Abstract
Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) is the most devastating insect-pest in cotton crop. It is vector of the cotton leaf curl virus (CLCV) and is responsible for huge losses to cotton industry in Pakistan and worldwide. It is mainly controlled by insecticides but the injudicious use of insecticides has resulted in insecticide resistance and population resurgence in addition to various harmful effects on the humans, non-target organisms and the environment. Transgenerational studies are very helpful to choose a best insecticidal option. In the current study, age-stage two-sex life table analysis was used to identify transgenerational effects of sublethal doses of afidopyropen. The adults of B. tabaci were treated with three concentrations of afidopyropen i.e., LC10, LC30 and LC50. The results indicated significant changes in the progeny i.e. the fecundity decreased in treated population; and female and male longevity of their progeny were more in control as compared to treated populations. Similarly, population parameters like intrinsic rate of growth (r), net reproductive rate (R0) and limiting rate of growth (λ) were significantly decreased in the treated adult progeny with values of 0.08–0.11, 4.85–7.46 and 1.09–1.12 per day, respectively. Based on the reduced biotic potential, afidopyropen can be suggested as an effective alternative option for the management of B. tabaci.
Similar content being viewed by others
Introduction
The cotton, Gossypium hirsutum L. (Malvaceae) is an important fibre and cash crop of tropical and sub-tropical countries with an annual economic impact of about $600 billion worldwide1. The cotton crop is attacked by different insect pests which decrease the yield by 30–40% annually2. The whitefly, Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) is one of the most devastating and challenging insect pests of cotton. It is highly polyphagous pest and can feed on more than 600 distinct plant species. It damages the plants both directly by sucking the phloem sap and indirectly by spreading begomoviruses. Additionally, it secretes honey dew which promotes the sooty mould growth on plant leaves and thus reduces the photosynthetic activity of plants3.
Insecticides are considered as an important and effective tool for managing insect pests in different crops4,5. Despite the many advantages of insecticides, their widespread and injudicious usage has resulted in serious harmful effects to humans and the environment6. Afidopyropen derived from the opportunistic fungal pathogen, Aspergillus fumigatus is very effective for the management of whiteflies7. The transient receptor potential vanilloid (TRPV) of insects is the target of this novel pyropene pesticide. It interfers with the feeding and other behavior leading to starvation and death of target insect8.
Traditional bioassays do not provide full information about the usage of pesticides over an extended period of time9. Insecticides should be thoroughly examined as a potential pest control strategy through transgenerational studies on the progeny of insects10. Physiological and life table parameters, feeding behaviour, fertility, and several other physical and genetic traits are affected by the sublethal effects of various insecticides11. For instance, hatching efficiency, eggs size, and reproductive biology in diamondback moth decreased at sublethal levels of spinosad12.
Certain insecticides such as imidacloprid, bifenthrin, buprofezin and cycloxaprid have been shown to have sublethal effects on whiteflies. Some of these effects include delayed development time and a decrease in the fertility of the F1 generation13. Afidopyropen has recently been introduced and registered in Pakistan for the management of aphids, whitefly and jassid. For this purpose, a thorough evaluation of the transgenerational effects of afidopyropen on B. tabaci must be carried out in addition to examining its impact on mortality of B. tabaci14. In the current study, sublethal effects of afidopyropen were examined on several life table parameters by using the Age-stage, Two-sex life table. For better comparison, a life table was constructed using the offspring of populations of exposed and unexposed B. tabaci.
Materials and methods
Rearing of B. tabaci
B. tabaci population was reared using methodology of Esmaeily et al.15 with some modifications. The commercially available cotton cultivar (MNH-1050) was obtained from the Cotton Research Institute, Multan, Pakistan and sown in earthen pots (15 L volume) in greenhouse of MNS University of Agriculture, Multan, Pakistan. These plant were maintained under standard agronomic procedures and covered with net mesh bag (60 × 60 × 60 cm) to prevent pest infestation. The adults’ B. tabaci were released on these plants after 30 days of sowing to obtain whitefly culture for further experimentation.
Insecticide
The insecticide, afidopyropen (Sefina™ 5% DC, manufactured and formulated by BASF Corporation, USA; imported by BASF Pakistan Pvt. Ltd. and marketed by Engro Fertilizers Limited, Pakistan) was purchased from the local market in Multan, Pakistan.
Lethal concentration estimation
The adult whitefly culture maintained in the greenhouse was used for laboratory studies. For this purpose, plants were grown in plastic pots (each measuring 15 × 45 × 15 cm). The bioassays were carried out using the methodology of Heydari et al.16 with little modification. About 14 days old plants were selected and placed in meshed cages in laboratory at photoperiod of 14:10 (L:D), 35 ± 5 °C temperature and 60 ± 5% relative humidity. Six concentration (causing 0–100% mortality) of afidopyropen were prepared in distilled water. The leaves were dipped in a specific concentration of insecticide for 20 s and dried for five minutes before releasing B. tabaci on plants. There were four plants (replication) for each concentration. Each treated plant was placed in a two-sided mesh plastic cages (measuring 15 × 15 × 15 cm) and10 adult B. tabaci were released on each plant. The mortality data was recorded after 72 h of exposure.
Sublethal effects of afidopyropen on B. tabaci
For this experiment, LC50, LC30, and LC10 concentrations of afidopyropen calculated from above bioassay study were used. About 3 weeks old plants with 4–5 leaves were selected and treated with specific concentrations using the methodology described for bioassay study. Each treated plant was placed in meshed cage and 40 adult B. tabaci were released into the cage for feeding on treated plants for 1 day. After 1 day, the treated plants were replaced with untreated plants. The adult whiteflies allowed to feed and breed on these untreated plants till end of the experiment15. The number of offspring produced by females, the length of oviposition cycles, and other population parameters were monitored daily.
Following the treatment of F0, the adult survival and the number of eggs that each female laid everyday were recorded until their death. The survival rate, longevity of various life stages, and adult emergence were also observed. The conditions for transgenerational experiments were 35 ± 5 °C temperature, 60 ± 5% relative humidity and 14:10 (L:D) photoperiod. Following the arrangement and evaluation of the findings, various lethal concentration levels were determined to construct Age-stage, Two-sex life table.
Life table analysis
Raw data on life table parameters were analysed according to Chi and Su17. The main life-table parameters that were calculated include age-stage survival rate (Sxj), age-specific survival rate (lx), probability of a newly laid egg surviving to age x, female age-specific fecundity (fx), age-specific fecundity (mx), mean fecundity of individuals at age x, age-specific maternity (lxmx), and age-stage life expectancy. The TWOSEX-MS Chart programme was used to determine demographic characteristics such as the net reproduction rate (R0), intrinsic rate of increase (r), finite rate of rise (λ), and mean generation time (T). With 100,000 bootstraps, we estimated the variances, standard errors, and means using the bootstrap technique (100,000 bootstraps produced less variable findings and a normal frequency distribution, which were not influenced by the difference in sample sizes). Paired bootstrap test was used to identify the results with significant differences18,19,20,21,22.
Ethical statement
All methods related to plants were conducted in accordance with relevant institutional and national guidelines in the “Materials and methods” section.
Results
Lethal concentration estimation
The population of B. tabaci was exposed to different concentrations of afidopyropen and the LC10, LC30 and LC50 values were calculated based on the mortality data collected after 72 h of exposure. The LC10, LC30 and LC50 values for the afidopyropen were 11.66, 74.69 and 270.25 µg/mL while the chi-square (χ2) value was 3.29 (Table 1).
Survival and fecundity of parental generation
Higher male survival rate was observed in the LC50-treated population, while the lower male survival rate was observed in LC30 and LC10-treated population of B. tabaci. Survival rate of females was higher in the control, and lower female survival was observed in all populations treated at LC10, LC30 and LC50 values. Daily fecundity ranged from 13 eggs/day in the LC50-treated population while higher fecundity (57 eggs/day) was observed in control, LC30 and LC10-treated population (Fig. 1).
Life table parameters of progeny of treated adults
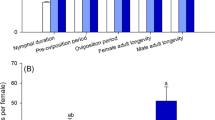
The effects of sublethal doses of afidopyropen on the B. tabaci population are presented in Table 2. Significant differences were recorded in different life table parameters i.e. pre-adult duration, female longevity, male longevity, oviposition period, total pre-oviposition period (TPOP), and fecundity (per female) in the progeny of whitefly individuals exposed to different concentrations (LC10, LC30, and LC50) and the control. Pre-adult duration was significantly higher (15.1 days) in the control population followed by populations treated with LC10 (14.96 days), LC30 (14.72 days) and LC50 (13.83 days) values. The female longevity was also higher in the control population (19.8 days) while it was the lowest in LC50-treated population (17.92 days). Similarly, male longevity was significantly higher in the control group (20.66 days) while significantly lower in LC50-treated population (18.04 days).
The adult pre-oviposition period (APOP) in the control was 0.47 days while in the LC50-treated population, APOP was 0.18 days. The total pre-oviposition period (TPOP) ranged from 15.24 to 15.35 days in the treated and control populations. the population in the control group had a higher value for oviposition days (3.85 days) while it was significantly lower in the LC50-treated population (3.12 days). The fecundity of B. tabaci was also significantly higher in the population that was taken as the control (16.29 eggs/female), as compared to the LC50-treated population (11.76 eggs/female).
Population parameter
The population parameters of B. tabaci in different treatments are shown in Table 3. There was non-significant difference among the values of intrinsic rate of increase (r), net reproductive rate (Ro) and limiting rate of growth (λ) while there was a significant difference in the values of mean generation time (T) in the progeny of B. tabaci individuals exposed to different concentrations (LC10, LC30, and LC50) and control. The intrinsic rate of increase (r) in the control was 0.11 per day while it was 0.08 per day in the LC50-treated population. Similarly, the net reproductive rate (Ro) was also higher in the control group (7.46 offsprings/day) as compared to (LC50-treated population (4.85 offsprings/day). The mean generation time (T) ranged from 17.36 to 18.08 days in the progeny of the LC50-treated and control population. The limiting rate of growth (ʎ) was relatively higher in the control group (1.12/day) than in the LC50-treated population (1.09/day). Based on population parameters, afidopyropen has no major effect on the next generation of a population that has been treated.
Age-stage specific survival rate (S xj)
Females in the control group had more age-stage specific survival rate (0.47) as compared to males (0.43) on day 16. On 18th day of LC10-treated population, the Sxj values in adult females and males peaked at 0.44 and 0.40, respectively. On 17th day, the males in LC30-treated group had Sxj values of around 0.42, whereas females in the same group had values of about 0.34. On 17th day of LC50-treated population, female and male adults had age-stage specific survival rate of 0.41 and 0.36, respectively. On day 21st, the Sxj in females dropped to zero, whereas on day 23rd, the same was true for males. Males exposed to LC30 had the lowest peak in Sxj (Fig. 2).
Age-stage life expectancy (e xj)
The age-stage specific expectancy (exj) represents the typical lifespan of a species or individual at age x and developmental stage j (Fig. 3). Values for exj were greater in the populations treated with LC10 (reaching 0 on the 23rd day) compared to those in control (reaching 0 on the 21st day).
Age-specific maternity (l x m x)
Age-specific maternity is another key indicator of population dynamics. A combination of the age-specific survival rate (lx) and the age-specific fecundity (mx) of the entire population determines the age-specific maternity (lxmx). The sum of these characteristics is depicted graphically in Fig. 4. Adults treated with LC50 had the lowest age-stage specific fecundity (fx), with just 5.66 offspring at 19 days of age compared to 20.33 offspring from the control treatment.
Age-stage reproductive values (v xj)
Reproductive age (vxj) has been shown in Fig. 5. On day 19th, the vxj value was 7.62 for the control population which dropped to 0.0 on day 20. Age-stage reproductive value in the LC50-treated population was 4.53 on the 16th day and dropped to zero by the 21st day.
Discussion
Afidopyropen is a recently launched chemical in Pakistan that could be used to protect crops from sucking insect pests especially whitefly, jassid and aphids23. For designing effective pest management strategy, it is crucial to understand the impacts of any pesticide24,25. With its high toxicity against B. tabaci and lack of cross-resistance, afidopyropen has been reported very effective even against resistant population of B. tabaci26. It was shown that sublethal concentrations of insecticides affect survival and growth (shortened oviposition period, decreased fecundity, and decreased hatchability of eggs) of insects.
Afidopyropen has been documented to have negative effects on insect pests14. Fecundity was relatively lower in the progeny of the treated population as compared to the B. tabaci population in control. These results are comparable to the findings of other researchers who have reported that afidopyropen reduce the fecundity of aphid27,28. The female longevity was also higher in the control population compared to the treated population. The intrinsic rate of increase (r), net reproductive rate (Ro), limiting rate of growth (λ) and age-stage specific survival rate (Sxj) were lower in the treated progeny of B. tabaci. These results are similar to the findings of Tang et al.28, who reported that afidopyropen decreased the values of female longevity, r, Ro, λ, and the age-stage specific survival rate (Sxj) of aphids. Sublethal doses of afidopyropen showed a significant decrease in vitellogenins (Vg) expression compared to the control group, as revealed by Liu et al.29. Overexpression of Vg and its receptors VgR in insects may lead to enhanced fertility. Additionally, different population parameters viz r, Ro, and λ, were decreased in the afidopyropen-treated population. Inhibitory effects such as lower fecundity, delayed growth, shortened lifespan, impaired motility, or learning, have traditionally been the focus of research on insects’ reactions to sublethal dosages of insecticides30. It can be attributed to the fact that afidopyropen has the ability to inhibit the activity of acylCoA: cholesterol acyltransferase31. Additionally, afidopyropen targets the transient receptor potential vanilloid (TRPV) of insects, which results in starvation, desiccation, and mortality32.
Mean generation time (T) was increased in control and decreased in insecticide-treated populations. While Liu et al.29 reported that T was increased in insecticide-treated populations in aphids. It may be due to the difference in biology of whitefly and aphid i.e. aphids reproduce by parthenogenesis while whitefly is an oviparous insect. The age-stage reproductive (Vxj) was higher in the control as compared to other treated populations. Age-specific maternity (lxmx) age-specific survival rate (lx), the overall population’s age-specific fecundity (mx) and age-stage specific fecundity (fx) were relatively lower in the control group as compared to the treated population. These results are comparable with the results of Esmaeily et al.15, who reported that lxmx, lx, mx and fx are increased under the sublethal concentration of pymetrozin against whiteflies. Nevertheless, it is known that stress had a profound impact on the population dynamics of insects. Insects may adapt by increasing their number of moults or moult duration, developmental time, and reproductive rate in response to these factors. Insects’ immunological reactions, including melanization, lysozyme levels, and phenoloxidase (PO), can be responsible for the alterations in physiology and morphology they exhibit in response to food, gases, and chemicals33. Afidopyropen is known to enhance glutathione s-transferases and P450 enzymes in the insects25, 34, which in documented to exert significant fitness cost in the afidopyropen-exposed whitefly population as compared to the control34.
Conclusion
Afidopyropen is an effective insecticide for the control of whiteflies. By examining the many factors during this experiment, it is indicated that this insecticide can be quite helpful population suppressor. To control this insect pest, it might be included in an integrated pest management program.
Data availability
Datasets used and/or analysed during the current investigation are available from the corresponding author upon reasonable request.
References
Ashraf, J. et al. Recent insights into cotton functional genomics: Progress and future perspectives. Plant Biotechnol. J. 16, 699–713 (2018).
Shah, S. I. A., Malik, T. H., Khan, I. R. & Hussain, Z. Screening of USDA cotton accessions against sucking insect pests complex and cotton leaf curl virus (CLCuV) disease with major emphasis on abiotic factors. Pak. J. Zool. 49, 1159–1173 (2017).
Gangwar, R. K. & Gangwar, C. Lifecycle, distribution, nature of damage and economic importance of whitefly, Bemisia tabaci (Gennadius). Acta. Sci. Agric. 2, 36–39 (2018).
Biondi, A., Desneux, N., Siscaro, G. & Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87(7), 803–812 (2012).
Damalas, C. A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 4(10), 945–949 (2009).
Tariq, M. I., Afzal, S., Hussain, I. & Sultana, N. Pesticides exposure in Pakistan: A review. Environ. Int. 33(8), 1107–1122 (2007).
Leichter, C. A., Thompson, N., Johnson, B. R. & Scott, J. G. The high potency of ME-5343 to aphids is due to a unique mechanism of action. Pestic. Biochem. Physiol. 107, 169–176 (2013).
Chen, X. D., Ashfaq, M. & Stelinski, L. L. Susceptibility of Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae) to the insecticide afidopyropen: A new and potent modulator of insect transient receptor potential channels. Appl. Entomol. Zool. 53, 453–461 (2018).
Tan, Y., Biondi, A., Desneux, N. & Gao, X. W. Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology 21, 1989–1997 (2012).
Betini, G. S., Wang, X. & Fryxell, J. M. Transgenerational plasticity mediates temperature effects on fitness in the water flea Daphnia magna. Can. J. Zool. 98, 661–665 (2020).
De França, S. M. et al. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects (ed. Shields, V. D. C.) 23–39 (InTech, 2017).
Yin, X. H., Wu, Q. J., Li, X. F., Zhang, Y. J. & Xu, B. Y. Sublethal effects of spinosad on Plutella xylostella (Lepidoptera: Yponomeutidae). Crop Prot. 27(10), 1385–1391 (2008).
Sohrabi, F., Shishehbor, P., Saber, M. & Mosaddegh, M. S. Lethal and sublethal effects of buprofezin and imidacloprid on the whitefly parasitoid Encarsia inaron (Hymenoptera:Aphelinidae). J. Crop Prot. 32, 83–89 (2012).
Desneux, N., Decourtye, A. & Delpuech, J. M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007).
Esmaeily, S., Samih, M. A., Zarabi, M. & Jafarbeigi, F. Comparative study of insecticides and C. procera extract on biological parameters of Bemisia tabaci (Genn.). Ann. Plant Prot. Sci. 20(1), 14–18 (2012).
Heydari, A., Moharrami, P. S., Pour Mirza, A. A. & Talebi, A. A. Effects of buprofezin, pymetrozin and fenpropathrin on reproductive parameters of Trialeurodes vaporariorum Westwood (Hom.: Aleyrodidae). Appl. Entomol. Phytopathol. 71(2), 29–46 (2003).
Chi, H. & Su, H. Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35(1), 10–21 (2006).
Huang, Y. B. & Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 137, 327–339 (2013).
Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985).
Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17(1), 26–34. https://doi.org/10.1093/ee/17.1.26 (1988).
Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. http://140.120.197.173/Ecology/Download/TWOSEX-MSChart-B100000.rar (National Chung Hsing University in Taiwan, 2020).
Tuan, S. J., Lee, C. C. & Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 70, 805–813 (2014).
Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest Manag. Sci. 77, 64–76 (2021).
Wang, R. et al. Characterization of field-evolved resistance to cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 18, 2571–2578 (2019).
Zhou, X. et al. Physiological and biochemical responses to sublethal concentrations of the novel pyropene insecticide, afidopyropen, in whitefly Bemisia tabaci MED (Q Biotype). Agronomy 11(11), 2260 (2021).
Zhang, Z. et al. Pymetrozine-resistant whitefly Bemisia tabaci (Gennadius) Populations in China remain susceptible to afidopyropen. Crop Prot. 149, 105757 (2021).
Ma, K. S., Tang, Q. L., Liang, P. Z., Li, J. H. & Gao, X. W. Sublethal concentration of afidopyropen suppressed the population growth of the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 21, 2055 (2021).
Tang, Q. L., Liang, P. Z., Li, J. H. & Gao, X. W. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 21(7), 2055–2064 (2022).
Liu, X. et al. Sublethal and transgenerational effects of afidopyropen on biological traits of the green peach aphid Myzus persicae (Sluzer). Pestic. Biochem. Phys. 180, 104981 (2022).
Guedes, R. N. C., Smagghe, G., Stark, J. D. & Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62 (2016).
Tomoda, H. et al. Pyripyropenes, novel ACAT inhibitors produced by Aspergillus fumigatus. IV. Structure elucidation of pyripyropene M to R. J. Antibiot. 49, 292–298 (1996).
Kandasamy, R. et al. Afidopyropen: New and potent modulator of insect transient receptor potential channels. Insect Biochem. Mol. Biol. 84, 32–39 (2017).
Khaliq, A. M., Javed, M., Sohail, M. & Sagheer, M. Environmental effects on insects and their population dynamics. J. Entomol. Zool. 2(2), 1–7 (2014).
Wang, R. et al. Characterization of field-evolved resistance to afidopyropen, a novel insecticidal toxin developed from microbial secondary metabolites, in Bemisia tabaci. Toxins 14(7), 453 (2022).
Acknowledgements
The authors especially Reem Atalla Alajmi extend appreciation to Researchers Supporting Project (RSP-2023/R99) King Saud University, Riyadh, Saudi Arabia for the study.
Author information
Authors and Affiliations
Contributions
M.N.N. and N.I. designed the experiment. M.S.S., M.U. and M.N.N. performed the experiments. S.S., H.R., A.D.A., M.S.S., M.A.B. and R.A.A. help in the data analysis. M.S.S., M.N.N., N.I., H.R. prepared the initial draft of the manuscript. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shafi, M.S., Iqbal, N., Naqqash, M.N. et al. Transgenerational effect of Afidopyropen on Bemisia tabaci Gennadius (Homoptera: Aleyrodidae). Sci Rep 13, 19988 (2023). https://doi.org/10.1038/s41598-023-46479-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46479-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.