Abstract
Fusarium head blight (FHB) is a critical fungal disease causes serious grain yield losses and mycotoxin contaminations. Currently, utilization of chemical fungicides is the main control method which has led to serious resistance. Development of novel synergist is an important strategy to reduce the usage of chemical fungicides and postpone the development of resistance, while natural components are interesting resources. In this study, the synergistic effect of Taxodium 'zhongshansha' essential oil (TZEO) was determined and the best synergistic ratio (SR) of 3.96 in laboratory which was observed when the weight ratio of TZEO and prothioconazole was 1 : 1 with the corresponding EC50 (half maximal effective concentration) value of Fusarium graminearum was 0.280 mg L−1. Subsequently, an increase of 6.31% on the control effect to FHB index in field test was observed when compared to the treatment with prothioconazole alone, though there was no significant difference between these treatments. Furthermore, we established an effective method to detect the mycotoxin contaminations in wheat grain with the limits of quantifications (LOQs) value of 5 µg kg−1 (DON, ZEN, 3-DON, and 15-DON) and 1 µg kg−1 (OTA) and the contents were less to the maximum residue limit (MRL) values. It was also shown that the application of 20% TZEO EW led to a 20% reduction in the use of prothioconazole, which was calculated based on the control effect values of 86.41% and 90.20% between the treatments of 30% prothioconazole OD (225 g a.i ha−1, recommend dosage) and 30% prothioconazole OD (180 g a.i ha−1) + 20% TZEO EW (225 mL ha−1), significantly. The initial residue of prothioconazole and prothioconazole-desthio was increased in the treatment with TZEO, which may play an important role in the synergistic effect on FHB. Moreover, none of the treatments posed a prothioconazole residue risk in the wheat grain and the environment. In addition, the essential oil has no any negative influence on wheat growth, which was revealed by a study of the chlorophyll content. These results provide an important botanical synergist for use with prothioconazole to control Fusarium head blight, and in-depth study to the synergistic mechanism of this oil is necessary in our future research.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L.), one of the most important cereal crops in the world, accounts for approximately 30% of average global grain consumption1. Fusarium head blight (FHB) is a common fungal disease of wheat caused by the Fusarium graminearum, which has a serious impact on grain yield and quality2. FHB also can lead to a serious contamination of the affected grains with mycotoxins such as deoxynivalenol (DON) and zearalenone (ZEN), which are toxic to humans and animals3,4. Chemical control to this fungal disease is an important and frequently-used method, which will lead to some serious risks including resistance and residue5,6.
It can be concluded that the development of resistance is the most important challenge to control FHB, the proportion of carbendazim-resistant strains in Anhui and Jiangsu provinces was reported to be 12.8% and 43.3%, which is caused by the long-term use of the fungicide in these provinces7. Moreover, carbendazim stress leads to the production of more mycotoxins8. At present, there are more than 300 kinds of fungicides have been registered for the control of FHB9. The triazolinthione fungicide prothioconazole is a broad-spectrum systemic demethylase inhibitor, which can be used in the control of several diseases of cereal grain crops10. However, it has been determined that prothioconazole and the main metabolite (prothioconazole-desthio) exhibit potent teratogenicity and endocrine-disrupting effects on mammals11. It is important to establish an effective strategy to reduce the application of prothioconazole and improve its effectiveness, which may be attained by the development and use of novel botanical synergists.
In China, some synergists have been in use for a long time, castor oil is widely used due to the excellent solubility12. Karanja oil also exhibits significant synergistic effects to specific organochlorine fungicides in the control of stored grain pests13. Moreover, Melaleuca alternifolia oil, eucalyptus oil, Cymbopogon citratus oil and some phytochemicals including green tea polyphenols, eugenol, or geraniol can be used as synergists with several chemical pesticides14,15. In our previous study, we have determined the insecticidal activities of Taxodium 'zhongshansha' essential oil (TZEO) which was extracted from T. 'zhongshansha' leaves by hydrodistillation method, while study to the synergistic effect of this oil is limited16.
This study aims to determine the synergistic effect of TZEO on F. graminearum in our laboratory. Then, it is followed by a field test to evaluate the effectiveness of a mixture of 20% TZEO EW (emulsion in water) and 30% prothioconazole OD (oil dispersion) in controlling FHB. Furthermore, the effect of this oil on wheat growth was assessed along with mycotoxin contamination. Finally, the residue situations of prothioconazole and prothioconazole-desthio in wheat were determined. These results provide an insight into the use of T. 'zhongshansha' essential oil as a green fungicide synergist, which will provide a creative strategy to reduce the application of chemical fungicides used to control FHB and postpone the development of resistance. In addition, further studies are needed to elucidate the synergistic mechanism responsible for its activity.
Results
Synergistic effect of T. ‘zhongshansha’ essential oil to prothioconazole in vitro
There was no obvious inhibitory effect of this oil against F. graminearum can be found when we evaluate the antifungal activity of TZEO (Table S1). A positive dosage effect was observed between the prothioconazole concentration and inhibitory rate, with an EC50 value of 1.124 mg L−1 (Table S2).
A preliminary evaluation of the synergistic effect of TZEO on prothioconazole was conducted with two dosages of the essential oil at 5 mg L−1 and 10 mg L−1. The EC50 of prothioconazole in the above treatments was determined as 0.607 and 0.940 mg L−1, respectively, which showed an obvious synergistic effect on prothioconazole for the inhibition of F. graminearum (Table S3). Based on the pre-experiment results, six treatments with different weight ratios of prothioconazole:TZEO (1:0.1, 1:0.5, 1:1, 1:5, 1:10, and 1:20) were performed. As shown in Fig. 1, the synergistic ratios were 1.45, 1.91, 3.96, 1.79, 1.46, and 0.74, with EC50 values of 0.780, 0.590, 0.280, 0.630, 0.770, and 1.500 mg L−1, respectively. The SR showed a trend of increasing first and then decreasing with weight ratio. In the all treatments, the best weight ratio between prothioconazole and TZEO was determined as 1:1, with a SR value of 3.96.
Optimal synergistic ratio of Taxodium 'zhongshansha' essential oil (TZEO) to prothionidazole against to Fusarium graminearum. (A–F) The linear regression between dosage and inhibitory rate when the ratio of prothioconazole : TZEO was 1 : 0.1, 0.5, 1, 5, 10, and 20, respectively, (G) the curve of synergistic ratios.
Impact of T. ‘Zhongshansha’ essential oil to the control of FHB in field study
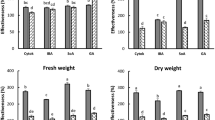
Based on the results in laboratory, a formulation of 20% TZEO EW was prepared for the field test (Fig. 2). Firstly, the use of TZEO has no negative influence on wheat growth, and none of the treatments were found to be associated with any phytotoxicity. This formulation showed an obvious synergistic effect on 30% prothioconazole OD according to the data obtained from the field tests which was repeated three times of each treatment. The disease index was 2.80 and the control effect value was 86.41% when only 30% prothioconazole OD (225 g a.i ha−1) was sprayed. In the treatments involving 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1) and 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1), the control effect was 92.72% and 91.75%, with an increase of 6.31% and 3.20% compared to the treatment of 30% prothioconazole OD alone used, respectively. Moreover, the control effect in the treatment of 30% prothioconazole OD (180 g a.i ha−1) + 20% TZEO EW (225 mL ha−1) and 30% prothioconazole OD (180 g a.i ha−1) + Maifei (225 mL ha−1) was 90.20% and 87.62%, respectively, with corresponding disease index values of 2.00 and 2.55. Though there was no significant difference between these treatments, the results showed that TZEO was effective in increasing the control efficacy of prothioconazole on FHB. It can be concluded that the efficiency was better than that of the commercial synergist Maifei, which has been shown to reduce the application of prothioconazole by more than 20%.
Control effect to Fusarium head blight in field test. (A) Disease index of Fusarium head blight in different treatments, (B) Control effect to Fusarium head blight in different treatments. Treatment-1, 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1); Treatment-2, 30% prothioconazole OD (180 g a.i ha−1) + 20% TZEO EW (225 mL ha−1); Treatment-3, 30% prothioconazole OD (225 g a.i ha−1) + “Maifei” (225 mL ha−1); Treatment-4, 30% prothioconazole OD (180 g a.i ha−1) + “Maifei” (225 mL ha−1); Treatment-5, 30% prothioconazole OD (225 g a.i ha−1); Treatment-6, Blank control.
In addition, we evaluated the influence on the chlorophyll content in leaves which can be used to growth condition of wheat using the SPAD value. The SPAD value of 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1) and 30% prothioconazole OD (180 g a.i ha−1) + 20% TZEO EW (225 mL ha−1) was 35.42 and 39.72. In the two treatments with Maifei and different dosage of prothioconazole, the value was 37.38 and 40.84, respectively. When only prothioconazole was sprayed, the SPAD value was 38.71, while the value was 38.98 in the blank control. It can be concluded that there was no significant difference between all treatments (Fig. S1).
Effect of T. ‘zhongshansha’ essential oil on the control of mycotoxin contamination
In this study, the limit of quantification (LOQ) value of DON, ZEN, 3-DON, and 15-DON was 5 µg kg−1, while that for OTA was 1 µg kg−1, which was satisfied to the maximum residue limit (MRL) values of DON, ZEN, and OTA at 1000, 60, and 5 µg kg−1. The content of DON, 3A-DON, 15A-DON, and OTA in blank control was 1205.55 µg kg−1, 259.68 µg kg−1, 290.05 µg kg−1, and 640.96 µg kg−1, respectively (Table 1)17. When 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1) was sprayed, only 41.03 µg kg−1 of 15A-DON was detected in the grains. DON (120.23 µg kg−1) and 15A-DON (50.15 µg kg−1) were found in the treatment of 30% prothioconazole OD (180 g a.i ha−1) + 20% TZEO EW (225 mL ha−1). Similarly, the content of 15A-DON was 52.35 µg kg−1 in the treatment of 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1), and the DON and 15A-DON content was 71.50 µg kg−1 and 51.60 µg kg−1 in the 30% prothioconazole OD (180 g a.i ha−1) + Maifei (225 mL ha−1). Moreover, DON content of 211.50 µg kg−1 and 15A-DON of 52.80 µg kg−1 were observed in the grains when only 30% prothioconazole OD (225 g a.i ha−1) was sprayed.
Effect of T. ‘zhongshansha’ essential oil on prothioconazole residues
As shown in Fig. 3A, the average initial residue of prothioconazole in wheat plants were 320.12 µg kg−1, 237.60 µg kg−1, and 172.12 µg kg−1 in the treatment with 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1), 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1), and 30% prothioconazole OD (225 g a.i ha−1), respectively. The content of prothioconazole decreased quickly on day 2 after spraying 30% prothioconazole OD together with the synergist, a similar phenomenon was observed on day 4 when only 30% prothioconazole OD was applied. At the 28th day, the average prothioconazole residue content after spayed 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1) was 58.84 µg kg−1, and the value was 56.70 µg kg−1 in the treatment of 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1) and 30% prothioconazole OD. In addition, no prothioconazole was observed in all treatments on harvest day, while the value was less than LOQ (5 µg kg−1) in the soil and grain samples.
Residue dynamics of prothioconazole (A) and prothioconazole-desthio (B) in wheat leaf. Treatment-1, 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1); Treatment-3, 30% prothioconazole OD (225 g a.i ha−1) + “Maifei” (225 mL ha−1); Treatment-5, 30% prothioconazole OD (225 g a.i ha−1).
The initial concentration of prothioconazole-desthio in wheat plant was 5970.50 µg kg−1 in the treatment of 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1), while the final residue value was 80.00 µg kg−1 (Fig. 3B). In the treatment of 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1) was sprayed, the initial residue of prothioconazole-desthio was 3062.54 µg kg−1 and the final value was 46.93 µg kg−1. The values of the initial and final residue concentration in the treatment of 30% prothioconazole OD were 1769.18 µg kg−1 and 129.80 µg kg−1, respectively. In addition, similar curves were observed in all treatments with two increasing trends on the 6th and 14th day, which may be because of the degradation and enrichment processes18. Moreover, the values of prothioconazole-desthio residue were less than LOQ in all solid samples, except the initial residue of 23.86 µg kg−1, 29.72 µg kg−1, and 26.72 µg kg−1 in treatment of 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1), 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1), and 30% prothioconazole OD, respectively. Finally, it was determined that there was no prothioconazole-desthio residue in wheat grains.
Discussion and conclusion
In this study, the inhibitory rate of T. ‘zhongshansha’ essential oil against F. graminearum was 11.48% at a treatment concentration of 5 mg/L, and 13.26% with 10 mg/L. Hence, it is difficult to develop this oil as a novel botanical fungicide, while the development as synergist may be a creative strategy owing to the physicochemical properties of the essential oil19,20. Then, the best synergistic ratio of 3.96 was observed when the weight ratio of TZEO and prothioconazole was 1:1, with an EC50 of 0.280 mg L−1. It can be concluded that the synergistic effect of TZEO was significant, which was caused by the characteristic liposolubility of essential oil. It has been reported that various essential oils have obvious synergistic effects to some fungicides, including tea tree essential oil, Cinnamomum cassia oil, Tagetes minuta oil, and neem oil21,22. Only a limited number of studies have reported data regarding the actual effect in field studies, our results may provide a basis for the utilization of essential oils as novel botanical synergists.
Then, field test was used to verify the synergistic effect to prothioconazole in the control of Fusarium head blight and mycotoxin contaminations. Since mycotoxins in wheat grains have serious negative influences on human and animal health, one of the important evaluation indicators of fungicides used against FHB is the control effect to mycotoxins23,24. The occurrence of mycotoxins is unpredictable and difficult to estimate, which pose a serious health risk to animals and humans25,26. It was concluded that the use of the synergist in this study has a significant effect on the control of mycotoxin contamination, and prothioconazole was an effective agent to reduce mycotoxin content27.
The efficiency of the essential oil was better than the commercial synergist Maifei in field, which can result in a more than 20% reduction in the application of prothioconazole. It has been reported that the natural phenolic agent octyl gallate enhanced the activities of kresoxim-methyl and fludioxonil on Penicillium expansum28, and 2,3-dihydroxybenzaldehyde showed similar activity to amphotericin B29. Volatile components such as eugenol, thymol, and carvacrol synergistically interacted with fluconazole, which can increase the penetration of the fungicide by inhibiting biofilm formation and creating pores in the cell membrane30. Diferu-loylmethane enhanced the antifungal activities of azoles against Candida albicans by increasing the levels of reactive oxygen species and regulating the genes related to fungal oxidative stress, which may lead to apoptosis31. Moreover, the use of synergist was a creative strategy to postpone the development of resistance32. The treatment with DMIs (Demethylase inhibitors) increased the expression levels of erg11 (the target gene of DMI fungicides) by 15.4 to 56.6-fold, then the treatment with a mixture of schizostatin and DMIs reverted the erg11 transcription levels to the pre-DMI treatment levels33,34. In addition, this oil had no negative influence on the chlorophyll content. It has been reported that the spraying of some essential oils easily leads to dehydration, hence the result of this study showed that T. ‘zhongshansha’ essential oil was safe to wheat growth35,36.
Finally, the TZEO also enhances the initial residue of prothioconazole and prothioconazole-desthio, which may play an important role in revealing the synergistic effect on FHB. It can be concluded that the use of synergist enhances the stability of this fungicide, which can be determined by the comparison of the initial residue content37. The use of a synergist has no residue risk, that was determined as the comparison with the MRL value of prothioconazole in wheat grain which was identified with the concentration of prothioconazole-desthio (100 μg kg−1) according to standard requirements38,39. As we all know, the differences between a larger scale in real agricultural model and our study are significant. Hence, more optimize study to this formulation and the related evaluation including in-depth exploration to the synergistic mechanism of this oil is necessary in our future research.
In summary, T. ‘zhongshansha’ essential oil showed significant synergistic effect to prothioconazole against F. graminearum with the best synergistic ratio value of 3.96. This essential oil also showed obvious synergistic effect in the control of Fusarium head blight and mycotoxin contaminations, which was better than the commercial synergist Maifei in field, while also can result in a more than 20% reduction in the application of prothioconazole. In addition, the essential oil had no negative influence on the chlorophyll content, which demonstrate that T. ‘zhongshansha’ essential oil was safe to wheat growth. This oil also enhanced the initial residue of prothioconazole and prothioconazole-desthio, which may play an important role in revealing the synergistic effect on FHB. These results provide a creative strategy for the use of T. ‘zhongshansha’ essential oil as a green fungicide synergist, which is important to reduce the application of chemical fungicides and postpone the development of fungicide resistance. Future studies are needed for the elucidation of the synergistic mechanism and evaluation of the synergistic effect of this oil on other chemical fungicides.
Materials and methods
Chemicals and reagents
The standards of prothioconazole, prothioconazole-desthio (purity ≥ 97.00%), and 30% prothioconazole OD were purchased from Jiuyi Agriculture Co., Ltd (Anhui, China). Mycotoxin standards (purity ≥ 98.00%) including DON, 3-acetyldeoxynivalenol (3A-DON), 15A-DON (15-O-acetyl-4-deoxynivalenol), ZEN, and OTA were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
TZEO was isolated from T. ‘zhongshansha’ leaves via the hydrodistillation method with distilled water at a 1:8 (solid:liquid) ratio, 5 mL n-hexane was added into the flask with a 8 h extraction procedure. The extraction temperature was set at 100 °C, and volatile components were evaporated and dissolved in n‐hexane via inverse flow, continuously. Finally, the n-hexane layer was collected and concentrated in vacuo at room temperature which was transferred to a brown bottle for storage.
The 20% TZEO EW was obtained from Anhui Province Engineering Laboratory for Green Pesticide Development and Application, which was stored at a room temperature. HPLC-grade acetone and methanol were purchased from Aladdin (Shanghai, China), while acetonitrile was obtained from TEDIA (Cincinnati, OH, USA). All the analytic-grade solvents used in this study were purchased from Anpel (Shanghai, China). A commercial synergist named Maifei was provided by Guangyuan Yinong Chemical Co., Ltd (Beijing, China).
Evaluation to synergistic effect of TZEO in laboratory
The phytopathogens F. graminearum was obtained from Anhui Province Engineering Laboratory for Green Pesticide Development and Application of Anhui Agricultural University. The phytopathogen was inoculated at the center of the plate and incubated in the dark at 28 °C. In addition, the strain was identified by Prof. Li Chen who has studied to the FHB decades. Firstly, we assayed the individual antifungal activity of prothioconazole and TZEO against F. graminearum in vitro via mycelial growth rate method. A standard solution of prothioconazole was diluted to prepare test solutions, the potato dextrose agar medium (PDA medium) was subsequently sub-packed in 6 sterilized petri dishes, with 100 mL of medium per dish. Based on the results of pre-experiments, the actual test dosage of prothioconazole was 8.0, 5.0, 2.5, 1.25, 0.625, 0.313, and 0.156 mg L−1. Simultaneously, the test dosage of TZEO was set as 20.0, 10.0, 5.0, 2.0, 1.0, and 0.5 mg L−1. The test solutions were prepared when we assayed the experiments, three replicates of every treatment were determined.
For evaluating the synergistic effect of TZEO and prothioconazole on the control of F. graminearum in laboratory, different concentrations of the essential oil were added into the fungicide solution with a volume ratio of 1:1, in order to obtain a combination solution. Based on the results of the pre-experiment, six treatments with a mass ratio of 1:0.1, 1:0.5, 1:1, 1:5, 1:10, and 1:20 (Prothioconazole : TZEO) were set up to evaluate the synergistic effect. Five dosages of every treatment were assayed and repeated 3 times. The treatment with prothioconazole alone was defined as control and the growth control (without any treatment) was set, simultaneously.
All treated plates were placed in the dark at 28 °C and photographed after 48 h. Thereafter, the synergistic effect was calculated by the synergistic ratio (SR). While SR value is more than 1 indicated synergism, a value near 1 indicated additive action and a value less than 1 indicated antagonism. The formula for the calculation of SR is as follows40:
Field test design and sampling
The test was conducted in accordance with Standard Operating Procedures on Pesticide Registration Residue Field Trials41. Six treatments were used and the design was determined by the results in laboratory (Table S4). The agent and dosage were 30% prothioconazole OD (225 g a.i ha−1, recommend dosage), 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1), 30% prothioconazole OD (180 g a.i/ha) + 20% TZEO EW (225 mL ha−1), 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1), 30% prothioconazole OD (180 g a.i ha−1) + Maifei (225 mL ha−1), and a blank control, respectively. All the test agents were sprayed using a UAV sprayer (P20, XAG Technology Co., Ltd., Guangzhou, China) with a spraying width of 5 m, the flying height and velocity was 2.6 m and 5 m s−1, respectively. Each plot measured 120 m (length) by 5 m (width) (total plot area = 600 m2) and separated by a buffer zone. The field test was assayed three replications of every treatment and conducted on flat terrain in Fengtai County, Anhui Province. The levels of soil fertility, cultivation and fertilization management were consistent with local agricultural production, which was satisfied to the requirements of this test. The first spraying was done at the beginning of the wheat flowering stage, and the second spraying was performed after 7 days.
For the residue risk assessment of prothioconazole under different treatment conditions, representative plant and soil samples in the treatment of 30% prothioconazole OD (225 g a.i ha−1, recommend dosage), 30% prothioconazole OD (225 g a.i ha−1) + 20% TZEO EW (225 mL ha−1), 30% prothioconazole OD (225 g a.i ha−1) + Maifei (225 mL ha−1) and blank control were collected at 2 h. Then, the samples were collected at 1st, 3rd, 5th, 7th, 10th, 14th, and 21st day after the application of fungicides. On the harvest day, the grain samples were collected along with the plant and soil samples for the final residue analysis. In all, samples of plant and soil weighing 5 kg were collected each time respectively and stored at − 20 °C until further use. The harvested grains (2 kg) from each treatment were obtained to determine the mycotoxin concentrations.
Evaluation to the effectiveness in controlling to Fusarium head blight
Twenty days after application, we assessed the effect of these treatments on FHB disease symptom development using the disease scale. Five points were chosen according to the diagonal distribution in each plot, and one hundred wheat plants in each point were fixed to investigate the disease index of FHB. Firstly, FHB severity was determined based on the percentage of surfaces exhibiting visible symptoms, with the scores ranging from 0 to 7 (0, 0%; 1, 0–25%; 3, 25–50%; 5, 50–75%; 7, 75–100%)42. Subsequently, the disease index (DI) and control effect to the DI were obtained using the follow formulars43:
In addition, the chlorophyll content in wheat leaf was determined using a SPAD-502 Chlorophyll Meter Model (Konica Minolta, Chiyoda city, Tokyo metropolis, Japan) with SPAD value. Ten leaves of similar size and consistent growth were collected from each plot on the fifth day after application, five points of each leaf were assayed to calculate the average value. The plant collection and use was in accordance with all the relevant guidelines, including the national standard “Method for Determining Chlorophyll Content” GB/T 15401-1995.
UPLC-MS/MS analysis of prothioconazole residue and mycotoxins
Based on the previous study41, the extraction and purification methods of prothioconazole and prothioconazole-desthio in different matrices were optimized. Two grams of the sample (plant, soil, and grain) was weighed and put into a centrifuge tube. After the addition of 5 mL deionized water and 10 mL acetonitrile, the sample was homogenized for 2 min. The extraction procedure was assayed with ultrasonic for 15 min. Four grams of NaCl was added and homogenized for 1 min. Centrifugation was subsequently carried out for 3 min at 20,627×g, following which 1 mL of supernatant was filtered through a 0.22-μm nylon membrane for UPLC–MS/MS analysis.
Liquid chromatography was carried out using a UPLC instrument (Waters, MA, USA) interfaced with a Xevo TQ-Smicro mass spectrometer (Waters, MA, USA). The mycotoxins and fungicides were separated using a UPLC® BEH C18 column (2.1 × 50 mm, 1.7 μm; Waters, MA, USA). Isocratic elution was carried out using water containing 0.1% (v/v) formic acid (A) and acetonitrile (B) at a flow rate of 0.4 mL min−1. The injection volume was 10 μL and column temperature was set at 40 °C. Mass spectrometric detection was performed in the multiple reaction monitoring (MRM) mode with electrospray negative ionization (ESI−, 3.0 kV). The main parameters were as follows (Fig. S2): cone source temperature, 150 °C; drying gas flow, 150 L h−1; sheath gas temperature, 500 °C; sheath gas flow, 1000 L h−1. For the detection of prothioconazole, the m/z value of the parent ion was 342.20, while the m/z values of quantification ions were 100.10 and 125.00. For prothioconazole-desthio, the parameters were as follows: parent ion, 312.20; quantification ions, 70.00 and 125.20.
To investigate mycotoxin contamination of grains, 10 g of grain powder was filtered through a 60-mesh sieve. It was transferred into a conical flask, followed by the addition of 20 mL water and 40 mL acetonitrile. Then, the solution was subsequently vibrated at 180 rpm for 10 min after homogenization for 1 min. 4 g of MgSO4 and 8 g of NaCl were added into a flask which was vibrated for 2 min. Two milliliter of the supernatant was transferred into a centrifuge tube. After centrifugation for 3 min at 20,627×g, the supernatant was filtered through a 0.22-μm nylon membrane for UPLC-MS/MS analysis. The m/z values of the observed UPLC-MS/MS peaks are detailed in Table S5, other detection conditions and parameters were the same as described above with electrospray positive ionization (ESI+, 3.0 kV) (Fig. S3).
Statistical analysis
The data was expressed as mean ± standard deviation (SD), and the differences in the mean values between treatment groups were calculated using SPSS 22.0 software (Chicago, IL, USA). DPS 7.5 software was used to calculate the EC50 value and its 95% confidence interval. Comparisons with p ≤ 0.05 were considered significantly different. All figures were created using GraphPad Prism 7 (San Diego, CA, USA).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Curtis, T. Y., Powers, S. J., Wang, R. & Halford, N. G. Effects of variety, year of cultivation and sulphur supply on the accumulation of free asparagine in the grain of commercial wheat varieties. Food Chem. 239, 304–313 (2018).
Rawat, N. et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 48, 1576–1580 (2016).
Macias-Montes, A. et al. Risk assessment of the exposure to mycotoxins in dogs and cats through the consumption of commercial dry food. Sci. Total Environ. 708, 134592 (2020).
Nacher-Mestre, J. et al. Occurrence and potential transfer of mycotoxins in gilthead sea bream and Atlantic salmon by use of novel alternative feed ingredients. Chemosphere 128, 314–320 (2015).
Jansen, C. et al. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 102, 16892–16897 (2005).
Donau, S. S., Bollmann, U. E., Wimmer, R. & Bester, K. Aerobic dissipation of the novel cyanoacrylate fungicide phenamacril in soil and sludge incubations. Chemosphere 233, 873–878 (2019).
Chen, H. Z. et al. Carbendazim-resistance of Gibberella zeae associated with Fusarium head blight and its management in Jiangsu Province. China. Crop Prot. 124, 104866 (2019).
Liu, S. M. et al. Baseline sensitivity of Fusarium graminearum from wheat fields in Henan, China, to metconazole and analysis of cross resistance with carbendazim and phenamacril. J. Phytopathol. 168, 156–161 (2020).
Liu, J. L. et al. Sensitivity and resistance risk assessment of Fusarium graminearum from wheat to prothioconazole. Plant Dis. 106, 2097–2104 (2022).
Tian, S. N. et al. Toxicity effects in zebrafish embryos (Danio rerio) induced by prothioconazole. Environ. Pollut. 255, 113269 (2019).
Zhang, Z. X. et al. Stereoselective endocrine-disrupting effects of the chiral triazole fungicide prothioconazole and its chiral metabolite. Environ. Pollut. 251, 30–36 (2019).
Gahukar, R. T. A review of castor-derived products used in crop and seed protection. Phytoparasitica 45, 655–666 (2017).
Nikolić, M. M., Jovanović, K. K., Marković, T. L., Marković, D. L., Gligorijević. N. N., Radulović, S. S., Kostić, M., Glamočlija, J. M. & Soković, M. D. Antimicrobial synergism and cytotoxic properties of Citrus limon L., Piper nigrum L. and Melaleuca alternifolia (Maiden and Betche) Cheel essential oils. J. Pharm. Pharmacol. 69, 1606–1614 (2017).
Pereira, V., Dias, C., Vasconcelos, M. C., Rosa, E. & Saavedra, M. J. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Ind. Crop Prod. 52, 1–7 (2014).
Zhang, J., Wu, H. H., Jiang, D., Yang, Y. A., Tang, W. J. & Xu, K. H. The antifungal activity of essential oil from Melaleuca leucadendra (L.) L. grown in China and its synergistic effects with conventional antibiotics against Candida. Nat. Prod. Res. 33, 2545–2548 (2019).
Liao, M. et al. Transcriptomic analysis of Sitophilus zeamais in response to limonene fumigation. Pest Manag. Sci. 78, 4774–4782 (2022).
Tangni, E. K., Waegeneers, N., Overmeire, I. V., Goeyens, L. & Pussemier, L. Mycotoxin analyses in some home produced eggs in Belgium reveal small contribution to the total daily intake. Sci. Total Environ. 407, 4411–4418 (2009).
Xie, Y. et al. Comparative toxicokinetics and tissue distribution of prothioconazole and prothioconazole-desthio in Chinese lizards (Eremias argus) and transcriptional responses of metabolic-related genes. Environ. Pollut. 247, 524–533 (2019).
Dassanayake, M. K. et al. Synergistic field crop pest management properties of plant-derived essential oils in combination with synthetic pesticides and bioactive molecules: A review. Foods 10, 2016 (2021).
O’Neal, S. T., Johnson, E. J., Rault, L. C. & Anderson, T. D. Vapor delivery of plant essential oils alters pyrethroid efficacy and detoxification enzyme activity in mosquitoes. Pestic. Biochem. Phys. 157, 88–98 (2019).
Camiletti, B. X., Asensio, C. M., Gadban, L. C., Pecci, M. D. L. P. G., Conles, M. Y. & Lucini, E. I. Essential oils and their combinations with iprodione fungicide as potential antifungal agents against withe rot (Sclerotium cepivorum Berk) in garlic (Allium sativum L.) crops. Ind. Crop Prod. 85, 117–124 (2016).
Togbé, C. E., Zannou, E., Gbèhounou, G., Kossou, D. & van Huis, A. Field evaluation of the synergistic effects of neem oil with Beauveria bassiana (Hypocreales: Clavicipitaceae) and Bacillus thuringiensis var. kurstaki (Bacillales: Bacillaceae). Int. J. Trop. Insect Sci. 34, 248–259 (2014).
Diao, X., Han, Y. Y. & Liu, C. L. The fungicidal activity of tebuconazole enantiomers against Fusarium graminearum and its selective effect on DON production under different conditions. J. Agric. Food Chem. 66, 3637–3643 (2018).
Duan, Y. B. et al. Molecular and biological characteristics of laboratory metconazole-resistant mutants in Fusarium graminearum. Pestic. Biochem. Phys. 152, 55–61 (2018).
Juan, C., Ritieni, A. & Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 141, 1747–1755 (2013).
Nugmanov, A. et al. Systems to reduce mycotoxin contamination of cereals in the agricultural region of Poland and Kazakhstan. Crop Prot. 106, 64–71 (2018).
Limay-Rios, V. & Schaafsma, A. W. Effect of prothioconazole application timing on Fusarium mycotoxin content in maize grain. J. Agr. Food Chem. 66, 4809–4819 (2018).
Kim, J. H. et al. Use of chemosensitization to overcome fludioxonil resistance in Penicillium expansum. Lett. Appl. Microbiol. 51, 177–183 (2010).
Kim, J. H., Faria, N. C., Martins, M. D. L., Chan, K. L. & Campbell, B. C. Enhancement of antimycotic activity of amphotericin B by targeting the oxidative stress response of Candida and Cryptococcus with natural dihydroxybenzaldehydes. Front. Microbiol. 3, 261 (2012).
Khan, M. S. A. & Ahmad, I. Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biot. 90, 1083–1094 (2011).
Sharma, M., Manoharlal, R., Negi, A. S. & Prasad, R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 10, 570–578 (2010).
Ullah, R. M. K. et al. Insights into the use of eco-friendly synergists in resistance management of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Insects 13, 846 (2022).
Dutta, S. et al. Control of anthracnose and gray mold in pepper plants using culture extract of white-rot fungus and active compound schizostatin. Mycobiology 47, 87–96 (2019).
Park, M. Y., Jeon, B. J., Kang, J. E. & Kim, B. S. Synergistic interactions of schizostatin identified from Schizophyllum commune with demethylation inhibitor fungicides. Plant Pathol. J. 36, 579–590 (2020).
da Silva, P. P. M., de Oliveira, J., dos Mares Biazotto, A., Parisi, M. M., da Glória, E. M. & Spoto, M. H. F. Essential oils from Eucalyptus staigeriana F. Muell. ex Bailey and Eucalyptus urograndis W. Hill ex Maiden associated to carboxymethylcellulose coating for the control of Botrytis cinerea Pers. Fr. and Rhizopus stolonifer (Ehrenb.: Fr.) Vuill. in strawberries. Ind. Crop Prod. 156, 112884 (2020).
Pouresmaeil, M., Nojadeh, M. S., Movafeghi, A. & Maggi, F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crop Prod. 155, 112785 (2020).
Shi, Y. H. et al. Removal of prothioconazole using screened microorganisms and identification of biodegradation products via UPLC-QqTOF-MS. Ecotox. Environ. Safe. 206, 111203 (2020).
Jiang, D. D. et al. Enantioselective separation and dissipation of prothioconazole and its major metabolite prothioconazole-desthio enantiomers in tomato, cucumber, and pepper. J. Agr. Food Chem. 67, 10256–10264 (2019).
Zhang, Z. X. et al. Toxification metabolism and treatment strategy of the chiral triazole fungicide prothioconazole in water. J. Hazard. Mater. 432, 128650 (2022).
Liao, M. et al. Anti-fungal activity of moso bamboo (Phyllostachys pubescens) leaf extract and its development into a botanical fungicide to control pepper phytophthora blight. Sci. Rep. 11, 1–10 (2021).
Gao, Q. et al. Effect of application method and formulation on prothioconazole residue behavior and mycotoxin contamination in wheat. Sci. Total Environ. 729, 139019 (2020).
Xiao, J. J. et al. Application method affects pesticide efficiency and effectiveness in wheat fields. Pest. Manag. Sci. 76, 1256–1264 (2020).
Sun, H. Y. et al. Evaluation of tebuconazole for the management of Fusarium head blight in China. Australas. Plant Path. 43, 631–638 (2014).
Acknowledgements
This work was supported by the Anhui Provincial Key Research and Development Plan (202004a06020060), the University Synergy Innovation Program of Anhui Province (GXXT-2021-059), the China Scholarship Council (Quan Gao, 202208340039), and the Excellent and Top-notch Talent Cultivation Project in Colleges and Universities (gxgwfx2022001).
Author information
Authors and Affiliations
Contributions
Conceptualization—Y.W., X.C., Y.Y., G.C. and Q.G.; data curation—Y.W., S.J. and X.C.; formal analysis—Y.W., Y.Y., G.C. and Y.Z.; funding acquisition—Q.G. and S.J.; methodology—Y.W., X.C., Y.Z., M.Z. and Q.G.; writing-original draft—Y.W., Y.Y. and Q.G.; writing—review and editing—Q.G. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Yin, Y., Chen, X. et al. Effect of novel botanical synergist on the effectiveness and residue behavior of prothioconazole in wheat field. Sci Rep 13, 20353 (2023). https://doi.org/10.1038/s41598-023-47797-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47797-z
This article is cited by
-
Antifungal and antivirulence effects of Citrus sinensis essential oil on Alternaria pathogens in orange
Journal of Plant Pathology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.