Abstract
Age-related macular degeneration (AMD) is a common cause of visual loss among the elderly. Genetic variants in the gene encoding complement factor H (CFH) have been identified as an AMD susceptibility gene, however, the mechanistic link is debated. Here, we investigated the link between the CFH Y402H genotype and low-grade inflammation. We recruited 153 healthy individuals, 84 participants with dry stages of AMD, and 148 participants with neovascular AMD. All participants were subjected to detailed retinal examination, and interview regarding comorbidities and lifestyle. Blood samples were analyzed for level of C-Reactive Protein (CRP), white blood cell differential count, and stained with fluorescent antibodies to differentiate CD4+ and CD8+ T cells. CFH Y402H genotyping was performed using an allele-specific polymerase chain reaction genotyping assay. Splenocytes from young and aged wild type and Cfh null mutant C57BL/6J mice were examined for CD4+ and CD8+ T cells. Healthy individuals with the CFH Y402H at-risk polymorphism HH had higher levels of CRP and lower proportions of CD4+ T cells compared to persons with the YH or YY polymorphism (P = 0.037, Chi-square). Healthy individuals with the HH polymorphism displayed lower proportions of CD4+ T cells with ageing (P < 0.01, one-way ANOVA), whereas both young and aged Cfh null mutant mice displayed lower proportions of CD4+ T cells (P < 0.001 and P < 0.05; unpaired t test). Participants with dry AMD and the HH polymorphism had similarly lower proportions of CD4+ T cells (P = 0.024, one-way ANOVA), but no difference in CRP-levels. In the neovascular stage of AMD, there was no difference in proportion of CD4+ cells or CRP levels according to genotype. The risk-associated CFH genotype is associated with an age-related decrease in proportion of CD4+ T cells and increased levels of CRP in healthy individuals. This indicates that decreased complement regulation results in extensive changes in innate and adaptive immune compartments that precede development of AMD.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a common debilitating retinal disease among the elderly, robbing them of visual function and quality of life. Recent research into the pathogenesis of AMD describes a complex disease mechanism with local and systemic inflammation. As such, nearly all parts of the immune system have been associated with AMD1, 2. Much focus has been drawn to the complement system because of a strong association between a small nucleotide polymorphism (SNP) in complement factor h (CFH) Y402H and development of AMD3. Several lines of research have also associated development of AMD with increased levels of CRP4, 5 as well as various phenotypically and functional alterations in circulating immune cells6,7,8,9,10,11.
CFH is a negative regulator of the complement system and CFH Y402H carriers have slightly increased activity in the complement system12,13,14. The complement is part of the innate immune system and works as a strong regulator of immune cells from both the innate and adaptive immune system15. The CD4+ T cells are a group of heterogeneous cells that play a pivotal role in the immune system through several surface receptors including complement receptors16. With age, various phenotypical and functional changes of the innate and adaptive arms of the immune system have been observed, a phenomenon known as immunosenescence. A relatively simple measure of immunosenescence is the decline of circulating CD4+ T cells with age17, 18.
Based on the increased complement activity and possible interactions with immune cells, we hypothesized that CFH Y402H carriers show signs of increased inflammation with ageing.
The purpose of the present study was to investigate how the CFH Y402H polymorphism alters markers of ageing and inflammation in a group of individuals with no AMD, in a murine model of CFH deficiency, and in individuals with AMD (Table 1).
Methods
Design
This prospective study was approved by the Regional Committee of Ethics in Research of the Region of Zealand (SJ-385/SJ-142). Oral and written informed consent was obtained from all participants prior to inclusion after explanation of the nature and possible consequences of the study. The study adhered to the tenets of the Declaration of Helsinki.
This study was designed as a series of observational studies, with immunological data obtained during two separate cohort studies in the period 2010–2013 and in 2015–2018.
Study participants and inclusion
Patients and their spouses attending the Department of Ophthalmology, Zealand University Hospital, were invited to participate. Individuals were not included if they had any cancer, infectious or autoimmune diseases, or if they received any immune-modulating medication. Participants underwent an interview regarding medical history, current medication use, height and weight, weekly alcohol consumption, and tobacco use.
Retinal diagnosis
All participants were subjected to a detailed retinal examination, including dilated funduscopic examination, digital color fundus photography (Carl Zeiss, Germany), spectral-domain optical coherence tomography, and autofluorescence imaging (Heidelberg Engineering, Heidelberg, Germany). Fluorescein and indocyanine green angiography was performed in cases suspected of neovascular AMD to confirm the final diagnosis.
Individuals were categorized based on the Clinical Age-Related Maculopathy Grading System (CARMS) described by Seddon et al.19 into three groups: No AMD—less than 10 small drusen without pigment abnormalities corresponding to CARMS grade 1. Dry AMD—one or more of the following signs: Drusen maculopathy, pigment abnormalities, atrophic lesions corresponding to CARMS grade 2–4. Neovascular AMD—non-drusenoid pigment epithelial detachments, serous retinal detachments, choroidal neovascular membrane with angiographic leakage corresponding to CARMS grade 5. The highest CARMS grade received from both eyes was used for participant classification.
Blood sampling and analysis
Fresh venous blood samples were obtained. One lithium-heparin-coated tube was used for measuring CRP (Dimension Vista 1500, Siemens Healthineers, Erlangen, Germany). One ethylenediaminetetraacetic acid (EDTA)-coated tube was used for automated white blood cell differential counts (Sysmex KX-21NTM, Sysmex Corporation, Kobe, Japan). Another EDTA-coated tube was used for genotyping purposes. Extraction of DNA was performed on EDTA-stabilized venous blood using Chemagic Magnetic Separation Module 1. The DNA samples were genotyped at LGC genomics, using the Kompetitive Allele Specific Polymerase chain reaction (KASP) genotyping assay. A third tube with EDTA-stabilized fresh venous blood was prepared for flow cytometric analysis. We used the differential count to obtain a fixed number of cells in the sample (500,000 leukocytes). The sample was lysed and washed using red blood cell lysis buffer (Nordic Biosite AB, Sweden). The sample was washed three times by centrifuging and resuspending in isotonic buffer. Fluorescent monoclonal antibodies were added to the test tube, and cells were incubated for 20 min in darkness. Antibodies used for CD4 were Peridinin-chlorophyll protein complex, IgG2a (FAB3791C-100, R&D Systems Inc., MN, USA), and for CD8 was Phycoerythrin-CY7, IgG1 (300914, BioLegend, CA, USA). After incubation, cells were washed and analyzed using flow cytometry (BD FACSCanto IITM, BD Biosciences, NJ, USA).
Animal studies
We used young (7–8 weeks of age) and aged (52–54 weeks) WT and Cfh−/− C57BL/6J mice. Six mice were in each group. WT mice purchased from Harlan Laboratories; Cfh−/− mice provided by Matthew Pickering20. All animal experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the UCL Institute of Ophthalmology Ethical Review Panel.
Following cervical dislocation, spleens were removed and meshed to single-cell suspensions. Cells were washed and stained for flow cytometry as stated above with the following antibodies: CD3-pacific blue IgG2b (100213, BioLegend, CA, USA), CD4-APC IgG2a (100515, BioLegend, CA, USA) and CD8-APC-Cy7 IgG2a (301016, BioLegend, CA, USA). Samples were analyzed using flow cytometry (BD LSR II, BD Biosciences, NJ, USA).
Data analysis and statistics
Participant characteristics were summarized in groups defined by stage of AMD. In each group, we compared demographics and measurements based on CFH Y402H polymorphism. We also included the calculated CD4/CD8 and neutrophil/lymphocyte ratios as these measures of immunosenescence and inflammation have been used by others18, 21. Statistical analysis was performed using the Statistical Package for the Social Sciences, SPSS version 25 (IBM Corporation, Armonk, NY, USA). Normally distributed data was presented using mean and standard deviation (SD) and comparisons were made using parametric tests. Not normally distributed data was presented using median and interquartile range (IQR) and comparisons were made using non-parametric tests. Categorical data was presented in percentage and tested using χ2 test, with the exception of tests with small categories (< 5 cases) in which case Fisher’s Exact test was used.
Results
Participants
A total of 380 participants were recruited for this study. Of these, 143 had neovascular AMD, 84 had dry AMD, and 153 were healthy individuals. The average age of all participants was 71 years (SD: 12.9), and 227 (59.7%) were female. The healthy individuals were younger than patients with AMD (mean age 62.5 years, SD: 14.3 vs. 76.8 years SD: 7.7). The at-risk SNP HH was more frequent among participants with neovascular AMD (40% of participants), compared to those with dry AMD (29.8% of participants), and those without AMD (13.1% of participants) (P < 0.001). The CFH Y402H SNP did not deviate from the Hardy-Weinbergs equilibrium in healthy individuals: (χ2 = 3.04, P = 0.10), participants with dry AMD (χ2 = 0.40, P = 0.53), or participants with neovascular AMD (χ2 = 0.52, P = 0.47).
The group of healthy individuals were defined as healthy based on the retinal analysis. While some of these individuals had hypertension, hyperlipidemia, cardiovascular disease, and/or type 2 diabetes, the frequency of systemic disease was the same across CFH Y402H genotypes (Table 2).
Does CFH polymorphism affect markers of inflammation and ageing in a population of healthy individuals?
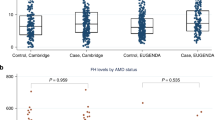
In the population of healthy individuals, the proportion of CD4+ T cells in individuals with the at-risk HH SNP was 41.0% (SD, 10.9), which was significantly lower than in individuals with the YY SNP 47.6% (11.0) (P = 0.022) and 48.2% (9.2) with YH SNP (P = 0.004) (Table 2). In a subgroup analyses based on age, it was seen that only individuals with the HH SNP aged 60 years or older demonstrated a lower proportion of CD4+ T cells (P < 0.01) (Fig. 1). Excluding the outlier in the group of HH SNP aged 60 years or older did not change the conclusion (P < 0.05, data not shown). None of the comorbidities or lifestyle choices were associated with the lower proportion of CD4+ T cells.
The proportion of CD8+ T cells was 22.6% (SD: 12.2) for all healthy individuals, and did not differ according to CFH SNP. Neither did the proportion of neutrophils and lymphocytes. Contrary, individuals with the HH SNP demonstrated elevated levels of CRP compared to individuals with the YY and YH SNPs (P = 0.037, Chi-square) (Table 2).
Does CFH deficiency affect T cell distribution in a rodent model?
To explore the effect of decreased CFH function in an animal model, we used mice with complete deficiency in Cfh. Compared to wild type, we found lower proportions of CD4+ T cells in both the group of young (P < 0.001) and aged (P < 0.05) mice deficient in Cfh (Fig. 2).
Does CFH polymorphism affect markers of inflammation and ageing in a population with dry AMD?
In the population of individuals with early and late dry AMD according to CARMS 2–4, the proportion of CD4+ T cells in individuals with the at-risk HH SNP was 39.9% (11.9), which was significantly lower compared to 46.4% (11.3) in individuals with the YY SNP (P = 0.046) and 47.5% (10.2) with YH SNP (P = 0.004). Contrary, the levels of CRP, neutrophils, and lymphocytes were similar across CFH genotypes in the group of participants with dry AMD (Table 3).
Does CFH polymorphism affect markers of inflammation and ageing in a population with neovascular AMD?
In the population of individuals with neovascular AMD (CARMS 5), the proportion of CD4+ T cells in individuals with the at-risk HH SNP was 47.5% (11.2), which did not differ from 51.8% (12.1) in individuals with the YY SNP (P = 0.199) and 44.6% (11.7) with YH SNP (P = 0.176). Similarly, no differences were noted in levels of CRP, neutrophils, or lymphocytes according to CFH SNP (Table 4).
Discussion
We measured immunological alterations associated with the CFH Y402H polymorphism to assess the mechanistic link between CFH and development of AMD. We found that the HH polymorphism was associated with increased levels of CRP and with age a lower proportion of CD4+ T cells in healthy individuals without AMD. Using a mouse model, we found a similar decrease in levels of CD4+ T cells in mice with deficiency in Cfh.
The at risk HH SNP has previously been associated with increased levels of circulating proinflammatory cytokines including IL6 and CRP22, 23. To the best of our knowledge, this is the first study on the association between a CFH genotype and systemic levels of T cells. Few studies exist on the association between leukocytes and CFH genotypes. In the Rotterdam Study, it was reported that the CFH Y402H polymorphism had no effect on the white blood cell count24, which is in agreement with our findings.
CFH is a negative regulator of the complement system and CFH Y402H carriers have slightly increased activity in the complement system with increased levels of complement split-products in plasma12,13,14. These split-products may interact with T cells and professional antigen-presenting cells via membrane-bound receptors and lead to stronger T cell responses15, 16. In immune homeostasis, this may be a beneficial modulation of an immune response, but given complement dysregulation the abundance of complement split-products may evade the T cell regulation and lead to chronic inflammation25 and lower levels of CD4+ T cells26.
Complement factor H binds to CRP and thereby dampen its proinflammatory activity. This binding is impaired in the at-risk variant27, 28. As a consequence, individuals who carry the at-risk polymorphism have higher concentrations of CRP both in plasma24, 28 and in the RPE-choroid layer compared to those who are homozygous for the non-risk variant29. This corresponds with the finding that CRP was increased in healthy individuals with the at-risk variant in the present study.
The systemic nature of the changes and the finding that it is demonstrated in healthy individuals with the AMD-risk conferring genotype supports the notion that systemic proinflammatory stimulation of the retinal pigment epithelium (RPE) is a contributor to development of AMD. As such the presence of inflammatory mediators in the highly perfused choroid has previously been demonstrated to activate the RPE in both in vitro and in vivo studies28, 30,31,32.
Further, it has been previously been shown that patients treated for AIDS, demonstrated increased incidence of early development of AMD33. While immune-reconstituted HIV represents a very specialized form of immune deviation, it does demonstrate that immune dysregulation, low CD4 counts and systemic inflammation may promote development of AMD.
The immune alterations with CFH-genotypes differed in AMD-patients compared to healthy individuals. We found that only the patients with dry AMD displayed an association between low levels of CD4+ T cells and the CFH at-risk polymorphism. During the progression of AMD numerous changes occur, and several cross-sectional studies have reported on higher levels of proinflammatory markers in both dry and neovascular AMD6, 11, 34. Therefore, it seems intuitive that the proinflammatory changes associated with the at-risk CFH polymorphism HH is overshadowed by the presence of AMD and the associated immunological alterations. The ocular immune recruitment is probably more prominent in neovascular AMD than in dry AMD35, which may contribute to the increased levels of CD4+ T observed in patients with neovascular AMD in our study.
Several limitations should be kept in mind when interpreting these findings. In this study we did not include any individuals with any known immune disease, inflammatory disorder, cancer, or persons who received immune modulating medication. Therefore, our results can only be generalized to this group of persons. Because complement factor H polymorphism and low CD4 count are both associated with autoimmune inflammatory disorders15, 26, this could potentially lead to a selection bias. Another limitation is the relative low numbers of patients with early dry AMD, which hinders subgroup analysis of patients with dry AMD. A large-scale study could validate our findings.
Further, CD4+ T cells are a group of heterogenous immune cells, and our data does not provide any insight into the differentiation and properties of these immune cells. Additional studies could examine possible functional or phenotypical differences in the CD4+ T cells according to CFH genotype.
In mice deficient in Cfh, we found lower proportions of CD4+ T cells in both young and aged mice. The rodent model of Cfh-deficiency is not identical to the decreased complement regulation with CFH Y402H polymorphisms. The Cfh deficient mice have complete loss of Cfh resulting in undetectable levels of plasma C320. This may explain why no age-effect is observed in mice and also demonstrate that the loss of complement regulation has an influence on the distribution of CD4+ T cells thereby strengthening our results.
It is well-known that carriers of the CFH Y402H have increased risk of AMD3, 24. An implication of our study is that these individuals demonstrate increased systemic inflammation at a stage before signs of AMD can be detected clinically. Thus, if an anti-inflammatory prevention/treatment should be available, this should be administered to individuals based on risk of disease rather than signs of disease.
In conclusion, we found that healthy individuals with the AMD-risk conferring CFH Y402H genotype displayed decreased levels of CD4+ T cells and increased levels of CRP in plasma. Mice with deficiency in Cfh also displayed decreased levels of CD4+ T cells. This indicates that decreased complement regulation results in extensive changes in innate and adaptive immune compartments that precede development of AMD.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Miller, J. W. Age-related macular degeneration revisited—Piecing the puzzle: The LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 155, 1-35.e13 (2013).
Rozing, M. P. et al. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 76, 100825 (2020).
de Jong, S., Tang, J. & Clark, S. J. Age-related macular degeneration: A disease of extracellular complement amplification. Immunol. Rev. 313, 279–297 (2023).
Feng, C., Krogh Nielsen, M., Sørensen, T. L. & Subhi, Y. Systemic levels of C-reactive protein in patients with age-related macular degeneration: A systematic review with meta-analyses. Mech. Ageing Dev. 191, 111353 (2020).
Hong, T., Tan, A. G., Mitchell, P. & Wang, J. J. A review and meta-analysis of the association between C-reactive protein and age-related macular degeneration. Surv. Ophthalmol. 56, 184–194 (2011).
Behnke, V., Wolf, A. & Langmann, T. The role of lymphocytes and phagocytes in age-related macular degeneration (AMD). Cell. Mol. Life Sci. 77, 781–788 (2020).
Faber, C. et al. Age-related macular degeneration is associated with increased proportion of CD56(+) T cells in peripheral blood. Ophthalmology 120, 2310–2316 (2013).
Krogh Nielsen, M., Hector, S. M., Allen, K., Subhi, Y. & Sørensen, T. L. Altered activation state of circulating neutrophils in patients with neovascular age-related macular degeneration. Immun. Ageing A 14, 18 (2017).
Krogh Nielsen, M. et al. Patients with a fast progression profile in geographic atrophy have increased CD200 expression on circulating monocytes. Clin. Exp. Ophthalmol. 47, 69–78 (2019).
Subhi, Y. et al. The transcriptome of peripheral blood mononuclear cells in patients with clinical subtypes of late age-related macular degeneration. Immun. Ageing A 16, 20 (2019).
Subhi, Y. et al. T-cell differentiation and CD56+ levels in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Aging 9, 2436–2452 (2017).
Reynolds, R. et al. Plasma complement components and activation fragments: Associations with age-related macular degeneration genotypes and phenotypes. Investig. Ophthalmol. Vis. Sci. 50, 5818–5827 (2009).
Scholl, H. P. N. et al. Systemic complement activation in age-related macular degeneration. PLoS One 3, e2593 (2008).
Smailhodzic, D. et al. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology 119, 339–346 (2012).
Kemper, C. & Atkinson, J. P. T-cell regulation: With complements from innate immunity. Nat. Rev. Immunol. 7, 9–18 (2007).
Török, K., Dezső, B., Bencsik, A., Uzonyi, B. & Erdei, A. Complement receptor type 1 (CR1/CD35) expressed on activated human CD4+ T cells contributes to generation of regulatory T cells. Immunol. Lett. 164, 117–124 (2015).
Provinciali, M., Moresi, R., Donnini, A. & Lisa, R. M. Reference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerontology 55, 314–321 (2009).
Wikby, A., Månsson, I. A., Johansson, B., Strindhall, J. & Nilsson, S. E. The immune risk profile is associated with age and gender: Findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 9, 299–308 (2008).
Seddon, J. M., Sharma, S. & Adelman, R. A. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 113, 260–266 (2006).
Pickering, M. C. et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 31, 424–428 (2002).
Niazi, S., Krogh Nielsen, M., Sørensen, T. L. & Subhi, Y. Neutrophil-to-lymphocyte ratio in age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. (Copenh) 97, 558–566 (2019).
Cao, S. et al. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am. J. Ophthalmol. 156, 1176–1183 (2013).
Mooijaart, S. P., Koeijvoets, K. M. C., Sijbrands, E. J. G., Daha, M. R. & Westendorp, R. G. J. Complement factor H polymorphism Y402H associates with inflammation, visual acuity, and cardiovascular mortality in the elderly population at large. Exp. Gerontol. 42, 1116–1122 (2007).
Despriet, D. D. G. et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 296, 301–309 (2006).
Hajishengallis, G., Reis, E. S., Mastellos, D. C., Ricklin, D. & Lambris, J. D. Novel mechanisms and functions of complement. Nat. Immunol. 18, 1288–1298 (2017).
Ahmad, D. S., Esmadi, M. & Steinmann, W. C. Idiopathic CD4 Lymphocytopenia: Spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J. Med. 3, 37–47 (2013).
Laine, M. et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J. Immunol. (Baltim Md) 2007(178), 3831–3836 (1950).
Molins, B. et al. Complement factor H binding of monomeric C-reactive protein downregulates proinflammatory activity and is impaired with at risk polymorphic CFH variants. Sci. Rep. 6, 22889 (2016).
Johnson, P. T. et al. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc. Natl. Acad. Sci. U.S.A. 103, 17456–17461 (2006).
Faber, C. et al. Chemokine expression in murine RPE/choroid in response to systemic viral infection and elevated levels of circulating interferon-γ. Investig. Ophthalmol. Vis. Sci. 60, 192–201 (2019).
Juel, H. B. et al. Retinal pigment epithelial cells upregulate expression of complement factors after co-culture with activated T cells. Exp. Eye Res. 92, 180–188 (2011).
Udsen, M., Tagmose, C., Garred, P., Nissen, M. H. & Faber, C. Complement activation by RPE cells preexposed to TNFα and IFNγ. Exp. Eye Res. 218, 108982 (2022).
Jabs, D. A., Van Natta, M. L., Pak, J. W., Danis, R. P. & Hunt, P. W. Incidence of intermediate-stage age-related macular degeneration in patients with acquired immunodeficiency syndrome. Am. J. Ophthalmol. 179, 151–158 (2017).
Krogh Nielsen, M. et al. Systemic levels of interleukin-6 correlate with progression rate of geographic atrophy secondary to age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 60, 202–208 (2019).
Ambati, J., Atkinson, J. P. & Gelfand, B. D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 13, 438–451 (2013).
Acknowledgements
We thank Stephen Moss, University College London, for providing Cfh null mutant mice.
Funding
This research was funded by grants from the Velux Foundation, Fight for Sight Denmark, the Region Zealand, and Synoptik-Fonden.
Author information
Authors and Affiliations
Contributions
C.F. and M.H.N. conceived the study. C.F. and M.K.N. analysed data, prepared figures, and wrote the manuscript. Y.S. revised the manuscript. All authors provided data and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nielsen, M.K., Subhi, Y., Falk, M. et al. Complement factor H Y402H polymorphism results in diminishing CD4+ T cells and increasing C-reactive protein in plasma. Sci Rep 13, 19414 (2023). https://doi.org/10.1038/s41598-023-46827-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46827-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.