Abstract
Patients with left main coronary artery disease (LMCAD) with a high SYNTAX score (SS) were excluded from randomized studies that comparing percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). We sought to compare PCI and CABG in the real-world practice and investigate the impact of SS I, SS II, and SS II 2020 on clinical outcomes. In total, 292 Patients with LMCAD (173 PCI, 119 CABG) treated between 2017 and 2021 were enrolled. The primary outcome was major adverse cardiovascular events (MACE), a composite of all-cause death, stroke, or myocardial infarction (MI). The mean SS I was high in both groups (PCI vs. CABG: 31.64 ± 11.45 vs. 32.62 ± 11.75, p = 0.660). The primary outcome occurred in 28 patients (16.2%) in the PCI group and in 19 patients (16.0%) in the CABG group without significant difference [adjusted hazard ratio, 95% CI = 0.98 (0.51–1.90), p = 0.97] over the follow-up period (26.9 ± 17.7 months). No significant difference was observed in all-cause mortality (11.6% vs. 11.8%, p = 0.93) or stroke rates (3.5% vs. 5.0%, p = 0.51) between groups. However, PCI was associated with higher MI (4.6% vs. 0.8%, p < 0.05) and revascularization rates (26% vs. 5.9%, p < 0.001). Prognostic value of the SS I, SS II and SS II 2020 on the primary outcome was not relevant in the PCI group. Among patients with LMCAD, PCI and CABG did not significantly differ in the composite endpoint of all-cause death, stroke, and MI. These results support the potential expansion of PCI indications in LMCAD management for whom are ineligible for CABG with complex coronary artery disease.
Similar content being viewed by others
Introduction
The optimal treatment for left main coronary artery disease (LMCAD), either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), remains disputed. The Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) study recommended CABG for Patients with LMCAD, particularly those with high SYNTAX scores (SS) I (≥33), citing higher adverse events rates with PCI1. However, 10-year follow-up results from SYNTAX study indicated no mortality differences between PCI and CABG2. Technological advancements in drug-eluting stents (DES), coronary devices, and interventional techniques has expanded the applicability of PCI. Landmark trials like EXCEL and PRECOMBAT showed comparable outcomes between PCI and CABG for patients with low to intermediate anatomical complexity3,4. Consequently, guidelines classify PCI as a class I recommendation for LMCAD with a low SS I (0–22), a Class IIA recommendation for LMCAD for an intermediate SS I (23–32), but a class IIIB recommendation for a high SS I5,6. However, high SS I patients with LMCAD, excluded from EXCEL and PRECOMBAT, may still treated by PCI in contemporary real-world practice7,8.
The SS I was developed as an angiographic scoring tool to classify anatomical complexity of coronary artery disease. Inherent limitations in the SS I have been repeatedly challenged and its applicability remains debatable9. After that, SS II incorporating anatomical complexity of coronary arteries and seven clinical characteristics was established to predict four-year all-cause mortality and determine the most appropriate revascularization strategy (PCI or CABG) in patients with multivessel disease or LMCAD10. Recently, SS II 2020 was developed using the ten-year outcomes reported in the extended SYNTAX(ES) study and has been externally validated using patient-level data from three landmark randomized trials11. Absolute risk deference (ARD) between PCI and CABG calculated using the SS II 2020 is being tested to support clinical decision making on revascularization12.
In this context, we sought to investigate clinical outcomes of patients with LMCAD undergoing PCI or CABG, including patients with high SS I and evaluate the impact of SS I, SS II, and SS II 2020.
Methods
Study population
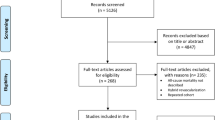
In this retrospective study, patients with chronic or acute coronary syndrome who underwent PCI or CABG for de novo LMCAD (defined as ≥ 50% left main artery stenosis) between January 2017 and December 2021 at the Taipei Veterans General Hospital, a high-volume referral center in Taiwan, were included. The revascularization strategy, incorporating factors like lesion complexity, comorbidities, surgical risk, and affordability of DES, was a shared decision by physicians and patients. The Heart Team approach was preferred for intermediate or high SS patients. PCI and CABG procedures were performed as per local practice norms with intravascular imaging and stenting strategies left to operator discretion. Post-PCI patients received dual antiplatelet therapy as per guidelines13, with contemporary new generation DES recommended.
This study has been approved by the research ethics committee of the Taipei Veterans General Hospital (No.2022-06-005BC) and was conducted in accordance with the Declaration of Helsinki. The research ethics committee approved a request to waive of informed consent since no more than minimal risk to study subjects.
SYNTAX score calculation
Two independent interventional cardiologists blinded to clinical outcomes (CC Chang and MJ Chuang) calculated SS I from coronary angiograms. SS II incorporated seven clinical parameters: age, creatinine clearance, left ventricular ejection fraction (LVEF), presence of LMCAD, gender, presence of chronic obstructive pulmonary disease (COPD), and presence of peripheral artery disease (PAD), alongside the SS I and predicted a mortality outcome for either PCI or CABG, leading to patient categorization into PCI, CABG, or equipoise groups. SS II 2020 incorporates two anatomical effect modifiers (SSI and the presence of three-vessel disease or LMCAD) and seven clinical prognostic factors of revascularization, including age, medically treated diabetes mellitus with or without insulin, COPD, PAD, current smoking, creatinine clearance and LVEF to predict 5-year major adverse cardiovascular events (MACE). SS II 2020 of the study population were analyzed by using the web calculator. The predicted 5-year MACE rates with PCI and 5-year MACE rates with CABG were provided. Absolute risk difference (ASD) between PCI and CABG was calculated (5-year MACE rate with PCI minus 5-year MACE rate with CABG).
Study endpoints
The primary endpoint was MACE during follow-up, defined as a composite of all-cause mortality, stroke, or MI as per the Fourth Universal Myocardial Infarction definition14. MACE was analyzed hierarchically. The complete revascularization was defined as no residual stenosis ≥ 70%15 either after the index procedure or after staged PCI within 60 days.
Statistical methods
Categorical variables are presented as percentages and numbers, continuous variables as mean ± standard deviation. Baseline characteristics and procedural data were compared using Student’s t-test for continuous variables and chi-square test for categorical data. Survival curves were constructed using Kaplan-Meier estimates and were compared using the log-rank test. Hazard ratios with 95% confidence intervals were reported based on the Cox regression model. A two-sided p value less than 0.05 was considered statistically significant. Data analysis used SPSS software (version 25, SPSS, Chicago, Illinois, USA).
Results
Patient characteristics
Between January 2017 and December 2021, 292 Patients with LMCAD were retrospectively enrolled: 173 underwent PCI, and 119 patients received CABG. Table 1 presents the baseline characteristics. Most of patents had LMCAD and three-vessel disease. The PCI group were older with more heart failure history and previous MI. However, other medical conditions and clinical presentations were similar between groups. Table 2 summarizes the SS I, SS II, and SS II 2020 between two groups. The anatomical complexity of coronary artery disease based on SS I was similar between groups (PCI vs. CABG: 31.64 ± 11.45 vs. 32.62 ± 11.75, p = 0.660). In the PCI group, 43.4% of patients had a high SS I, whereas 20.2% with a low score in the CABG group. Despite similar SS II-PCI scores and predicted 4-year PCI mortality rates across groups, SS II-CABG scores and predicted 4-year CABG mortality were significantly lower in the CABG group. Most SS II recommendations were equipoised between PCI and CABG. In the PCI group, only 80.9% of patients were treated following SS II recommendations, compared with 92.4% in the CABG group.
The SS II 2020 predicted 5-year MACE rates with PCI and 5-year MACE rates with CABG were both significantly higher in the PCI group then the CABG group (Fig. 1 and Table 2). ARD between PCI and CABG were similar in both groups.
Supplementary Table S1 summarizes details of PCI procedures. Of PCI patients, 89.1% used intravascular imaging (either intravascular ultrasound (IVUS) or optical coherence tomography (OCT)), 70.5% received one stent for LMCAD and proximal optimization technique (POT) was performed in 87.9% of cases. Complete revascularization was achieved in 68.2% patients. We used DES in 96.5% of the patients. In the CABG group, 73.1% received arterial grafts, averaging 3.0 ± 0.8 grafts per patient.
Major clinical outcome
Table 3 presents clinical outcomes. After an average follow-up of 26.9 ± 17.7 months, the primary outcome occurred in 16.2% of PCI patients and 16.0% of CABG patients, a statistically nonsignificant difference. The hazard ratio (HR) (PCI vs. CABG) was 1.00 (95% confidence interval [CI], 0.56–1.80, p = 0.99) (Fig. 2). After adjusting for covariates (age, gender, diabetes, hypertension, chronic kidney disease, end-stage renal disease, known heart failure, prior MI), cumulative hazard rates of the primary outcome remained similar in both treatments, with an adjusted HR of 0.98 (95% CI, 0.51 to 1.90, p=0.97). Regarding the individual components of the primary outcome and other clinical endpoints, all-cause mortality (11.6% in PCI vs. 11.8% in CABG, p=0.93), stroke (3.5% in PCI vs. 5.0% in CABG, p=0.51), and cardiac death rates (6.9% in PCI vs. 6.7% in CABG, p=0.95) did not differ significantly. However, the MI rates (4.6% in PCI vs. 0.8% in CABG, p<0.05) and repeat revascularization rates (26% in PCI group vs. 5.9% in CABG, p < 0.001) were significantly higher in the PCI group.
Table 4 shows the subgroup analysis. The event rate of the primary outcome did not significantly differ between PCI and CABG groups, irrespective of SS I and SS II classifications. The treatment effect of PCI versus CABG was consistent across all subgroups.
Cox-regression analyses were further performed to investigate the association between MACE and SS I, SS II, and SS II 2020 subgroups in the PCI cohort respectively. Patients in the PCI group were stratified by SS I (<33 or ≥ 33), SS II recommendations (PCI, CABG, or equipoise) or SS II 2020 (ARD ≥ 4.5% or < 4.5%). Supplementary Table S2 shows the baseline characteristics of the PCI group divided by SS I (<33 or ≥ 33). Prognostic value of the SS I, SS II and SS II 2020 on the primary outcome was not relevant in our PCI cohort (Fig. 3 and Supplementary Table S3).
Discussion
Main findings
In this observational study, we identified that the incidence of all-cause death, stroke, or MI in patients with LMCAD undergoing either PCI or CABG was similar, even in those with a high SS I. Additionally, the rates of MI and repeat revascularization were significantly higher in the PCI group, corroborating the NOBLE trial’s results16.
The original SYNTAX trial suggested CABG as the preferable method for patients with LMCAD with high SS I17,18. Technological advancements, such as new drug coatings for DES and thinner metallic platforms19, have improved stent design, reducing the rate of in-stent restenosis and target lesion revascularization.
Moreover, the prevalent use of intravascular imaging, such as IVUS or OCT, has improved PCI outcomes18,20,21. The success of PCI for LMCAD relies on thorough pre-procedural planning, correct stent apposition, optimal stent expansion, and appropriate wire positioning during rewiring. Both IVUS21,22,23 and OCT24,25 can provide valuable information when performing PCI for LMCAD. Our cohort reported that 89.1% of patients undergoing PCI for LMCAD used intravascular imaging, higher than the 77.2% reported in the EXCEL trial26. Current guidelines recommend IVUS for LMCAD as a Class IIA intervention27.
These advancements in PCI techniques and technology have improved clinical outcomes, a conclusion supported by the results of the SYNTAX II study28. This progress also allows for a greater likelihood of complete revascularization, further improving clinical outcomes29. The complete revascularization rate of PCI in our study was 68.2%, which could explain the comparative outcome between PCI and CABG. In our study, most patients in the PCI group received DES (96.5%), all of which were second-generation or third-generation DES. This contemporary PCI approach for patients with LMCAD might explain the comparable short-term and mid-term outcomes between the PCI and CABG groups. As such, the applicability of only using the SS I to guide decision making on revascularization is questionable. It is noteworthy that real-world data show the advancements have made PCI an alternative choice for patients with LMCAD and high SS I30. Moreover, clinical factors such as diabetes or EuroSCORE were more relevant to outcomes instead of the SS I31.
Likewise, the mean SS I was 32.0 ± 11.6 in our study and 45.5% of patients had high SS I (SS I ≥ 33), supported PCI as a reasonable choice in high anatomical complexity cases who are ineligible for CABG.
Regarding the SS II 2020, the predicted 5-year MACE rates with PCI/CABG were both significantly higher in our PCI group than in the CABG group. It is noteworthy that patients receiving PCI in our study were older, with higher prevalence of heart failure and previous MI. This observation may reflect the fact that patients with a high risk may prefer PCI over CABG or even were not suitable for CABG after heart team evaluation.
Nevertheless, the PCI group had higher risks for MI and repeat revascularization, aligning with prior research28,32,33,34. Among the eight patients experiencing MI in the PCI group of our study, five incidents related to target lesion revascularization (TLR). In contrast, the CABG group reported a markedly lower MI rate. PCI primarily addresses flow-limiting lesions, but further events may occur due to either target lesions or non-target lesions. With CABG treatment, graft vessels usually bypass the entire disease vessels which might help avert future MI events35. Beside the diseased part of the target vessel, previous study suggested left internal mammary artery (LIMA) grafting was associated with lower risk of down-stream disease progression compared to PCI36. The EXCEL trial also confirmed significantly higher non-periprocedural MI rates in the PCI group compared with the CABG group at five years (6.8% vs. 3.5%)3.
In our cohort, most patients in the PCI group underwent repeat revascularization non-emergently, often for non-left main lesions. The risk of repeat revascularization and TLR did not vary between high and low to intermediate SS I group (supplementary Table S3). Furthermore, we suggest implementing standardized postoperative care after revascularization (PCI or CABG) in future studies due to potential disparities in postoperative care among surgeons and interventionalists.
The SS II and SS II 2020 incorporate clinical factors to predict long-term outcomes after revascularization. In our study, SS II recommendations did not significantly discriminate the risk of MACE in the PCI group. Similarly, a cut off value of ARD ≥ 4.5% or <4.5% calculated from the SS II 2020 did not associate with MACE. These observations may be influenced by a limited sample size without adequate statistical power. In addition, we used categorical variables rather than the absolute estimated mortality in this study, which limited the prognostic prediction. Patients with high estimated mortality in both PCI and CABG could be classified as equipoise in SS II and as ARD < 4.5% in SS II 2020, although their prognosis was expected to be poorer. The utility of SS II 2020 warrants further evaluation.
We acknowledge that our study, being retrospective and observational, may have inherent limitations, including potential selection bias and confounding factors. Periprocedural MI was not included due to debates over its definition after revascularization. Clinical events were obtained by reviewing medical records without formal adjudication. Bleeding events were not systematically collected and reported. The residual SYNTAX score was not provided in this study.
Conclusion
In this single-center retrospective study, we observed no significant difference in the composite outcome of all-cause death, stroke, or MI between PCI and CABG in patients with LMCAD, irrespective of SS I. These results support the potential expansion of PCI indications in LMCAD management for whom are ineligible for CABG with complex coronary artery disease.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Reference
Serruys, P. W. et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360, 961–972. https://doi.org/10.1056/NEJMoa0804626 (2009).
Thuijs, D. et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 394, 1325–1334. https://doi.org/10.1016/s0140-6736(19)31997-x (2019).
Stone, G. W. et al. Five-year outcomes after PCI or CABG for left main coronary disease. N. Engl. J. Med. 381, 1820–1830. https://doi.org/10.1056/NEJMoa1909406 (2019).
Park, D. W. et al. Ten-year outcomes after drug-eluting stents versus coronary artery bypass grafting for left main coronary disease: Extended follow-up of the PRECOMBAT trial. Circulation 141, 1437–1446. https://doi.org/10.1161/circulationaha.120.046039 (2020).
Neumann, F.-J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165. https://doi.org/10.1093/eurheartj/ehy394 (2018).
Lawton, J. S. et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization. J. Am. College Cardiol. 79, e21–e129. https://doi.org/10.1016/j.jacc.2021.09.006 (2022).
Kipp, R. et al. Patient preferences for coronary artery bypass graft surgery or percutaneous intervention in multivessel coronary artery disease. Catheter. Cardiovasc. Interv. 82, 212–218. https://doi.org/10.1002/ccd.24399 (2013).
Ohlow, M. A., Farah, A., Kuntze, T. & Lauer, B. Patients’ preferences for coronary bypass grafting or staged percutaneous coronary intervention in multi-vessel coronary artery disease. Int. J. Clin. Pract. 72, e13056. https://doi.org/10.1111/ijcp.13056 (2018).
Morice, M. C. Has the SYNTAX score become obsolete?. J. Am. Coll. Cardiol. 72, 1330–1331. https://doi.org/10.1016/j.jacc.2018.07.023 (2018).
Farooq, V. et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 381, 639–650. https://doi.org/10.1016/S0140-6736(13)60108-7 (2013).
Takahashi, K. et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: Secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet 396, 1399–1412. https://doi.org/10.1016/S0140-6736(20)32114-0 (2020).
Hara, H. et al. External validation of the SYNTAX Score II 2020. J. Am. Coll. Cardiol. 78, 1227–1238. https://doi.org/10.1016/j.jacc.2021.07.027 (2021).
Neumann, F. J. et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165. https://doi.org/10.1093/eurheartj/ehy394 (2019).
Thygesen, K. et al. Fourth universal definition of myocardial infarction (2018). Circulation 138, e618–e651. https://doi.org/10.1161/CIR.0000000000000617 (2018).
Mehta, S. R. et al. Complete revascularization with multivessel PCI for myocardial infarction. N. Engl. J. Med. 381, 1411–1421. https://doi.org/10.1056/NEJMoa1907775 (2019).
Holm, N. R. et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. The Lancet 395, 191–199. https://doi.org/10.1016/S0140-6736(19)32972-1 (2020).
Mohr, F. W. et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381, 629–638. https://doi.org/10.1016/s0140-6736(13)60141-5 (2013).
Yoon, Y. H. et al. Impact of SYNTAX score on 10-year outcomes after revascularization for left main coronary artery disease. JACC Cardiovasc. Interv. 13, 361–371. https://doi.org/10.1016/j.jcin.2019.10.020 (2020).
Hassan, S., Ali, M. N. & Ghafoor, B. Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach. J. Cardiothoracic Surg. 17, 65. https://doi.org/10.1186/s13019-022-01812-y (2022).
Hong, S.-J. et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JAMA 314, 2155–2163. https://doi.org/10.1001/jama.2015.15454 (2015).
de la Torre Hernandez, J. M. et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J. Am. Coll. Cardiol. 58, 351–358. https://doi.org/10.1016/j.jacc.2011.02.064 (2011).
Ladwiniec, A. et al. Intravascular ultrasound to guide left main stem intervention: A NOBLE trial substudy. EuroIntervention 16, 201–209. https://doi.org/10.4244/eij-d-19-01003 (2020).
Wang, Y. et al. Meta-analysis and systematic review of intravascular ultrasound versus angiography-guided drug eluting stent implantation in left main coronary disease in 4592 patients. BMC Cardiovasc. Disorders 18, 115. https://doi.org/10.1186/s12872-018-0843-z (2018).
Amabile, N. et al. Optical coherence tomography to guide percutaneous coronary intervention of the left main coronary artery: The LEMON study. EuroIntervention 17, e124–e131. https://doi.org/10.4244/eij-d-20-01121 (2021).
Cortese, B. et al. Optical coherence tomography, intravascular ultrasound or angiography guidance for distal left main coronary stenting. The ROCK cohort II study. Catheter. Cardiovasc. Interv. 99, 664–673. https://doi.org/10.1002/ccd.29959 (2022).
Stone, G. W. et al. Five-year outcomes after PCI or CABG for left main coronary disease. New Engl. J. Med. 381, 1820–1830. https://doi.org/10.1056/NEJMoa1909406 (2019).
Neumann, F. J. et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165. https://doi.org/10.1093/eurheartj/ehy394 (2019).
Banning, A. P. et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur. Heart J. 43, 1307–1316. https://doi.org/10.1093/eurheartj/ehab703 (2022).
Ahn, J. M. et al. Comparison of stenting versus bypass surgery according to the completeness of revascularization in severe coronary artery disease: patient-level pooled analysis of the SYNTAX, PRECOMBAT, and BEST trials. JACC Cardiovasc. Interv. 10, 1415–1424. https://doi.org/10.1016/j.jcin.2017.04.037 (2017).
Scudiero, F. et al. Outcomes of left main revascularization after percutaneous intervention or bypass surgery. J. Interv. Cardiol. 2022, 6496777. https://doi.org/10.1155/2022/6496777 (2022).
Jou, Y. L. et al. Comparison of the predictive value of EuroSCORE, SYNTAX score, and clinical SYNTAX score for outcomes of patients undergoing percutaneous coronary intervention for unprotected left main coronary artery disease. Catheter. Cardiovasc. Interv. 80, 222–230. https://doi.org/10.1002/ccd.23450 (2012).
Giacoppo, D. et al. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis: A systematic review and meta-analysis. JAMA Cardiol. 2, 1079–1088. https://doi.org/10.1001/jamacardio.2017.2895 (2017).
Sliman, H. et al. Clinical features and outcomes of revascularization in very old patients with left main coronary artery disease. Coronary Artery Disease 30, 584–589. https://doi.org/10.1097/mca.0000000000000744 (2019).
Lee, J. et al. Prognostic effect of the SYNTAX Score on 10-year outcomes after left main coronary artery revascularization in a randomized population: Insights from the extended PRECOMBAT trial. J. Am. Heart. Assoc. 10, e020359. https://doi.org/10.1161/jaha.120.020359 (2021).
Doenst, T. et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J. Am. Coll. Cardiol. 73, 964–976. https://doi.org/10.1016/j.jacc.2018.11.053 (2019).
Zhang, M. et al. Left internal mammary artery versus coronary stents: Impact on downstream coronary stenoses and conduit patency. J. Am. Heart Assoc. 5, e003568. https://doi.org/10.1161/JAHA.116.003568 (2016).
Author information
Authors and Affiliations
Contributions
W.T.S., C.C.C. and P.H.H. wrote the main manuscript text. M.J.C., Y.L.T. and R.H.C. collected and analyzed the data. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sung, WT., Chuang, MJ., Tsai, YL. et al. Impacts of the SYNTAX score I, II and SYNTAX score II 2020 on left main revascularization. Sci Rep 14, 1073 (2024). https://doi.org/10.1038/s41598-024-51192-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51192-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.