Abstract
Octopus vulgaris (Cuvier, 1797) is a cephalopod species with great economic value. In western Asturias (northwest of Spain), O. vulgaris artisanal fisheries are relatively well monitored and conditionally eco-labeled by the Marine Stewardship Council (MSC). Despite this, the Asturian octopus stocks have not been genetically assessed so far. In order to improve the current fishery plan and contrast the octopus eco-label validity in Asturias, 539 individuals from five regions of the O. vulgaris geographic distribution, including temporal samplings in Asturias, were collected and genotyped at thirteen microsatellite loci. All the samples under analysis were in agreement with Hardy–Weinberg expectations. Spatial levels of genetic differentiation were estimated using F-statistics, multidimensional scaling, and Bayesian analyses. Results suggested that the O. vulgaris consists of at least four genetically different stocks coming from two ancestral lineages. In addition, temporal analyses showed stability in terms of genetic variation and high NE (> 50) for several generations in different localities within Asturias, pointing out to indeed sustainable fishery exploitation levels. Even though, the current Asturias fishery plan shows no significant genetic damages to the stocks, the regional-specific management plans need systematic genetic monitoring schemes as part of an efficient and preventive regional fishery regulation strategy.
Similar content being viewed by others
Introduction
Marine biological resources are limited, yet the global increasing demand for seafood products has led to the overexploitation of fisheries, which is highlighted as one of the current main threats to marine species. It has been argued that the fraction of fish stocks that are within biologically sustainable levels decreased by more than a 30% from 1974 to 2017, whereas the percentage of stocks fished at biologically unsustainable levels increased, especially in the late 1970s and 1980s, from 10% in 1974 to 34.2% in 20171. Although overfishing is undoubtedly the greatest threat to marine biodiversity2,3,4, it is clear that the depletion of the world’s fish stocks cannot be attributed solely to fishing. Habitat destruction5,6, pollution7,8, anthropogenic climate change9 or invasive species10 also have an impact on fish populations. However, the high economic growth observed in recent years has triggered a global increase in consumption, which in turn has had a damaging effect on the natural environment11 and the subsequent loss of the ocean biodiversity12. Fortunately, over the past few decades there has been an increasing trend in global awareness regarding this emerging issue12, prompting several proposed programs for establishing sustainable fishery plans13,14,15 as well as the development of tools that can educate consumers about the impact of products on the natural environment throughout their life cycle, but which at the same time can also provide producers with the opportunity to inform consumers about the benefits of their products11.
Eco-labels are “seals of approval” given to products that are deemed to have low or no negative impacts on the environment16. The FAO recognized that eco-labels could contribute to improved fisheries management and convened a technical consultation in 1998, which led to the development of the “Guidelines for the Ecolabelling of Fish and Fishery Products from Marine Capture Fisheries”17. Since then, numerous programes have been proposed for eco-labeling seafood products in an effort to encourage fisheries managers to create sustainable fisheries16. These initiatives aim to provide a market-based incentive for sustainable fisheries management. Processors, wholesalers, and retailers who purchase products from these accredited fisheries can acquire the right to affix an eco-label, informing consumers that the product has been caught in a sustainable fishery. Hypothetically, if there were a demand for environmental quality, consumers would respond by purchasing those products with an eco-label, thereby reducing demand for those without and causing price devaluation on unlabeled products. This may result in fishermen putting pressure on fisheries managers to achieve sustainability accreditation and thus receive a higher percentage of the price16. One of the most recognized and prestigious ecolabels in use today is the Marine Stewardship Council (MSC).
Designing sustainable fishery management requires deep understanding of the current conservation status of species and valid biological data. Stock assessment is crucial, but it can be challenging due to methodology difficulties, financial cost and intensive data requirements, so its application is usually limited to industrial fisheries18. Effective and sustainable management plans for artisanal fisheries also need to be established to reduce potential threats to marine resources and ensure environmental protection and sustainability19,20. Unfortunately, the vast majority of fished artisanal stocks are unassessed21,22, and their status, although highly uncertain, is generally considered worse than that of data-rich populations23,24.
Stock has been historically defined as an intraspecific group of randomly mating individuals with temporal and spatial integrity25,26. Defining stock boundaries for fisheries is fundamental and it is linked to the central idea that each stock has a harvestable surplus, and fisheries that comply with this limit will not compromise the stock’s natural perpetuation. Stocks that are scientifically assessed are usually in better condition than stocks that are unassessed18,27, being either exploited at sustainable rates or being re-built. Since January 2014, the reformed Common Fisheries Policy (CFP) of the European Union prescribes the end of overfishing and the rebuilding of all stocks above levels that can produce maximum sustainable yields (MSY). Although the global number of sustainable fishery products is increasing, there is reported evidence that the types of data upon which most assessments are established can be insufficient and misleading22,28,29. In this matter, there is an emerging need for radical changes in monitoring and collecting precise biological data from marine stocks; moreover, there is a direct link between fishing pressure and loss of species gene pools30,31. Genetic factors play an important role in fishery resource conservation because the latter are the product of their genes, the environment, and the interactions between the two32. Failure to detect biological characteristics of a stock within a population can lead to genomics changes and the subsequent loss of populations genetic diversity33.
Traditionally, fisheries conservation and management have been based on abundance data, productivity estimates, and information on stock dynamics34,35. However, genetics offers a diverse collection of versatile and useful tools to inform fisheries management on issues that have a biological basis36. Despite this, genetics has not yet been effectively integrated into fishery management schemes mainly due to the existing gap between managers, decision-makers, fisheries scientists and geneticists, resulting in less application of fisheries genetics37. Significant progress and findings in the field of marine genomics as well as the proven importance and validity of molecular genetic data, have made them vital tools for species identification; fisheries stock structure; resolving mixed-stock fisheries; age biomarkers; ecosystem monitoring; estimating harvest rates and abundance; genetic diversity, population abundance, and resilience; evolutionary responses to fishing; genetic effect of stock enhancement; detection of pathogens and invasive species; and product provenance and fisheries surveillance36.
The use of molecular genetic techniques in fisheries research has increased dramatically in the last few decades. Microsatellites and mitochondrial DNA markers have been used at an increasing rate in fisheries and today the application of new techniques to fisheries research, such as mass sequencing-derived markers, has grown significantly38. However, there is a clear species bias where marine invertebrates continue to lack genetic and genomic resources compared to other widely studied groups such as fish39. The importance of accurate genetic data is even more tangible when it comes to marine resources with huge market interest such as Octopus vulgaris Cuvier, 1797.
O. vulgaris, also known as common octopus, is a cephalopod species with a very strong market interest in Europe, and specifically in Spain, the current major supplier of octopus in the global seafood market40,41. The great commercial interest in O. vulgaris along with the growing demand for this species, increase the potential risk of fundamental changes in its population structure, which can lead to stock collapse due to overexploitation. Recent studies have reported a significant decrease in octopus global captures, mostly caused by unmonitored fishing activities in some regions42. Particularly, the information obtained from the analysis of genetic markers in this species can be useful in assessing the level of the marine stock healthiness, and subsequently in framing more sustainable fishery plans43. It is vitally important that this valuable species is sustainably managed, as it is not only an important food but also provides a significant source of income to local communities and families31.
A co-management system was established for the small-scale fishery (SSF) of octopuses with baited traps in Asturias (NW Spain) from 2000 to 2001 covering from the Eo estuary to the Nalon estuary44. The management measures include limiting entry to licensed boats from any of the eight legally recognized fishers’ associations, seasonal closures (almost always between mid-July to mid-December), type and number of gears and boat regulations, minimum landing octopus weight of 1 kg, and a maximum catch per season44,45. In the last two fishery seasons, Asturias management set the annual global catch as equal to the mean latent productivity (instead of the Maximum Sustainable Yield (MSY) criteria) minus two times the standard error of the estimate as a precautionary, sustainable and economically viable annual harvest rate46.
The artisanal octopus fishing with traps in western Asturias was certified with the Marine Stewardship Council (MSC) eco-certification in 2016, which made it the world’s first cephalopod fishery with this accreditation. However, the BUREAU VERITAS IBERIA (the certifying body) noted some weaknesses when recommending the MSC sustainability certificate for this fishery. They reported that “biological information on the resource was still scarce”, explicitly recommending that “information on the knowledge of octopus populations need to be improved”47. Having passed the first 5 years of its initial certification, and with explicit use of a precautionary annual harvest rate46, the octopus fishery of western Asturias has achieved MSC recertification in 2021. In the case of the western Asturias octopus fishery, the eco-label validated the fishery as sustainable and environmentally friendly since it has a minimum impact on the marine ecosystem48.
Over the past twenty years, some research has been conducted on studying the Atlantic O. vulgaris population using DNA markers31,49,50,51. However, the genetic status of O. vulgaris stocks in the southern area of the Bay of Biscay is still poorly studied. In this work, the main objectives have been to determine, both spatially and temporally, the levels and characteristics of genetic variation in octopus samples from areas localized within the MSC-certified octopus management plan in western Asturias, in comparison with other areas of the species geographic distribution, as well as to assess the stability of gene frequencies and carry out the first estimations of effective population sizes for this valuable species. All this data can address questions of direct relevance for the sustainable management of the species.
Results
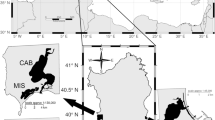
In this work, we have conducted temporal and spatial genetic analysis in O. vulgaris samples from several areas of the species’ geographic distribution (Basque Country, Asturias, Galicia, Algarve, Canary Islands, and Catalonia) and including localities where the MSC-certified western Asturias octopus fishery is established (i.e. Tapia de Casariego, Puerto de Vega and Cudillero; Fig. 1).
Microsatellites genetic variation in common octopus (O. vulgaris)
A total of 13 microsatellite loci were arranged into 2 multiplex PCRs (M1, M2) (Table 1). The number of alleles per locus (k) varied from 3 to 31 between loci, with an average of 13.77, and yielded an average allelic richness (AR) of 7.03 (ranging from 1.36 to 15.65) (Table 1). The observed heterozygosity and the within-population gene diversity across loci ranged from HO = 0.04 (M1; Vulg12) and HS = 0.06 for the same locus, to HO = 0.89 (M2; OV10) and HS = 0.92 (M1; OCT08). The global averages for observed heterozygosity and within-population gene diversity were 0.49 and 0.51, respectively (Table 1). Only the marker OCT08 (FIS = 0.113 p < 0.05), showed significant deviations from the Hardy–Weinberg equilibrium (13 loci average FIS = 0.04 p < 0.05) whereas the other twelve loci (92%) agreed with Hardy Weinberg expectations (Table 1). The potential presence of null alleles (Brookfield 1 statistic q > 0.05) was detected for only four loci (31%), specifically for the locus OCT08 (Brookfield 1996, B = 0.059); Vulg15 (B = 0.114); Vulg12 (B = 0.056) and Ovul08 (B = 0.090) (Table 1). The mean overall FST values for the 13 microsatellites was FST = 0.07 (p < 0.05) and four of them showed higher and significant FST values: Vulg15 (FST = 0.340, p < 0.05); Vulg12 (FST = 0.469, p < 0.05); Vulg13 (FST = 0.083, p < 0.05); Ovul08 (FST = 0.260, p < 0.05) (Table 1). The analysis conducted with BayeScan v2.1 for outlier detection resulted in no loci under selection or biased by species admixture and hybridization, which have the same expectations in terms of outliers; however, when we found 1 locus with positive α-values, the q-value was higher than 0.05 and not fall apart from neutral expectations for the analyzed genetic data.
The comparative analyses for levels of genetic variation among samples by locations revealed Olhão (Algarve) showing the highest values for allelic richness (7.621) while Cudillero (Asturias) showed the highest number of private alleles for the 2020–2021 fishery season (Ap = 7) (Table 2). Significant differences in allelic richness and observed heterozygosity were found in this work (p < 0.05) ranging from AR = 6.079 (Pasaia, Basque Country, Spain; 2020–2021) to AR = 7.621 (Olhão, Algarve, Portugal; 2020–2021) and from HO = 0.444 (Puerto de Vega, Asturias, Spain; 2020–2021) to HO = 0.571 (Olhão, Algarve, Portugal; 2006–2007) (Table 2). The expected heterozygosity was also significantly differentiated (p < 0.01) among the samples and San Andrés (Canary Islands, Spain; 2020–2021) showed the highest values (HE = 0.613) whereas Cudillero (Asturias, Spain; 2020–2021) showed the lowest ones (HE = 0.461) (Table 2). Comparative temporal analyses revealed no significant differences in the levels of genetic variation during the past 14 years (2007–2021) (approximately 9 generations, considering the life-span of 1.5 years for O. vulgaris). That was the case of Puerto de Vega (Asturias, Spain) and Olhão (Algarve, Portugal) and the same non-significant result was also obtained for the time range between 2018 and 2021 for the Ribadesella, Cudillero, Puerto de Vega, and Tapia de Casariego populations (Asturias); reflecting stable levels of genetic variation for the stocks in these locations for the mentioned period (Table 2).
The highest level of discrepancies between observed and expected heterozygosity was noticed in San Andrés (Canary Island, Spain) but none of the samples disagreed with Hardy Weinberg expectations (Table 2). The population bottleneck hypothesis was tested with the software Bottleneck and no significant excess of predicted heterozygotes was observed under the TPM model. Results from Wilcoxon’s test showed no sign of significant recent bottlenecks in the nine populations under study (Table 2).
Spatial and temporal genetic analyses in common octopus (O. vulgaris)
A significant spatial structuring pattern was found in this work for O. vulgaris samples (Fig. 2). The pairwise FST values revealed high genetic differentiation among samples from four separated geographic areas: (1) Bay of Biscay (including Basque Country and Asturias) and also the northern Atlantic samples from Galicia; (2) Southern Portugal; (3) Canary Islands and 4) Catalonia (Figs. 2, 3). Within the Bay of Biscay and adding the sample from Bueu (Galicia, 2020–2021) we found genetic homogeneity by FST values, except for one sample from Puerto de Vega (Asturias, Spain; 2020–2021), showing a significant differentiated genetic pattern with the rest of the samples (Fig. 2). These patterns of genetic differentiation increased with geographic distance between locations. The results of the partial Mantel tests indicated a correlation between genetic and geographic distances, with R2 = 0.81 and p-value = 0.004, presenting a significant Isolation by Distance (IBD) pattern.
FST heatmaps (based on Weir and Cockerham55) following genetic analyses of O. vulgaris using microsatellites along the Iberian Peninsula and the Canary Islands. Labels indicate the fishery season (first two digits) and the locality (Pasaia (PS), Ribadesella (RB), Cudillero (CU), Puerto de Vega (PV), Tapia de Casariego (TP), Bueu (BU), Olhão (OL), San Andrés (SA), Barcelona (BC)). The darker the color, the higher the FST value. Asterisks indicate significant p-values (p < 0.05) after Bonferroni correction. Temporal comparisons are highlighted by black rectangles.
Genetic clustering using Discriminant Analysis of Principal Components (DAPC) of O. vulgaris populations using microsatellites along the Iberian Peninsula and the Canary Islands. (A) Global analysis, (B) Enlarged detail of localities in the Basque Country, Asturias, Galicia and Portugal. Labels indicate the fishery season (first two digits) and the locality (Pasaia (PS), Ribadesella (RB), Cudillero (CU), Puerto de Vega (PV), Tapia de Casariego (TP), Bueu (BU), Olhão (OL), San Andrés (SA), Barcelona (BC)).
Temporal genetic differentiation was also tested by FST values (Fig. 2) and by DAPC (Fig. 3a,b), for those locations where data from more than one fishery season were available (Figs. 2, 3). The data revealed no significant temporal genetic changes in samples from Olhão (Portugal) within the period of 14 years (approximately 9 generations considering O. vulgaris life spam). No significant temporal differences were found between 2018 and 2021 (2 generations) for the rest of the locations, except for the samples from Puerto de Vega collected between 2007 and 2018, with respect to the last sampling collected during 2020–2021 in the same locality (21PV) (Figs. 2, 3).
The Neighbour Joining tree obtained after Nei genetic distances DA estimations57 clearly separated the Canary Islands and Barcelona from the rest of the samples, and separated Portugal from the rest of the other populations located further north (see the map in Figs. 1, 4a,b). The Bayesian analyses for structuring in O. vulgaris showed 2 main genetic clusters (Evanno’s k = 2, Lnʹ(K) = 733.240) (Fig. 4c) representing 2 ancestral lineages (i.e., Algarve, Canary Islands, and Mediterranean, and then the rest of Atlantic samples including the Bay of Biscay) (Fig. 4).
Neighbor-joining trees using DA distance57 and bayesian analysis of O. vulgaris populations in this study. (A) Global analysis for the 9 localities showing all the fishery seasons, (B) global analysis for all localities showing only the 2020–2021 fishery season, (C) Structure bar-plot showing the assignment probabilities for each genotyped individual from 2020–2021 fishery season under admixture model. Each bar corresponds to one individual. Labels indicate the fishery season (first two digits) and the locality (Pasaia (PS), Ribadesella (RB), Cudillero (CU), Puerto de Vega (PV), Tapia de Casariego (TP), Bueu (BU), Olhão (OL), San Andrés (SA), Barcelona (BC)).
Effective population sizes were analyzed with NeEstimator 2.158 using the temporal methods based on allelic variances59. The analyses showed estimates of NE-Puerto de Vega(2007–2018) = ∞ (95% CIs for NE, Jackknife on Loci: 1042.5–∞); NE-Puerto de Vega(2007–2021) = 182.3 (27.8–∞) and NE- Olhão(2007–2021) = 1651.3 (292.7–∞) for approx. 7 to 9 generations (2007–2018–2021). The shorter period from 2018 to 2021 (approx. 2 generations) revealed the following estimates in Asturias NE-Ribadesella(2018–2021) = ∞ (248.3–∞); NE-Cudillero(2018–2021) = ∞ (261.5–∞); NE-Puerto de Vega(2018–2021) = 46.6 (8.0–∞) and finally NE-Tapia de Casariego(2018–2021) = 137.6 (54.6–∞).
Discussion
This study aims to shed light on the genetic status of the populations of Octopus vulgaris, a species of great commercial interest, and on the efficiency of the current O. vulgaris management plan in Asturias, northern Spain.
Genetic variation and management units (MU) for exploited O. vulgaris stocks in the eastern Atlantic area
Genetic diversity is the key element for maintaining a species’ potential to adapt to environmental changes, when there is undeniable evidence that the environment is changing mainly due to anthropogenic activity60. In this work, the levels of genetic variability found when using a set of 13 microsatellite markers on samples from 2007, 2018, and 2021 (mean K = 13.7, AR = 7.0, HE = 0.51, HO = 0.49) did not show significant temporal changes. The reported values were just slightly lower than those previously reported by De Luca et al.43 (13 microsatellites, mean K = 15.8, AR = 7.4, HE = 0.65, HO = 0.50) for Mediterranean O. vulgaris samples. Melis et al.42 (five microsatellites, Mean K = 27.6, AR = 16.8, HE = 0.90, HO = 0.83) and Cabranes et al.50 (five microsatellites, Mean K = 18.3, HE = 0.87, HO = 0.75) reported higher genetic variation values for Mediterranean and Atlantic populations, respectively; but using a very low number of genetic markers. Significant loss of genetic variation will lead to loss of species’ evolutionary potential61. It is well established that overfishing is seen as the major threat to the loss of marine populations genetic diversity within populations and it has the potential (when fishing is highly selective) to permanently change the characteristics within a population, usually in directions of less economic value62,63. Negative consequences of losing genetic diversity will not only affect the fishing industry, but also disturb the respective predator–prey populations and eventually the entire marine ecosystem64.
The Hardy–Weinberg principle depends on a number of assumptions namely simple Mendelian inheritance in a diploid organism with discrete generations, random mating, an infinite population, and no mutation, migration, or selection65. None of the populations in our study showed significant deviations from the Hardy–Weinberg Equilibrium (HWE), and at the same time, no signals of recent bottlenecks were found in those localities under study. These results could suggest that those populations (all of them under fishery exploitation) are at rest and not perturbed in a significant way.
Our data includes a representation of the eastern Atlantic and Mediterranean area octopus populations (Basque Country, Asturias, Galicia, Portugal, Canary Islands, and Catalonia). The Bayesian analyses suggested two ancestral gene pools (1: Bay of Biscay and Galicia and 2: Algarve, Canary Islands, and Mediterranean areas). Contemporary genetic heterogeneity is evident at smaller geographic scales among Portugal, Canary Islands, and Mediterranean samples. Some samples (i.e. Puerto de Vega 2020–2021 in Asturias) showed peculiarities that revealed punctual genetic heterogeneity within Galicia and the Bay of Biscay samples analyses at very small distance scales (Figs. 1, 2). Note that, although the presence of null alleles (common when working with microsatellites) could lead to overestimation of both, FST and genetic distances56, our results remain the same when using the ENA correcting method. Previous studies on genetic structuring in O. vulgaris have been carried out based on mitochondrial and nuclear DNA datasets in the Mediterranean42,43,49,66,67 and Atlantic areas31,49,50,51. Using both types of markers, De Luca et al.43 found a pronounced differentiation of the Atlantic and Sicilian specimens supporting the isolation of the biota within the Strait of Messina and significant differentiation within the Mediterranean Sea as was previously suggested by Casu et al.66. Moreover, Melis et al.42 highlighted high variability and low but significant genetic differentiation among populations in a very small geographic scale in the Mediterranean, where samples clustered into four groups in the coasts of Sardinia. In the Atlantic area, significant subpopulation structure was also identified using only five microsatellites, consistent with an isolation-by-distance (IBD) model for Atlantic populations, but the genetic differences between pairs of samples separated by < 200 km were not significant50. Recently, Quinteiro et al.51 used mitochondrial DNA and suggested a significant differentiation in their study including insular and continental samples from the Galicia and Morocco coasts, with the exception of pairwise comparisons for samples from Madeira and the Canaries populations. These results pointed out the existence of genetically differentiated octopus populations in the Mediterranean and Atlantic areas and the necessity of local regulations for the appropriate management of octopus stocks. The octopus stocks of the Bay of Biscay had not been genetically monitored for more than fifteen years until the present study.
Our study area is also located in the Canaries-Iberian upwelling system. Even when upwelling was early viewed as dispersive environments for the larval stages, these systems are now considered more retentive areas than previously thought, where the larvae are capable to regulate their transport by exploiting the circulation patterns and later recruiting close to their natal habitats68. Genetic similarity within marine species is directly related to individual dispersal capability31. The movements of adult O. vulgaris have been found highly limited and within 1 km in most of the recaptures (84–86%) in a capture-recapture experiment conducted by Mereu et al.69,70 where high site fidelity and the necessity for creating small no-take areas were suggested. While it seems that planktonic O. vulgaris paralarvae have an oceanic strategy in upwelling systems rather than the coastal-shelf strategy of other neritic species (Loliginidae and Sepiidae families)71; pre-recruits usually are distributed in specific and differential grounds, as has been found in Portugal (8 pre-recruit grounds), where its western zone adjacent to Ria Formosa lagoon (southern coast) was identified as the main recruitment ground for O. vulgaris along the Portuguese coast72. Our Portuguese samples were coming from this area (Algarve) and as it is mentioned above, they showed high levels of genetic diversity. It seems also that environmental factors are fundamental to the behavior of the dispersal ability of paralarvae in O. vulgaris73 and wide inter-annual fluctuations in O. vulgaris abundances have been described in distinct geographic areas in connection with environmental drivers (reviewed by Roa-Ureta et al.46). In Galicia, it seems that a large fraction of the annual variability in catch is due the impact of upwelling on the survival of planktonic life stages74. Characterizing possible local paralarvae retentions in the Asturian coast as a consequence of hydrographic conditions as well as identifying recruitment grounds are still pending. Moreover, it had been recently argued that the Asturias O. vulgaris stock presents a rich dynamic that results from intrinsic properties of the stock, as well as from small perturbations from a combination of moderate fishing removals and possibly environmental forces that determine strong density-dependent and overcompensations that cause fluctuations in O. vulgaris stocks abundance46. These facts can explain punctual genetic heterogeneity within the Bay of Biscay area, where we have found a global pattern of genetic homogeneity (considering Basque Country, Asturias, and even Galicia) which is congruent with previous studies on the area (i.e.: Cabranes et al.50). Despite this, periodic genetic monitoring on these exploited and fluctuating stocks seems to be advisable. Deeper studies using genomic tools (i.e.: SNP studies covering wider areas within the octopus genome) will help in the future to re-assess structuring and management units in this small geographic area.
The relevance of temporal genetic data on assessments for the MSC eco-labeled sustainable fisheries of the Asturias O. vulgaris fishing stock
In this work, we have found stable levels of genetic variation for the localities where temporal analyses were possible (Ribadesella, Cudillero, Puerto de Vega, Tapia de Casariego, all of them located in Asturias; and Olhão, which is located in Portugal). It is worth mentioning that temporal replicates were all in HWE. Temporal analyses are a powerful tool in population genetics and in its application to relevant problems, such as assessments of fishery stock status. Temporal studies allow to discerning between real genetic signals and noise artifacts, meaning that genetic patterns that are consistent through time are unlikely to be sampling artifacts59. Moreover, sampling adult population over generations and looking for statistically significant shifts in allelic frequencies that cannot be explained by evolutionary forces, such as mutation, selection, migration, or varying year-class strengths in populations, help to identify Sweepstakes Reproductive Success (SRS) patterns, common in marine species, where extremely large variance in individual reproductive success is due to sweepstakes-like chances of matching reproductive activity with oceanographic conditions75. In addition, temporal replicates allow estimating of effective population sizes, which is a relevant parameter that gives clues about the health state of an endangered/exploited population determining the rate of loss of genetic diversity, fixation of deleterious alleles, and the efficiency of natural selection at maintaining beneficial alleles76.
In the Portuguese samples (Olhão, Algarve, Portugal), the temporal replicates were genetically homogeneous and showed high diversities. Besides being a relevant recruitment zone, it seems that a relevant co-management plan has been reported in the area and has shown promising results for the sustainable use of fishery resources77,78. O. vulgaris stock assessment is difficult due to distinct life history features such as short life cycles, semelparous reproduction, high natural mortality rates, rapid growth, and complex population structures79. A keystone in any attempt at species conservation and/or management is the effective size of a population (NE). Fifty individuals (NE = 50) have been considered necessary for a population’s immediate survival avoiding inbreeding depression80. In the Algarve, Portugal, high effective population sizes NE-OL(2018–2021) = 1651.3 (292.7–∞) were found. In three out of the four Asturias locations temporally sampled in this work, our data revealed also high effective population sizes (NE-Ribadesella (2018–2021) = ∞ (248.3–∞); NE-Cudillero (2018–2021) = ∞ (261.5–∞); and NE-Tapia de Casariego (2018–2021) = 137.6 (54.6–∞)). The decrease in the effective population size in the case of Puerto de Vega is the only noticeable case, as in the time range between 2007 and 2018 the value is infinite (NE-PV(2007–2018) = ∞ (1042.5–∞)) while from 2018 to 2021, it drops sharply to 46.4 (NE-PV(2018–2021) = 46.6 (8.0–∞). It has been said that octopus fisheries in Asturias exercise a low pressure on the stability and renewal capacity of the stocks47. During the first few years of the management plan (2000–2001 to 2007–2008), total annual landings averaged 180 tons, and total annual effort normally exceeded 3000 days of O. vulgaris fishing, but later (2008–2009 to 2018–2019), landings decreased, averaging 102 tons, and effort decreased as well to less than 2000 days46. Recently, Roa-Ureta et al.46 estimated abundances for octopus’ stocks within the Asturias management plan using depletion models and reported densities ranging from 1250 to nearly 5000 per km2 considering the area of the fishing grounds off in Asturias (228 to 397 km2). Moreover, they predicted recruitment from spawning abundance observations for female spawning stock and recruitment dynamics in O. vulgaris in this area. These temporal analyses indicate significant oscillations in fishing efforts and recruitments (see Fig. 5b in Roa-Ureta et al.46). Moreover, taking into account the entire available time range (2007–2021), the NE-PV(2007–2021) = 182.3 (27.8–∞) is still high. The case of Puerto de Vega can be punctual, or an indicator of restricted gene flow81, or a signal about current environmental and/or fishery pressures on the specific zone perturbing the octopus populations. Discerning this will need more studies, including replication, to be evaluated.
Conclusions
Findings from this study can give a better vision of the spatial and temporal distributions of genetic variation in common octopus in the Atlantic area, and of the efficiency of the current O. vulgaris management plan in Asturias, as well as of the healthiness levels of these fishery stocks. The Atlantic O. vulgaris populations show significant genetic structuring at a large geographical scale that fits with a classical isolation by distance model, where the probability of individuals mating with one another is restricted and local retention of paralarvae makes populations small in comparison to the total species distribution. That reinforces the necessity of local and regional plans to guarantee long-term sustainability. Results from our work can provide a baseline for further genomic studies on Asturian common octopus and therefore for sustainable exploitation. To the best of our current knowledge the Asturias O. vulgaris fishery plan seems to be currently adequate, since our data is not detecting recent harms to the fished stock and, accordingly, the validity of the MSC label seems to be rational. More data will be needed to assess if the Asturias management plan may require a more specific regional approach including smaller spatial scales.
Materials and methods
Ethics declaration
No use of live animals was required for this study. All samples used for the present study came from animals fished for commercial purposes or from the collections of other research centers. For more information, please see the Acknowledgements section.
Samples, DNA extractions, and microsatellite amplifications
A total of 539 O. vulgaris individuals were collected in nine localities across the Bay of Biscay (Pasaia in the Basque Country, Spain; Ribadesella, Cudillero, Puerto de Vega, and Tapia de Casariego in Asturias, Spain), Portuguese waters (Bueu in Galicia, Spain; Olhão in Algarve, Portugal), Macaronesia (San Andrés in Canary Islands, Spain) and the Mediterranean sea (Barcelona in Catalonia, Spain) during the last fishery seasons (2020–2021) (Fig. 1).
Moreover, samples from the fishery campaign 2006–2007 from Portugal and Asturias (Puerto de Vega) and from the season 2017–2018 in Asturias (Tapia de Casariego, Puerto de Vega, Cudillero, Ribadesella) were also available for analyses. All the procedures were conducted using preserved or freshly small tissues from specimens collected by local fishermen and fixed in pure ethanol (100%). No use of live animals was required for this study.
DNA was extracted using EZNA® Mollusc Kit (Omega Bio-Tek Inc., Norcross, GA, USA). Approximately 25 mg from octopus tissues were cut and chopped into small pieces, put into 1.5 ml microcentrifuge tubes and processed following the manufacturer’s instructions. The resulting DNA was visualized on agarose gel 1% and stored in a − 20 °C freezer for further applications. Thirteen microsatellite loci were amplified reliably and arranged into two multiplex PCRs using Multiplex Manager 1.2 software82 according to dye colors and expected amplicon sizes. Microsatellite amplifications were carried out by combining 13 loci (previously tested in single PCRs) in two multiplex PCR reactions (Table 1), using QIAGEN Multiplex PCR Kit (QIAGEN Inc., Venlo, Netherlands) at the following conditions: 15 min at 95 °C, 40 cycles at 94 °C for 30 s, 55 °C for 1 min and 30 s, 72 °C for 1 min, and a final extension at 60 °C for 30 min. Each PCR reaction was conducted in a final volume of 13 μl. Forward primers were 5’ labeled using fluorescent dyes: 6-FAM™, NED™, VIC®, and PET® (Applied Biosystems, Foster City, CA, USA) (Table 1). PCR products were run on the Automated Capillary Electrophoresis Sequencer 3130XL Genetic Analyzer (Applied Biosystems) after a 1:10 dilution.
Genetic variation
Microsatellite genotyping was conducted locus per locus by two different and independent readers using the automatic procedure implemented in Geneious Prime® 2020.2 and manually corrected. Possible genotyping errors and null allele frequency estimates were determined using Micro-Checker 2.2.383 and FreeNA56, with the number of replicates fixed to 10,000. The data set corrected for null alleles was used as a final input file for further statistical analysis. Allele frequencies, the number of alleles per locus (k), the mean number of alleles (NA) per locus, the observed heterozygosity, within-population gene diversity and the overall gene diversity (HO and Hs, and HT respectively), were calculated using “adegenet”84, “pegas”85 and “hierfstat”86 packages implemented in R version 4.1.2 through RStudio 2021.09.2 + 382 “Ghost Orchid” Release. The “PopGenReport” package87 in R was used to calculate the allelic richness (AR) per locus and per population and also the number of private alleles (AP) within populations. Spatial and temporal comparisons for levels of genetic variation were conducted using a two-sided statistical analysis included in the FSTAT 2.94 software88 for several statistics (AR, HO, HE).
Spatial and temporal genetic differentiation and clustering analyses
The F statistics following Weir and Cockerham55 and possible deviations from expected proportions in Hardy Weinberg’s equilibrium for each locus and population were assessed using FSTAT 2.94 software88. Significance levels of FIS were estimated by permutating alleles between genotypes within samples 10,000 times, and adjusted following a Bonferroni correction89. Additionally, FST (ENA) values were estimated using FreeNA, which estimated the unbiased FST following the ENA method56. The bottleneck hypothesis was investigated using the program BOTTLENECK v 1.2.02 under the two-phased model of mutation (TPM)90, taking into account 90% single stepwise mutations with a variance of 12. The “Wilcoxon sign-rank test” was used to determine whether a population exhibits a significant number of loci with heterozygosity excess91. Pairwise FST values between samples and corresponded p-values were calculated using FSTAT 2.94 software where for significance levels of FST, multi-locus genotypes were randomized between pairs of samples (10,000 permutations) and calculated after Bonferroni correction88,89. Comparisons between regions and between fishery seasons were conducted using a two-sided statistical analysis included in the FSTAT software for several statistics [AR, HO, HE, FIS, FST, relatedness (R), and corrected relatedness].
A discriminant analysis of principal components (DAPC) using the R working package “adegenet”84, was conducted to cluster the samples in groups. Besides this, the population structure was also assessed with Bayesian clustering population structure analysis in STRUCTURE 2.3.492. In order to fasten the procedure, structure analysis was performed through “ParallelStructure” package93 in R. Structure analysis was run among all nine populations, without taking temporal data into account. The settings used were an admixture model from K = 1 to K = 18 in 20 runs94,95. Assignment clusters were made with a length burn-in period of 20,000 and 200,000 Markov Chain Monte Carlo repetitions. The most likely value of K was chosen using the delta K statistic94, using the STRUCTURE HARVESTER software96. A Neighbour‐Joining (NJ) tree based upon pairwise Nei's genetic distance DA57 was constructed with the software POPTREEW97 using 10,000 bootstraps and visualized in The Interactive Tree of Life (https://itol.embl.de)98.
The Package “ade4”99 was used to perform a partial Mantel test to study if the observed patterns of genetic structure found here conformed to the isolation by distance model (IBD) explaining genetic isolation between populations100. The geographic distances between each pair of sampling localities (Kms) were calculated on the basis of a spherical earth (ignoring ellipsoidal effects), using the haversine formula59 and they were related with Edwards' distance101. The software BayeScan 2.1.102 was used to identify candidate loci deviating from neutral expectations from genetic data, using differences in allele frequencies between populations. Twenty pilot runs of 5000 iterations each, followed by an additional burn-in of 50,000 iterations, and then 5000 samplings with a thinning interval of 10 were conducted. Loci with α-value significantly > 0 and q-values < 0.05 were defined as “outliers”—i.e., loci putatively under directional selection. Loci with α-value significantly < 0 were considered putatively under balancing selection. The remaining loci were classified as neutral.
Effective population sizes
Finally, temporal change in allelic frequencies was used for estimating the effective population sizes (NE) using the Temporal Method, Nei/Tajima, Plan II103 with the NeEstimator software58. The lifespan of O. vulgaris from several regions in the Atlantic frequently exceeds 1 year and may reach a maximum of nearly 2 years104,105. A generation interval of 1.5 years for O. vulgaris was used for NE estimations, consequently, the 2007 fishing season was considered as the starting year (generation 0), while 2018 was considered generation 7 and 2021 generation 9.
Data availability
The datasets generated and/or analyzed during the current study are available in the Zenodo repository: https://doi.org/10.5281/zenodo.6635759. Microsatellite markers (GenBank accession numbers, [reference]): OCT08 (AF19713250); VULG15 (LC00303551); VULG14 (LC00303451); VULG07 (LC00302851); OVUL10 (JN57969952); VULG12 (LC003032,51); VULG13 (LC00303351); VULG06 (LC00302751); OVUL09 (JN57969852); VULG04 (LC00302651); OVUL08 (JN57969752); OV10 (AF19713450); VULG10 (LC00303051).
References
FAO. El estado mundial de la pesca y la acuicultura 2020 (FAO, 2020).
Jackson, J. B. C. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001).
Scheffer, M., Carpenter, S. & de Young, B. Cascading effects of overfishing marine systems. Trends Ecol. Evol. 20, 579–581 (2005).
Coll, M., Libralato, S., Tudela, S., Palomera, I. & Pranovi, F. Ecosystem overfishing in the ocean. PLoS ONE 3, e3881 (2008).
Peterson, M. S. & Lowe, M. R. Implications of cumulative impacts to estuarine and marine habitat quality for fish and invertebrate resources. Rev. Fish. Sci. 17, 505–523 (2009).
Claudet, J. & Fraschetti, S. Human-driven impacts on marine habitats: A regional meta-analysis in the Mediterranean Sea. Biol. Cons. 143, 2195–2206 (2010).
Smith, V. H., Tilman, G. D. & Nekola, J. C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100, 179–196 (1999).
Derraik, J. G. B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 44, 842–852 (2002).
Doney, S. C. et al. Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37 (2012).
Molnar, J. L., Gamboa, R. L., Revenga, C. & Spalding, M. D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 6, 485–492 (2008).
Wojnarowska, M., Sołtysik, M. & Prusak, A. Impact of eco-labelling on the implementation of sustainable production and consumption. Environ. Impact Assess. Rev. 86, 106505 (2021).
Yan, H. F. et al. Overfishing and habitat loss drive range contraction of iconic marine fishes to near extinction. Sci. Adv. 7, 6026 (2021).
Bastardie, F. et al. Spatial planning for fisheries in the Northern Adriatic: Working toward viable and sustainable fishing. Ecosphere 8, e01696 (2017).
Arkema, K. K. et al. Integrating fisheries management into sustainable development planning. Ecol. Soc. 24, 0201 (2019).
Aguión, A. et al. Establishing a governance threshold in small-scale fisheries to achieve sustainability. Ambio. https://doi.org/10.1007/s13280-021-01606-x (2021).
Gudmundsson, E. & Wessells, C. R. Ecolabeling seafood for sustainable production: Implications for fisheries management. Mar. Resour. Econ. 15, 97–113 (2000).
FAO. Guidelines for the Ecolabelling of Fish and Fishery Products from Marine Capture Fisheries. Revision 1 (FAO, 2009).
Hilborn, R. & Ovando, D. Reflections on the success of traditional fisheries management. ICES J. Mar. Sci. 71, 1040–1046 (2014).
Casey, J., Jardim, E. & Martinsohn, J. T. H. The role of genetics in fisheries management under the E.U. common fisheries policy. J. Fish Biol. 89, 2755–2767 (2016).
MSC. MSC Fisheries Standard v2.01. https://www.msc.org/docs/default-source/default-document-library/for-business/program-documents/fisheries-program-documents/msc-fisheries-standard-v2-01.pdf?sfvrsn=8ecb3272_9 (2018).
Costello, C. et al. Status and solutions for the world’s unassessed fisheries. Science 338, 517–520 (2012).
Hilborn, R. et al. Effective fisheries management instrumental in improving fish stock status. PNAS 117, 2218–2224 (2020).
Worm, B. & Branch, T. A. The future of fish. Trends Ecol. Evol. 27, 594–599 (2012).
Palomares, M. L. D. et al. Fishery biomass trends of exploited fish populations in marine ecoregions, climatic zones and ocean basins. Estuar. Coast. Shelf Sci. 243, 106896 (2020).
Ihssen, P. E. et al. Stock identification: Materials and methods. Can. J. Fish. Aquat. Sci. 38, 1838–1855 (1981).
Carvalho, G. R. & Hauser, L. Molecular genetics and the stock concept in fisheries. In Molecular Genetics in Fisheries (eds Carvalho, G. R. & Pitcher, T. J.) 55–79 (Springer, 1995).
Worm, B. et al. Rebuilding global fisheries. Science 325, 578–585 (2009).
Gough, C. L. A., Dewar, K. M., Godley, B. J., Zafindranosy, E. & Broderick, A. C. Evidence of overfishing in small-scale fisheries in Madagascar. Front. Mar. Sci. 7, 317 (2020).
Widjaja, S. et al. Illegal, Unreported and Unregulated Fishing and Associated Drivers 60 (2020).
Walters, C. & Martell, S. J. D. Stock assessment needs for sustainable fisheries management. Bull. Mar. Sci. 70, 629–638 (2002).
Moreira, A. A., Tomás, A. R. G. & Hilsdorf, A. W. S. Evidence for genetic differentiation of Octopus vulgaris (Mollusca, Cephalopoda) fishery populations from the southern coast of Brazil as revealed by microsatellites. J. Exp. Mar. Biol. Ecol. 407, 34–40 (2011).
Allendorf, F. W., Ryman, N. & Utter, F. M. Genetics and fishery management. In Population Genetics and Fishery Management 1–19 (1987).
Oosthuizen, A., Jiwaji, M. & Shaw, P. Genetic analysis of the Octopus vulgaris population on the coast of South Africa. S. Afr. J. Sci. 100, 603–607 (2004).
Botsford, L. W., Castilla, J. C. & Peterson, C. H. The management of fisheries and marine ecosystems. Science 277, 509–515 (1997).
Hilborn, R., Orensanz, J. M. & Parma, A. M. Institutions, incentives and the future of fisheries. Philos. Trans. R. Soc. B Biol. Sci. 360, 47. https://doi.org/10.1098/rstb.2004.1569 (2005).
Ovenden, J. R., Berry, O., Welch, D. J., Buckworth, R. C. & Dichmont, C. M. Ocean’s eleven: A critical evaluation of the role of population, evolutionary and molecular genetics in the management of wild fisheries. Fish Fish. 16, 125–159 (2015).
Aguirre-Sarabia, I. et al. Evidence of stock connectivity, hybridization, and misidentification in white anglerfish supports the need of a genetics-informed fisheries management framework. Evol. Appl. 14, 2221 (2021).
Grover, A. & Sharma, P. C. Development and use of molecular markers: Past and present. Crit. Rev. Biotechnol. 36, 290 (2016).
Valenzuela-Quiñonez, F. How fisheries management can benefit from genomics? Brief. Funct. Genom. 15, 352–357 (2016).
Khoufi, W., Jabeur, C. & Bakhrouf, A. Stock assessment of the common octopus (Octopus vulgaris) in Monastir; the Mid-eastern Coast of Tunisia. Int. J. Mar. Sci. 2, 1 (2012).
Pita, C. et al. Fisheries for common octopus in Europe: Socioeconomic importance and management. Fish. Res. 235, 105820 (2021).
Melis, R. et al. Genetic population structure and phylogeny of the common octopus Octopus vulgaris Cuvier, 1797 in the western Mediterranean Sea through nuclear and mitochondrial markers. Hydrobiologia 807, 277–296 (2018).
De Luca, D., Catanese, G., Procaccini, G. & Fiorito, G. Octopus vulgaris (Cuvier, 1797) in the Mediterranean Sea: Genetic diversity and population structure. PLoS ONE 11, e0149496 (2016).
Fernández-Rueda, P. & García-Flórez, L. Octopus vulgaris (Mollusca: Cephalopoda) fishery management assessment in Asturias (north-west Spain). Fish. Res. 83, 351–354 (2007).
Gobierno del Principado de Asturias. BOPA núm. 233 de 03-XII-2021, Vol. 233 (2021).
Roa-Ureta, R. H. et al. Estimation of the spawning stock and recruitment relationship of Octopus vulgaris in Asturias (Bay of Biscay) with generalized depletion models: Implications for the applicability of MSY. ICES J. Mar. Sci. https://doi.org/10.1093/icesjms/fsab113 (2021).
González, A. F., Macho, G., de Novoa, J. & García, M. Western Asturias Octopus Traps Fishery of Artisanal Cofradías 181 (2015).
Sánchez, J. L. F., Fernández Polanco, J. M. & Llorente García, I. Evidence of price premium for MSC-certified products at fishers’ level: The case of the artisanal fleet of common octopus from Asturias (Spain). Mar. Policy 119, 104098 (2020).
Murphy, J. M., Balguerías, E., Key, L. N. & Boyle, P. R. Microsatellite DNA markers discriminate between two Octopus vulgaris (Cephalopoda: Octopoda) fisheries along the northwest African coast. Bull. Mar. Sci. 71, 545–553 (2002).
Cabranes, C., Fernandez-Rueda, P. & Martínez, J. L. Genetic structure of Octopus vulgaris around the Iberian Peninsula and Canary Islands as indicated by microsatellite DNA variation. ICES J. Mar. Sci. 65, 12–16 (2008).
Quinteiro, J., Rodríguez-Castro, J., Rey-Méndez, M. & González-Henríquez, N. Phylogeography of the insular populations of common octopus, Octopus vulgaris Cuvier, 1797, in the Atlantic Macaronesia. PLoS ONE 15, e0230294 (2020).
Greatorex, E. C. et al. Microsatellite markers for investigating population structure in Octopus vulgaris (Mollusca: Cephalopoda). Mol. Ecol. 9, 641–642 (2000).
De Luca, D., Catanese, G., Fiorito, G. & Procaccini, G. A new set of pure microsatellite loci in the common octopus Octopus vulgaris Cuvier, 1797 for multiplex PCR assay and their cross-amplification in O. maya Voss & Solís Ramírez, 1966. Conserv. Genet. Resour. 7, 299–301 (2015).
Zuo, Z., Zheng, X., Liu, C. & Li, Q. Development and characterization of 17 polymorphic microsatellite loci in Octopus vulgaris Cuvier, 1797. Conserv. Genet. Resour. 4, 367–369 (2012).
Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358 (1984).
Chapuis, M. P. & Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 24, 621–631 (2007).
Nei, M. & Takezaki, N. Estimation of Genetic Distances and Phylogenetic Trees from DNA Analysis 8 (1983).
Do, C. et al. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol. Ecol. Resour. 14, 209–214 (2014).
Waples, R. S. Separating the wheat from the chaff: Patterns of genetic differentiation in high gene flow species. J. Hered. 89, 438–450 (1998).
Taboada, F. G. & Anadón, R. Patterns of change in sea surface temperature in the North Atlantic during the last three decades: Beyond mean trends. Clim. Change 115, 419–431 (2012).
Ellegren, H. & Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 17, 422–433 (2016).
Sinclair, M. & Valdimarsson, G. Responsible Fisheries in the Marine Ecosystem (CABI, 2003).
Pinsky, M. L. & Palumbi, S. R. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 23, 29–39 (2014).
Bradbury, I. R., Laurel, B., Snelgrove, P. V. R., Bentzen, P. & Campana, S. E. Global patterns in marine dispersal estimates: The influence of geography, taxonomic category and life history. Proc. R. Soc. B Biol. Sci. 275, 1803–1809 (2008).
Waples, R. S. Testing for Hardy-Weinberg proportions: Have we lost the plot? J. Hered. 106, 1–19 (2015).
Casu, M. et al. Genetic structure of Octopus vulgaris (Mollusca, Cephalopoda) from the Mediterranean Sea as revealed by a microsatellite locus. Ital. J. Zool. 69, 295–300 (2002).
Fadhlaoui-Zid, K. et al. Genetic structure of Octopus vulgaris (Cephalopoda, Octopodidae) in the central Mediterranean Sea inferred from the mitochondrial COIII gene. C.R. Biol. 335, 625–636 (2012).
Queiroga, H. et al. Oceanographic and behavioural processes affecting invertebrate larval dispersal and supply in the western Iberia upwelling ecosystem. Prog. Oceanogr. 74, 174–191 (2007).
Mereu, M. et al. Mark–recapture investigation on Octopus vulgaris specimens in an area of the central western Mediterranean Sea. J. Mar. Biol. Assoc. U.K. 95, 131–138 (2015).
Mereu, M. et al. Movement estimation of Octopus vulgaris Cuvier, 1797 from mark recapture experiment. J. Exp. Mar. Biol. Ecol. 470, 64–69 (2015).
Roura, Á. et al. Life strategies of cephalopod paralarvae in a coastal upwelling system (NW Iberian Peninsula): Insights from zooplankton community and spatio-temporal analyses. Fish. Oceanogr. 25, 241–258 (2016).
Moreno, A. et al. Essential habitats for pre-recruit Octopus vulgaris along the Portuguese coast. Fish. Res. 152, 74–85 (2014).
Chédia, J., Widien, K. & Amina, B. Role of sea surface temperature and rainfall in determining the stock and fishery of the common octopus (Octopus vulgaris, Mollusca, Cephalopoda) in Tunisia. Mar. Ecol. 31, 431–438 (2010).
Otero, J. et al. Bottom-up control of common octopus Octopus vulgaris in the Galician upwelling system, northeast Atlantic Ocean. Mar. Ecol. Prog. Ser. 362, 181–192 (2008).
Hedgecock, D. & Pudovkin, A. I. A. I. Sweepstakes reproductive success in highly fecund marine fish and shellfish: A review and commentary. Bull. Mar. Sci. 87, 971–1002 (2011).
Kalinowski, S. T. & Waples, R. S. Relationship of effective to census size in fluctuating populations. Conserv. Biol. 16, 129–136 (2002).
Sonderblohm, C. P., Pereira, J. & Erzini, K. Environmental and fishery-driven dynamics of the common octopus (Octopus vulgaris) based on time-series analyses from leeward Algarve, southern Portugal. ICES J. Mar. Sci. 71, 2231–2241 (2014).
Sonderblohm, C. P. et al. Participatory assessment of management measures for Octopus vulgaris pot and trap fishery from southern Portugal. Mar. Policy 75, 133–142 (2017).
Arkhipkin, A. I. et al. Stock assessment and management of cephalopods: Advances and challenges for short-lived fishery resources. ICES J. Mar. Sci. 78, 714–730 (2021).
Franklin, I. R. Evolutionary change in small populations. In Conservation Biology: An Evolutionary-Ecological Perspective (eds Soulé, M. E. & Wilcox, B. A.) 395 (Sinauer Associates, 1980).
Slatkin, M. Rare alleles as indicators of gene flow. Evolution 39, 53–65 (1985).
Holleley, C. E. & Geerts, P. G. Multiplex manager 1.0: A cross-platform computer program that plans and optimizes multiplex PCR. Biotechniques 46, 511–517 (2009).
Van Oosterhout, C., Hutchinson, W. F., Wills, D. P. M. & Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 4, 535–538 (2004).
Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405 (2008).
Paradis, E. Pegas: An R package for population genetics with an integrated-modular approach. Bioinformatics 26, 419–420 (2010).
Goudet, J. HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 5, 184–186 (2005).
Adamack, A. T. & Gruber, B. PopGenReport: Simplifying basic population genetic analyses in R. Methods Ecol. Evol. 5, 384–387 (2014).
Goudet, J. FSTAT (Version 1.2): A computer program to calculate F-STATISTICS. J. Hered. 86, 485–486 (1995).
Rice, W. R. Analyzing tables of statistical tests. Evolution 43, 223 (1989).
Piry, S., Luikart, G. & Cornuet, J. M. M. Bottleneck: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 90, 502–503 (1999).
Luikart, G., Allendorf, F. W., Cornuet, J.-M.M. & Sherwin, W. B. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. https://doi.org/10.1093/jhered/89.3.238 (1998).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Besnier, F. & Glover, K. A. ParallelStructure: A R package to distribute parallel runs of the population genetics program STRUCTURE on multi-core computers. PLoS ONE 8, e70651 (2013).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 14, 2611–2620 (2005).
Gilbert, K. J. et al. Recommendations for utilizing and reporting population genetic analyses: The reproducibility of genetic clustering using the program structure. Mol. Ecol. https://doi.org/10.1111/j.1365-294X.2012.05754.x (2012).
Earl, D. A. & VonHoldt, B. M. Structure harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361 (2012).
Takezaki, N., Nei, M. & Tamura, K. POPTREEW: Web version of POPTREE for constructing population trees from allele frequency data and computing some other quantities. Mol. Biol. Evol. 31, 1622–1624 (2014).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Dray, S. & Dufour, A.-B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 22, 1–20 (2007).
Slatkin, M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47, 264–279 (1993).
Cavalli-Sforza, L. L. & Edwards, A. W. F. Phylogenetic analysis. Models and estimation procedures. Am. J. Hum. Genet. 19, 233–257 (1967).
Foll, M. & Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 180, 977–993 (2008).
Waples, R. S. A generalized approach for estimating effective population size from temporal changes in allele frequency. Genetics 121, 379–391 (1989).
Katsanevakis, S. & Verriopoulos, G. Seasonal population dynamics of Octopus vulgaris in the eastern Mediterranean. ICES J. Mar. Sci. 63, 151–160 (2006).
Jereb, P. et al. Cephalopod Biology and Fisheries in Europe: II Species Accounts 360 (ICES, 2015).
Acknowledgements
The authors would like to thank to all the Asturian anonymous fishers who helped us during the fieldwork. They would like also to thank Aitor Ibabe Arrieta from UniOvi (Asturias, Spain) and Unai Cotano Basabe from AZTI (Basque Country, Spain); Adriana García Rellán and Rosana Solla Pazos from Cofradía de Pescadores “San Martiño” de Bueu (Galicia, Spain); Eduardo Almansa Berro and Beatriz C. Felipe Paramio from IEO-CSIC, C. O. Canarias (Tenerife, Spain); Prof. Karim Erzini and Monika J. Szynaka from CCMAR-UAlg (Faro, Portugal), Alberto Martín (MSC-Spain) and Oscar Escolar Sánchez from ICM-CSIC (Barcelona, Spain) for their significant and generous help with samples acquisitions. Aida Dopico García (Translator and English reviewer, UniOvi) kindly revised language and spelling mistakes in this manuscript.
Funding
This research was funded by the project ECOSIFOOD (MCI-20-PID2019-108481RB-I00/AEI/10.13039/501100011033) and GRUPIN-AYUD/2021/50967. Romero-Bascones A. was funded by the FPU program of the Spanish Ministry of Science, Innovation and Universities (MU-21-FPU20/06628). This work was partially developed in a Master Thesis presented by Pirhadi, N. and the Professional Practices conducted by R. Thoppil in the IMBRSea Master Program (imbrsea.eu) with the support of the Erasmus + program of the European Union. This is a contribution of the Marine Observatory of Asturias (OMA) and the Biotechnology Institute of Asturias (IUBA).
Author information
Authors and Affiliations
Contributions
N.P.: methodology, formal analysis, investigation, writing—original draft, and writing—review and editing; M.P.: methodology, formal analysis, investigation, writing—original draft, and writing—review and editing; A.R.: methodology and writing—review and editing; R.T.: methodology and writing—review and editing; J.L.M.: methodology and writing—review and editing M.P.F.R.: conceptualization, writing—review and editing; I.M.: conceptualization, writing—review and editing; L.G.F.: conceptualization, writing—review and editing; E.D.: conceptualization, funding acquisition, writing—review and editing; T.P.: conceptualization, supervision and writing—review and editing; Y.B.: conceptualization, supervision, funding acquisition and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pirhadi, N., Parrondo, M., Romero-Bascones, A. et al. Genetic monitoring on the world’s first MSC eco-labeled common octopus (O. vulgaris) fishery in western Asturias, Spain. Sci Rep 13, 2730 (2023). https://doi.org/10.1038/s41598-023-29463-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29463-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.