIR) spectrum of NiCo2S4/Ch was recorded using the Perkin Elmer spectrum 100 instrument.
Photodegradation tests
All the photodegradation tests were carried out at room temperature (33 ± 2 °C) in a wooden cabinet equipped with a Philips 23 W white LED bulb as the source of visible light with a light intensity of 52.13 W/m2. The photodegradation reactions were conducted in a 100 mL beaker with 50 mL dye solution placed 10 cm from the LED light. The light intensity was measured using a Lux meter placed at the base of the beaker.
Typically, in an experiment, the beaker containing 50 mL 35 ppm of dye solution and 22 mg of NiCo2S4/Ch was irradiated under LED light by placing it in a wooden chamber. The reaction cell was kept in the dark at a constant stirring speed for 30 min to attain adsorption–desorption equilibrium before irradiating it for a prescribed time duration. The dye degradation was examined by tracking the decrease in the absorbance of CR dye at λmax = 497 nm using a UV–visible spectrophotometer (GENESYS 10 S UV–Visible spectrophotometer).
The photodegradation efficiency was calculated from the following equation33:
The kinetics of the photodegradation of CR under optimum conditions was evaluated according to the following equation:
C0 and C are the initial and final concentrations at time t of CR dye, respectively, and k is the pseudo-first-order rate constant.
Experimental design
The effect of the reaction factors such as pH (A), photocatalyst dosage (B), initial dye concentration (C) and time (D) on the photocatalytic activity of NiCo2S4/Ch catalyst was examined using response to surface methodology (RSM) employing central composite design (CCD). As CR dye is an indicator and is blue in an acidic medium, the study of pH is limited to alkaline conditions. Design Expert Software (DOE) version 13 was used to create the design of the experimental analysis. The RSM is a collection of mathematical and statistical methods that help investigate the effect of individual parameters on the response34. These experimental data were fitted to achieve the best degradation efficiency. RSM gives a set of reaction conditions which help examine the effect of single-parameter and multi-parameter interactions and the relationship between operating parameters on the photodegradation rate of CR dye to achieve the optimum conditions35. According to the RSM analysis, an experimental design of 30 runs (16-factor points + 8 axial points + 6 replicates) was considered as the empirical model according to the following equation:
N, Number of experimental runs; and k, Number of input variables (parameters).
A quadratic equation predicted the relation between CR photodegradation and the individual factors. The response was predicted using the following equation36:
where Xi is the coded value of the ith individual parameter, β0, βi, and βij are the equation’s zero, first and second-order coefficients, respectively.
The analysis of variance (ANOVA) and F-test were used to appraise the results, and the coefficients of R2 and R2adj were used to assess the polynomial model's fitness. A careful comparison between the experimental and predicted outcomes was examined at the conclusion to demonstrate the predicted model's statistical significance.
Results and discussion
XRD analysis
The XRD is a useful technique to access the crystal structure of the nanocomposites. The XRD spectra of NiCo2S4 and NiCo2S4/Ch are shown in Fig. 1. The XRD spectrum of NiCo2S4 showed the broad and major diffraction peaks at 2θ = 31.47°, 38.19° and 55.10° could be assigned to (311), (500), and (440) planes of cubic NiCo2S4, respectively, matching with the JCPDS card No. 43-1477, having lattice parameter a = b = c = 9.417 Å37. The broad peaks in the XRD spectrum indicate the amorphous nature of the sample38. The XRD spectrum of NiCo2S4/Ch showed a shift of the peaks to a higher angle because of the distortion in the growth phase of NiCo2S4 due to the presence of chitosan39, and all the peaks corresponding to (311), (500), and (440) planes of cubic NiCo2S4 could be identified. The sift of the peaks and the appearance of a new peak at 2θ = 20.0° appeared, indicating the incorporation of chitosan in the nanocomposite40. The average crystallite size of the NiCo2S4/Ch nanocomposite was found to be 8.17 nm using Debye–Scherrer's equation.
Morphological and elemental analysis
The SEM images of the NiCo2S4/Ch nanocomposite illustrated in Fig. 2a–c displayed that the surface of the prepared sample was rough due to the dispersion of small spherical NiCo2S4 nanoparticles over the chitosan surface. The presence of small granular NiCo2S4 nanoparticles could be seen in the high-resolution SEM images. The TEM images of NiCo2S4/Ch nanocomposite showed an irregular distribution of NiCo2S4 nanoparticles over the chitosan (Fig. 2d). Clear lattice planes with lattice fringes of spacing 0.3 nm corresponding to (400) lattice plane of NiCo2S4 could be identified and marked in the HRTEM images of NiCo2S4/Ch nanocomposite (Fig. 2e,f). The size distribution histogram was plotted, and the average particle size could be calculated from the histogram seen in Fig. 2h. An average particle of the NiCo2S4/Ch nanocomposite was observed to be 10.0 nm in accordance with the average crystallite size. The EDAX spectrum (Fig. 2g) revealed the presence of nickel, cobalt, sulfur, oxygen and carbon with relative atomic percentages of 5.72%, 12.47%, 22.25%, 31.73%, and 27.83%. At the same time, a higher amount of carbon and oxygen is due to the chitosan41.
XPS analysis
The oxidation states and elemental composition of the NiCo2S4/Ch nanocomposite were analyzed through XPS. The survey spectrum of NiCo2S4/Ch showed the presence of Ni 2p, Co 2p, S 2p, C 1s, O 1s and N 1s as expected (Fig. 3a). As seen in Fig. 3b, the short scan of C 1s showed a triplet of peaks appearing at 284.4 eV, 258.8 eV and 288.2 eV. The peaks at lower binding energies, i.e. 284.4 eV and 258.8 eV, are attributed to the carbon as in C–C/C–H and C–O/C–N bonds, respectively42. The higher binding energy peak at 288.2 eV shows the presence of sp2 hybridized carbon in C=O or C–O–C bonds43. The short scan spectrum of O 1s (Fig. 3c) showed a doublet of peaks appearing at 531.3 eV assigned to C=O and 532.2 eV due to C–O–C bonds44. A shift in the binding energies to greater values for C 1s and O 1s in NiCo2S4/Ch could be attributed to the insertion of NiCo2S4 nanoparticles, which increased the electron density near C and O, causing a shift in the binding energies to higher values45. The core-level spectrum of N 1s is shown in Fig. 3d. Here, a doublet of peaks was also observed, similar to the O 1s spectrum. The peaks appeared at 399.8 eV and 400.2 eV, which could be assigned to C–NH and C–NH2 groups, respectively46. Figure 3e illustrates the deconvoluted spectrum of Ni 2p, showing the presence of a pair of doublets and satellite (Sat.) peaks. The 856.5 eV and 874.5 eV peaks correspond to Ni 2p3/2 and Ni 2p1/2, respectively, characteristic of the + 3 oxidation state of Ni atoms, while the peaks at 853.0 eV and 876.2 eV are distinctive of Ni+2 atoms, indicting the co-existence of Ni+2 and Ni+3 ions in NiCo2S4/Ch composite47. Figure 3f shows the deconvoluted short scan of Co 2p, which is quite similar to Ni 2p spectra. The strong peaks at 782.0 eV could be assigned to Co 2p3/2, and the peak at 798.1 eV corresponds to Co 2p1/2, indicating the presence of Co+2 ions, while the peaks at 778.0 eV and 793.1 eV are characteristic of Co+3 ions which is also present in small amounts, suggesting the co-existence of Co+2 and Co+3 ions in the composite48. The peaks at 784.5 eV and 802.5 eV are the satellite peaks of Co 2p. The typical short scan spectra of S 2p showed two main peaks and a satellite peak (Fig. 3g). The peaks at 161.2 eV and 162.7 eV are the characteristic peaks of S 2p3/2 and S 2p1/2, respectively, attributed to the − 2 oxidation state of sulfur49,50.
Functional group analysis
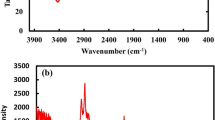
The presence of the chitosan and the surface functional group were also analyzed using FTIR spectroscopy. The FTIR spectrum of NiCo2S4 and NiCo2S4/Ch nanocomposite is illustrated in Fig. 4. Pure NiCo2S4, being an inorganic compound, showed two peaks at 1110 and 1068 cm−1 attributed to C–O stretching in adsorbed carbon dioxide. A wide peak in the region 3500–3000 cm−1 occurred due to the stretching of O–H and N–H bonds of chitosan overlapping in the same region. Two small peaks at 2917 cm−1 and 2852 cm−1 are the characteristic stretching bands of C–H groups present in chitosan51. The occurrence of C=O bond stretching is indicated by a broad and strong peak at 1599 cm−1. The peak at 1438 cm−1 was ascribed to the bending vibrations of CH2 groups. A strong band between 1029 and 1002 cm−1 corresponds to the stretching of C–O bonds. The bands at 914 cm−1 and 719 cm−1 indicate the out-of-plane bendings of C–H groups. All these bands in the FTIR spectrum of NiCo2S4/Ch nanocomposite agree with the previous literature and confirm the existence of chitosan in the prepared material52. Meanwhile, a small band at 584 cm−1 arises due to metal–oxygen stretchings53.
Optical properties
The optical and light harvesting behaviour of NiCo2S4 nanoparticles and NiCo2S4/Ch nanocomposite were investigated using UV-DRS. The absorption spectra of NiCo2S4 nanoparticles and NiCo2S4/Ch nanocomposite are shown in Fig. 5. Both the samples showed maximum absorption in the range of 300–310 nm. However, NiCo2S4/Ch nanocomposite showed higher absorption intensity compared to pure NiCo2S4 nanoparticles, indicating improved light harvesting properties. Additionally, both the samples showed strong absorption in the whole UV–visible region, suggesting the excellent light absorption tendency of NiCo2S4. The band gap of the photocatalyst was calculated using Tauc's plot according to the following equation:
here α, Coefficient of absorption; h, Planck's constant; ν, Frequency of light; A, Constant; Eg, Optical bandgap of the material, and n is ½ for indirect and 2 for direct allowed transitions, respectively.
NiCo2S4 nanoparticles displayed a bandgap of 2.2 eV, which is in good agreement with the formerly published studies54. The bandgap reduced from 2.2 to 2.0 eV for the NiCo2S4/Ch nanocomposite, possibly due to increased particle size after forming the nanocomposite55. Additionally, a low bandgap of the material indicates high light-harvesting abilities.
One useful method for determining the visible light photocatalytic activity of the produced nanocomposites is photoluminescence (PL) spectroscopy. The higher intensity of the PL peak indicates a faster rate of electron–hole recombination and a lower photocatalytic activity, while a low-intensity PL peak has a slower charge recombination rate and a higher photocatalytic activity56. In the PL spectrum, pristine NiCo2S4 displayed the maximum intensity, indicating the highest charge recombination rate (Fig. 5c). When chitosan and NiCo2S4 are combined, the PL peak's intensity drops. Higher photocatalytic activity requires a substantially lower charge recombination rate, which is indicated by the drop in PL intensity57. As expected, the NiCo2S4/Ch nanocomposite displayed the highest photocatalytic activity and the lowest intensity in the PL spectrum, indicating the slowest charge recombination rate.
Optimization of CR degradation using RSM
RSM was used to examine how response factors affected CR's degradation rate. The experimental design was formulated by CCD to attain maximum degradation efficiency of CR in aqueous solutions by adjusting the reaction parameters. CCD formulated an experimental array of 30 runs, and the predicted and actual responses, along with the four reaction parameters, i.e. pH (A), catalyst dosage (B), initial dye concentration (C) and reaction time (D), are shown in Table 1. The predicted equation of CR degradation was as follows:
The equation above represents the combined effect of several factors on the CR degrading efficiency (%). Contrarily, the response is affected favourably and unfavourably by the coefficients with positive and negative values, respectively.
ANOVA was used extensively to examine the impact of the constructed RSM model on the CR degradation efficiency58. The ANOVA results and the summary of the quadratic model are given in Table 2. As seen in Table 2, a low P-value (< 0.0001), an F-value of 219.11, and a high sum of square values of 2600.50 indicated that the quadratic model is the most suitable. The results reproducibility and the model's implication are generally assessed based on the coefficient of variance (CV) percentage. A CV % ranging between 0.5 and 13.5% is considered an optimum value. Here, a low CV value of just 1.11% indicated a high reproducibility of the proposed quadratic model. Furthermore, a higher value of correlation coefficients (R2 = 0.995, Adj R2 = 0.990 and Pred R2 = 0.961) indicated a well-fitted model59. The adequate precision of 43.12 for the degradation of CR shows a good response. A ratio larger than 4 is preferable, showing that the model's mean can deliver the expected performance60. The linear fit of actual vs predicted values for CR degradation (R2 = 0.995) is shown in Fig. 6a. The plot of residuals vs experimental runs (Fig. 6b,c) depicted all residuals are within ± 4%, indicating the model can predict a good response. The Box-cox plot (Fig. 6d) for power transforms showed a lambda value of 1, demonstrating that the response does not require transformation for the degradation of CR. These results confirm that this model could be used to predict the degradation efficiency of CR dye.
The relation between various independent factors on the degradation of CR was assessed by developing 3D response surfaces. The results of the interactions among the four parameters are shown in Fig. 7. Figure 7a shows the relation between the pH and catalyst dosage. The degradation process is not considerably impacted by pH. However, the degradation efficiency increases with increasing photocatalyst dosage and starts to decline above the optimum value. The upsurge in the photocatalyst dosage instigated a temporary rise in degradation efficiency brought on by the more active sites. However, the decline in the degradation efficiency of the dye was due to the increased opacity of the solution hindering the photons from reaching the catalyst's surface61. Figure 7b illustrates the relationship between pH and dye concentration affecting the rate of CR degradation. A low dye concentration causes a higher degradation rate. The plot of pH and time relating to degradation efficiency shows an initial increase in the degradation, which slumps after the optimum time duration (Fig. 7c). Figure 7d shows the relation between catalyst dosage and dye concentration. The degradation increases at decreasing CR concentration and increasing photocatalyst loading. The relation of catalyst dosage and dye concentration with time is illustrated in Fig. 7e,f. The photodegradation efficiency increases with increasing catalyst dosage and decreasing dye concentration. Similar trends were also observed in various studies investigating the photodegradation of dyes62,63. Optimization of these parameters is essential to maximize degradation efficiency and reduce the time and cost of operation. Therefore, in order to get the highest CR dye degradation efficiency, all four parameters were optimized.
The RSM has predicted the optimum pH to be 9, catalyst dosage of 21.5 mg, CR concentration of 35 ppm and radiation time of 67 min to attain a maximum degradation efficacy of 93.51% with a desirability value of 1.00. The NiCo2S4/Ch nanocomposite was shown to be a potential photocatalytic material for the degradation of CR by the desirability factor of 1.00. The optimum value of the independent factor and the desirability value is illustrated in Fig. 8. The actual degradation efficiency of CR was observed to be 93.46% according to run number 16, which is fairly consistent with the value predicted (93.51%). Meanwhile, pure NiCo2S4 nanoparticles showed 60.23% degradation under similar conditions. The results show the RSM model's significance for optimizing the reaction conditions for CR degradation.
The photodegradation experiment of CR dye was carried out at pH of 9, initial dye concentration of 35 ppm and catalyst dosage of 22 mg, and the degradation kinetics was plotted. Figure 9 shows the profile of the photodegradation of CR and the kinetics. The degradation of CR followed a pseudo-first-order model with a rate constant of 0.043 min−1. The synthesized NiCo2S4/Ch has a higher photodegradation rate than pure NiCo2S4 nanoparticles. The photocatalytic degradation of CR proceeds via the formation of aromatic intermediate products detected by the HRLCMS, as shown in Fig. 10. The attack of electrons, hydroxyl radicals and the addition of protons formed the intermediates. A plausible mechanism of the photocatalytic degradation pathway of CR dye based on the LCMS analysis is shown in Fig. 10b.
Scavenging tests
The concentration of ROS attacking the target molecules considerably impacts the rate of degradation processes62. To examine the role of the photogenerated electrons, holes, OH∙ and O2−∙ radicals on the photodegradation of CR, some sacrificial reagents were added before irradiating the reaction mixture to trap these species and to know which one of them was responsible for the most CR degradation11. Sodium carbonate (Na2CO3) as an OH∙ scavenger, benzoquinone (BQ) as an O2−∙ scavenger, potassium persulfate (K2S2O8) as an e− scavenger and disodium EDTA (Na2EDTA) as an h+ scavenger were used. As seen in Fig. 11a, BQ and Na2CO3 significantly retarded the degradation of CR, signifying that O2−∙ and OH∙ played a major role in the degradation of CR dye by NiCo2S4/Ch nanocomposite. Similar results were also reported in the literature64.
Performance and reusability studies
The photocatalytic performance of NiCo2S4/Ch nanocomposite for the degradation of other dyes has also been investigated. The photocatalytic degradation of congo red (CR), methylene blue (MB), methyl orange (MO), rhodamine B (RhB), crystal violet (CV) and rose Bengal (RB) were also investigated. As seen in Fig. 11b, the prepared photocatalyst showed excellent photocatalytic activity towards the degradation of these dyes with efficiency greater than 80%, suggesting great potential for the removal of dyes from the aqueous phase.
The recyclability and stability of the synthesized NiCo2S4/Ch nanocomposite were investigated, and the results are shown in Fig. 11c. The catalyst was centrifuged, washed with an ethanol solution, and dried in the oven at 60 °C for 2 h before being utilized for another cycle. The NiCo2S4/Ch nanocomposite could be reused and recycled for four successive cycles and showed an efficiency of 79.62 ± 1.23% after the fourth run. A minor decrease in the photocatalytic efficiency was attributed to the blockage of active sites by the degradation products of the CR dye and disruption in the pore structure of the photocatalyst during recycling. The XRD and SEM images of the reused photocatalyst are shown in Fig. 11d,e. The photocatalyst showed no significant change in the phase and morphology. All the peaks corresponding to NiCo2S4 were visible in the XRD spectrum of the reused photocatalyst. It is worth mentioning that the intensity of the peak of chitosan at 20° was decreased, possibly due to the accumulation of degradation products over the surface of the photocatalyst. However, the SEM image showed the NiCo2S4 nanoparticles were dispersed over a chitosan sheet, indicating the prepared photocatalyst maintained its structure after four cycles. Additionally, the absorbance spectra of reused NiCo2S4/Ch photocatalyst (Fig. 11f) showed a slight increase in the intensity in the UV region, which could be due to the adsorption of degradation intermediate products over the surface of the photocatalyst. The high stability and recyclability of the NiCo2S4/Ch photocatalyst was attributed to the support of the chitosan matrix.
Mechanism of photodegradation of CR by NiCo2S4/Ch
The valence and conduction band edge potential of NiCo2S4 plays a critical role in understanding the photocatalytic degradation mechanism of CR dye. The band positions of NiCo2S4 were calculated using Mullikan theory65. The bandgap and the absolute electronegativity of NiCo2S4 were calculated to be 2.2 eV66. The calculated valance band and conduction band positions of NiCo2S4 are + 1.93 eV and − 0.27 eV, respectively. A schematic illustration of the photodegradation mechanism of CR dye is shown in Fig. 12. When NiCo2S4/Ch was irradiated under visible light, the e− are excited from the valance band (VB) to the conduction band (CB) of NiCo2S4. The excitation of electrons leaves a positive vacancy (h+) in the VB. The electron-rich functional groups delocalize the photogenerated holes over the chitosan surface67. The photogenerated electrons are absorbed by the oxygen molecules, generating O2−∙ radicals. Meanwhile, the water molecules absorb the holes, producing OH∙ radicals. These radicals initiate a series of photocatalytic reactions attacking the CR molecules and bringing their degradation into simpler molecules68. The surface of chitosan and the functional groups helps to retain CR dye molecules over the photocatalyst's surface, while the ROS generated by NiCo2S4 degrade it. The hydroxyl groups of chitosan strongly attract the positively charged nitrogen of the CR dye69. Moreover, chitosan helps in charge of delocalization and enhances photocatalytic efficiency70. The photocatalytic reactions responsible for the degradation of CR are as follows71:
Comparison with other reported photocatalysts
Various materials have been reported to remove CR from the aqueous phase. As prepared, NiCo2S4/Ch nanocomposite outperformed most of the reported photocatalysts to degrade CR, as shown in Table 3.
Conclusion
The present study was consistent in assessing the photocatalytic performance of the fabricated NiCo2S4/Ch nanocomposite and the impacts of different reaction parameters on the photodegradation of CR dye. The effect of pH, photocatalyst dosage, initial dye concentration and irradiation time on the photodegradation of congo red was examined using RSM employing a CCD. According to the ANOVA analysis, a maximum degradation of 93.51% could be attained at the optimum pH of 9, photocatalyst dosage of 22 mg, and CR concentration of 35 ppm in 60 min with a desirability value of 1. Additionally, the statistical parameters like Adj R2, Pred R2, CV % and Adq precision justified the adequacy of the suggested quadratic model. The synthesized NiCo2S4/Ch nanocomposite showed a photodegradation efficiency of 93.46% within 60 min under prescribed conditions. The degradation mechanism was studied, and it was found that the composite effectively generated hydroxyl and superoxide radicals that contributed to the photodegradation process. The study emphasizes the possibility of NiCo2S4/Ch nanocomposite as a recyclable and cost-effective photocatalyst for treating dye-contaminated wastewater.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Gadore, V. & Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process Eng. 41, 101910 (2021).
Mishra, S. R. & Ahmaruzzaman, M. Tin oxide based nanostructured materials: Synthesis and potential applications. Nanoscale 14, 1566–1605 (2022).
Issa, M. A. & Abidin, Z. Z. Sustainable development of enhanced luminescence polymer-carbon dots composite film for rapid Cd2+ removal from wastewater. Molecules 25, 3541 (2020).
Mishra, S. R. & Ahmaruzzaman, M. Microplastics: Identification, toxicity and their remediation from aqueous streams. Sep. Purif. Rev. https://doi.org/10.1080/15422119.2022.2096071 (2022).
Jabbar, Z. H. & Esmail Ebrahim, S. Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 17, 100666 (2022).
Issa, M. A. et al. Facile synthesis of nitrogen-doped carbon dots from lignocellulosic waste. Nanomaterials 9, 1500 (2019).
Jabbar, Z. H., Graimed, B. H., Okab, A. A., Alsunbuli, M. M. & Al-husseiny, R. A. Construction of 3D flower-like Bi5O7I/Bi/Bi2WO6 heterostructure decorated NiFe2O4 nanoparticles for photocatalytic destruction of Levofloxacin in aqueous solution: Synergistic effect between S-scheme and SPR action. J. Photochem. Photobiol. A Chem. 441, 114734 (2023).
Mishra, S. R. & Ahmaruzzaman, M. CuO and CuO-based nanocomposites: Synthesis and applications in environment and energy. Sustain. Mater. Technol. 33, e00463 (2022).
Gadore, V., Mishra, S. R. & Ahmaruzzaman, M. Bio-inspired sustainable synthesis of novel SnS2/biochar nanocomposite for adsorption coupled photodegradation of amoxicillin and congo red: Effects of reaction parameters, and water matrices. J. Environ. Manag. 334, 117496 (2023).
Bhuyan, A. & Ahmaruzzaman, M. Metal-organic frameworks: A new generation potential material for aqueous environmental remediation. Inorg. Chem. Commun. 140, 109436 (2022).
Gadore, V., Mishra, S. R. & Ahmaruzzaman, M. Facile green synthesis of SnS2 nanoparticles using Tulsi extract: insight into the optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 1–18 (2023).
Jabbar, Z. H., Ammar, S. H. & Esmail Ebrahim, S. Enhanced visible-light photocatalytic bacterial inhibition using recyclable magnetic heterogeneous nanocomposites (Fe3O4@SiO2@Ag2WO4@Ag2S) in core/shell structure. Environ. Nanotechnol. Monit. Manag. 16, 100601 (2021).
Gadore, V. & Ahmaruzzaman, M. M. Fly ash–based nanocomposites: A potential material for effective photocatalytic degradation/elimination of emerging organic pollutants from aqueous stream. Environ. Sci. Pollut. Res. 28, 46910–46933 (2021).
Gadore, V., Mishra, S. R. & Ahmaruzzaman, M. Bandgap engineering approach for synthesising photoactive novel Ag/HAp/SnS2 for removing toxic anti-fungal pharmaceutical from aqueous environment. J. Hazard. Mater. https://doi.org/10.1016/J.JHAZMAT.2023.132458 (2023).
Mishra, S. R., Gadore, V. & Ahmaruzzaman, M. Inorganic–organic hybrid quantum dots for AOP-mediated photodegradation of ofloxacin and para-nitrophenol in diverse water matrices. NPJ Clean Water 6, 1–24 (2023).
Ranjan Mishra, S., Gadore, V. & Ahmaruzzaman, M. Novel 3D sphere-like β-In2S3/Biochar nanoflowers for remediation of dyes in single and binary systems and interpretation using statistical physical modeling. Environ. Nanotechnol. Monit. Manag. 20, 100807 (2023).
Sunaina, et al. Surface photosensitization of ZnO by ZnS to enhance the photodegradation efficiency for organic pollutants. SN Appl. Sci. 3, 689 (2021).
Song, Z. et al. Magnetic grinding synthesis of copper sulfide-based photocatalytic composites for the degradation of organic dyes under visible light. New J. Chem. 47, 2286–2295 (2023).
Zhang, L. et al. Effective charge separation and enhanced photocatalytic activity by the heterointerface in MoS2/reduced graphene oxide composites. RSC Adv. 6, 60318–60326 (2016).
Ahmaruzzaman, M. & Gadore, V. MoS2 based nanocomposites: An excellent material for energy and environmental applications. J. Environ. Chem. Eng. 9, 105836 (2021).
Thambidurai, M. et al. Studies on optical absorption and structural properties of Fe doped CdS quantum dots. Solid State Sci. 12, 1554–1559 (2010).
Lu, F. et al. Engineering sulfur vacancies and impurities in NiCo2S4 nanostructures toward optimal supercapacitive performance. Nano Energy 26, 313–323 (2016).
Shen, J. et al. Controlled synthesis and comparison of NiCo2S4/graphene/2D TMD ternary nanocomposites for high-performance supercapacitors. Chem. Commun. 52, 9251–9254 (2016).
Xia, C., Li, P., Gandi, A. N., Schwingenschlögl, U. & Alshareef, H. N. Is NiCo2S4 really a semiconductor?. Chem. Mater. 27, 6482–6485 (2015).
Li, W. et al. In situ electronic redistribution tuning of ZnIn2S4 nanosheets on NiCo2S4 hollow tube for boosted photocatalytic hydrogen evolution. Appl. Surf. Sci. 598, 153801 (2022).
Wang, Z., Nengzi, L. C., Zhang, X., Zhao, Z. & Cheng, X. Novel NiCo2S4/CS membranes as efficient catalysts for activating persulfate and its high activity for degradation of nimesulide. Chem. Eng. J. 381, 122517 (2020).
Pandey, N., Shukla, S. K. & Singh, N. B. Water purification by polymer nanocomposites: An overview. Nanocomposites 3, 47–66. https://doi.org/10.1080/20550324.2017.1329983 (2017).
Sirajudheen, P. & Meenakshi, S. Facile synthesis of chitosan-La3+-graphite composite and its influence in photocatalytic degradation of methylene blue. Int. J. Biol. Macromol. 133, 253–261 (2019).
Khan, A., Badshah, S. & Airoldi, C. Biosorption of some toxic metal ions by chitosan modified with glycidylmethacrylate and diethylenetriamine. Chem. Eng. J. 171, 159–166 (2011).
Bandara, S., Du, H., Carson, L., Bradford, D. & Kommalapati, R. Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomater 10, 1903 (2020).
Thakur, V. K. & Voicu, S. I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 146, 148–165 (2016).
Hamad, H., Bassyouni, D., El-Ashtoukhy, E. S., Amin, N. & Abd El-Latif, M. Electrocatalytic degradation and minimization of specific energy consumption of synthetic azo dye from wastewater by anodic oxidation process with an emphasis on enhancing economic efficiency and reaction mechanism. Ecotoxicol. Environ. Saf. 148, 501–512 (2018).
Mishra, S. R., Gadore, V., Ghotekar, S. & Ahmaruzzaman, M. Insights into the enhanced photocatalytic and antioxidant properties of novel biogenically synthesised β-In2S3 quantum dots. Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2023.2186228 (2023).
Hasanpour, M., Motahari, S., Jing, D. & Hatami, M. Statistical analysis and optimization of photodegradation efficiency of methyl orange from aqueous solution using cellulose/zinc oxide hybrid aerogel by response surface methodology (RSM). Arab. J. Chem. 14, 103401 (2021).
Sakkas, V. A., Islam, M. A., Stalikas, C. & Albanis, T. A. Photocatalytic degradation using design of experiments: A review and example of the Congo red degradation. J. Hazard. Mater. 175, 33–44 (2010).
Bose, S., Tripathy, B. K., Debnath, A. & Kumar, M. Boosted sono-oxidative catalytic degradation of brilliant green dye by magnetic MgFe2O4 catalyst: Degradation mechanism, assessment of bio-toxicity and cost analysis. Ultrason. Sonochem. 75, 105592 (2021).
Jiang, J. et al. A PEGylated deep eutectic solvent for controllable solvothermal synthesis of porous NiCo2S4 for efficient oxygen evolution reaction. Green Chem. 19, 3023–3031 (2017).
Rhim, J. W., Hong, S. I., Park, H. M. & Ng, P. K. W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 54, 5814–5822 (2006).
Rajesh Kumar, B. & Hymavathi, B. X-ray peak profile analysis of solid-state sintered alumina doped zinc oxide ceramics by Williamson-Hall and size-strain plot methods. J. Asian Ceram. Soc. 5, 94–103 (2017).
Mahmoud, Z. H., Hamrouni, A., Kareem, A. B., Mostafa, M. A. & Majeed, A. H. Synthesis and characterization of chitosan sheet modified by varied weight ratio of anatase (TiO2) nano mixture with Cr (VI) adsorbing. Kuwait J. Sci. (2023).
Sirajudheen, P., Resha Kasim, V. C., Nabeena, C. P., Basheer, M. C. & Meenakshi, S. Tunable photocatalytic oxidation response of ZnS tethered chitosan-polyaniline composite for the removal of organic pollutants: A mechanistic perspective. Mater. Today Proc. 47, 2553–2559 (2021).
Vieira, R. S., Oliveira, M. L. M., Guibal, E., Rodríguez-Castellón, E. & Beppu, M. M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A Physicochem. Eng. Asp. 374, 108–114 (2011).
Yang, X. et al. Perylene-functionalized graphene sheets modified with chitosan for voltammetric discrimination of tryptophan enantiomers. Microchim. Acta 186, 1–12 (2019).
Mishra, S. R., Roy, P., Gadore, V. & Ahmaruzzaman, M. A combined experimental and modeling approach to elucidate the adsorption mechanism for sustainable water treatment via In2S3-anchored chitosan. Sci. Rep. 13, 1–24 (2023).
Briggs, D. Handbook of X-ray photoelectron spectroscopy. In Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979 (eds. Wanger, C. D. Riggs, W. M., Davis, L. E., Moulder J. F. & Muilenberg G. E.), 190 pp. $195. Surf. Interface Anal. 3, v–v (1981).
Gieroba, B. et al. Spectroscopic studies on the temperature-dependent molecular arrangements in hybrid chitosan/1,3-β-D-glucan polymeric matrices. Int. J. Biol. Macromol. 159, 911–921 (2020).
Jiang, K. et al. Noble metal-free NiCo2S4/CN sheet-on-sheet heterostructure for highly efficient visible-light-driven photocatalytic hydrogen evolution. J. Alloys Compd. 853, 157284 (2021).
Li, C. et al. NiCo2S4 decorated gC3N4 nanosheets for enhanced photocatalytic hydrogen evolution. J. Nanoparticle Res. 22, 1–12 (2020).
Mishra, S. R. et al. In2S3 incorporated into CO32−@Ni/Fe/Zn trimetallic LDH as a bi-functional novel nanomaterial for enzymatic urea sensing and removal of sulfur-containing pharmaceutical from aqueous streams. Chem. Eng. J. https://doi.org/10.1016/J.CEJ.2023.146207 (2023).
Mishra, S. R., Gadore, V. & Ahmaruzzaman, M. Insights into persulfate-activated photodegradation of tinidazole and photoreduction of hexavalent chromium through β-In2S3 anchored on Ag-doped fish scale-derived HAp composite quantum dots. J. Clean. Prod. 427, 139221 (2023).
Melo-Silveira, R. F. et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by Xylan extracted from corn cobs. Int. J. Mol. Sci. 13, 409–426 (2011).
Taha, A. et al. Green synthesis of an activated carbon-supported Ag and ZnO nanocomposite for photocatalytic degradation and its antibacterial activities. Molecules 25, 1586 (2020).
Pandian, S. R. K., Deepak, V., Kalishwaralal, K. & Gurunathan, S. Biologically synthesized fluorescent CdS NPs encapsulated by PHB. Enzym. Microb. Technol. 48, 319–325 (2011).
Zhu, Y. et al. Mesoporous NiCo2S4 nanoparticles as high-performance electrode materials for supercapacitors. J. Power Sources 273, 584–590 (2015).
Gadore, V., Mishra, S. R. & Ahmaruzzaman, M. Green and environmentally sustainable fabrication of SnS2 quantum dots/chitosan nanocomposite for enhanced photocatalytic performance: Effect of process variables, and water matrices. J. Hazard. Mater. 444, 130301 (2023).
Gadore, V., Ranjan Mishra, S. & Ahmaruzzaman, M. SnS2 modified carbonate-intercalated Ni–Zn–Fe trimetallic LDH as a novel photocatalyst for persulfate activation: Influence of operational parameters, co-existing ions and organic compounds. Chem. Eng. J. 475, 146157 (2023).
Yadav, G. et al. Biogenic growth of egg shell–derived CaMn2O4 over tailored FLY ASH surface for synergistically photodegradation of ofloxacin: materialistic and chemical studies. Biomass Convers. Biorefinery 1–18 (2023).
Idrees, S. A. & Ibrahim, M. K. Optimization of congo-red photo-catalytic degradation by central composite design. In 2018 International Conference on Advanced Science and Engineering (ICOASE) 389–393 (IEEE, 2018).
Tripathi, P., Tiwari, S., Sonwani, R. K. & Singh, R. S. A step towards enhancing the efficiency of biofilm mediated degradation of brilliant green dye in packed bed bioreactor: Statistical and toxicity analysis. Process Saf. Environ. Prot. 170, 1228–1239 (2023).
Hosseini-Nasab, S. J., Saber-Tehrani, M., Haghgoo, M. & Aberoomand-Azar, P. Control of porous properties of ambient dried sodium silicate-based aerogels using response surface methodology. J. Thermoplast. Compos. Mater. 0892705719876316 (2019).
Dey, A. K., Mishra, S. R. & Ahmaruzzaman, M. Solar light–based advanced oxidation processes for degradation of methylene blue dye using novel Zn-modified CeO2@biochar. Environ. Sci. Pollut. Res. 30, 53887–53903 (2023).
Gadore, V., Mishra, S. R. & Ahmaruzzaman, M. One-pot synthesis of CdS/CeO2 heterojunction nanocomposite with tunable bandgap for the enhanced advanced oxidation process. Sci. Rep. 13, 7708 (2023).
Mishra, S. R., Gadore, V. & Ahmaruzzaman, M. A critical review on In2S3-based nanomaterial for emerging contaminants elimination through integrated adsorption-degradation technique: Effect of reaction parameters and co-existing species. J. Hazard. Mater. Lett. 4, 100087 (2023).
Wang, J., Wang, G., Cheng, B., Yu, J. & Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 42, 56–68 (2021).
Cao, W., Chen, L. & Qi, Z. Microwave-assisted synthesis of Ag/Ag2SO4/ZnO nanostructures for efficient visible-light-driven photocatalysis. J. Mol. Catal. A Chem. 401, 81–89 (2015).
Peng, J., Xu, J., Wang, Z., Ding, Z. & Wang, S. Developing an efficient NiCo2S4 cocatalyst for improving the visible light H2 evolution performance of CdS nanoparticles. Phys. Chem. Chem. Phys. 19, 25919–25926 (2017).
Liang, H., Lv, C., Chen, H., Wu, L. & Hou, X. Facile synthesis of chitosan membranes for visible-light-driven photocatalytic degradation of tetracycline hydrochloride. RSC Adv. 10, 45171–45179 (2020).
Yadav, G., Mishra, S. R., Gadore, V., Yadav, N. & Ahmaruzzaman, M. A smart and sustainable pathway for abatement of single and binary mixtures of dyes through magnetically retrievable Ca4Fe9O17 anchored on Biochar matrix. Sci. Rep. 131(13), 1–21 (2023).
Chatterjee, S., Lee, M. W. & Woo, S. H. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 101, 1800–1806 (2010).
Honarmand, M., Naeimi, A., Rezakhani, M. S. & Chaji, M. A. Ni/NiO doped chitosan-cellulose based on the wastes of barley and shrimp for degradation of ciprofloxacin antibiotic. J. Mater. Res. Technol. 18, 4060–4074 (2022).
Ahmaruzzaman, M. & Mishra, S. R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 143, 111417 (2021).
Jabbar, Z. H. et al. Photocatalytic degradation of Congo red dye using magnetic silica-coated Ag2WO4/Ag2S as Type I heterojunction photocatalyst: Stability and mechanisms studies. Mater. Sci. Semicond. Process. 153, 107151 (2023).
Saini, D., Aggarwal, R., Anand, S. R. & Sonkar, S. K. Sunlight induced photodegradation of toxic azo dye by self-doped iron oxide nano-carbon from waste printer ink. Sol. Energy 193, 65–73 (2019).
Bhagwat, U. O., Wu, J. J., Asiri, A. M. & Anandan, S. Photocatalytic degradation of congo red using PbTiO3 nanorods synthesized via a sonochemical approach. ChemistrySelect 3, 11851–11858 (2018).
Arumugam, B., Muthukutty, B., Chen, S.-M., Amanulla, B. & Ramaraj, S. K. Sustainable one-pot synthesis of strontium phosphate nanoparticles with effective charge carriers for the photocatalytic degradation of carcinogenic naphthylamine derivative. New J. Chem. 45, 15437–15447 (2021).
Adam, R. E., Pozina, G., Willander, M. & Nur, O. Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostruct. Fundam. Appl. 32, 11–18 (2018).
Acknowledgements
The authors are thankful to the Director, National Institute of Technology Silchar, for his help and continuous support for the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
V.G. wrote the main manuscript text. A.K.S. modified, revised and edited the manuscript. S.R.M. prepared figures and edited the manuscript and M.A. discussed results, commented, revised and corrected the whole manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gadore, V., Singh, A.K., Mishra, S.R. et al. RSM approach for process optimization of the photodegradation of congo red by a novel NiCo2S4/chitosan photocatalyst. Sci Rep 14, 1118 (2024). https://doi.org/10.1038/s41598-024-51618-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51618-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.