Abstract
Warming ocean temperatures are severely compromising the health and resilience of coral reefs worldwide. Coral bleaching can affect coral physiology and the energy available for corals to reproduce. Mechanisms associated with reproductive allocation in corals are poorly understood, especially after a bleaching event occurs. Using isotopic labeling techniques, we traced the acquisition and allocation of carbon from adults to gametes by autotrophy and heterotrophy in previously bleached and non-bleached Montipora capitata and Porites compressa corals. Experiments revealed that both species: (1) relied only on autotrophy to allocate carbon to gametes, while heterotrophy was less relied upon as a carbon source; (2) experienced a trade-off with less carbon available for adult tissues when provisioning gametes, especially when previously bleached; and (3) used different strategies for allocating carbon to gametes. Over time, M. capitata allocated 10% more carbon to gametes despite bleaching by limiting the allocation of carbon to adult tissues, with 50–80% less carbon allocated to bleached compared to non-bleached colonies. Over the same time period, P. compressa maintained carbon allocation to adult tissues, before allocating carbon to gametes. Our study highlights the importance of autotrophy for carbon allocation from adult corals to gametes, and species-specific differences in carbon allocation depending on bleaching susceptibility.
Similar content being viewed by others
Introduction
Understanding variation in resource allocation is important for identifying traits that enhance organismal survival during stressful environmental conditions1 and for predicting population responses to global climate change2. How parents manage exposure to environmental stressors can have a significant impact on the ability of their offspring to cope with similar stressors in the future3,4,5,6,7. Since reef-building corals live close to their thermal tolerance limits, they are especially sensitive to anthropogenic stressors, including ocean warming due to climate change8. Coral bleaching or the loss of photosynthetic symbionts due to thermal stress is expected to become more frequent and severe worldwide9,10, with annual bleaching predicted for most reefs by 205011. More than 80% of reefs are expected to experience harmfully frequent bleaching events through the end of this century12,13.This increase in the frequency and severity of bleaching is predicted to affect coral physiology, compromising both successful reproductive cycles and the potential for species adaptation. To enhance our predictive capacity about the future of coral reef ecosystems, it is critical to assess recovery strategies and the reproductive potential of corals following environmental stress (reviewed in6).
In corals, delayed gametogenesis, decreased egg size, low fecundity, and reduced likelihood of spawning may occur when elevated sea surface temperatures cause corals to bleach14,15,16,17,18,19,20,21,22. However, some species can continue sexual reproduction despite bleaching15,18,19,23,24. Successful reproduction after bleaching may be due to the mixotrophic ability of adult corals to obtain carbon from different sources. Both autotrophic and heterotrophic pathways are relied upon by adult corals as they recover from bleaching events25. In adult colonies it is well known that coral bleaching results in decreased rates of photosynthesis and increased metabolism of stored lipid reserves26,27 that in some species may be supplemented by increased rates of heterotrophy25,28,29. While trophic strategies vary among corals and are thought to influence bleaching resistance and thermal tolerance in adults30, their influence on gamete development and any resulting parental trade-offs remains largely uninvestigated. Carbon acquisition by parents and the provisioning of carbon to their offspring may contribute to the varied outcome in coral reproduction following bleaching.
Carbon stored as lipids in eggs31 is particularly important for species with lecithotrophic development, including corals, as larval nutrition is limited to resources contained within the egg32. To date, it is not known whether the carbon that is translocated to coral eggs is the result of autotrophic or heterotrophic carbon acquisition by the parent. Baumann et al.33 hypothesized that heterotrophic carbon may be disproportionately allocated to lipids in released coral eggs, independent of bleaching status of the parent. In the coral Pocillopora verrucosa, heterotrophy seemed to negatively impact gamete development34, while there was a positive relationship in the gorgonian Paramuricea clavata35. To date, only one prior study has traced photosynthate products from adult coral colonies of Stylophora pistillata to their released planulae (i.e., larvae)36. In this brooding species with fertilization and larval development occurring within parental polyps, there was greater transfer of autotrophic carbon from parents to planula than to eggs36, suggesting that the species may prioritize transfer of fixed carbon to offspring during embryogenesis. It is unknown how carbon is provisioned by corals that are broadcast spawners, where fertilization occurs in the water column after release of eggs/sperm from polyps. Furthermore, it is unknown how carbon is provisioned by parent to offspring following environmental stress for any coral reproductive strategy.
To better understand the link between the type of carbon acquisition by parents (autotrophic vs. heterotrophic) and subsequent allocation to offspring, we performed a series of experiments to follow carbon from parents to eggs in two coral species. We compared provisioning of 13C in colonies that had previously bleached and recovered with those colonies that did not bleach during thermal stress (Fig. 1). Specifically, we: (1) traced carbon allocation in adult corals after a bleaching event; (2) traced parental provisioning of carbon in adult corals to gametes; (3) assessed provisioning to gametes via autotrophic and heterotrophic pathways; and (4) focused on broadcast spawners with hermaphroditic (Montipora capitata) and gonochoric (Porites compressa) reproductive strategies.
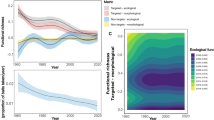
Experimental design and reproductive cycle in Hawaiian corals. (a) Bleached and non-bleached colonies were tagged during a bleaching event in Kāne'ohe Bay in Aug-Sept 201541 and monitored for 8 months until recovery. (b) In June 2016, four fragments were collected from each colony and the pulse phase of the experiment was conducted for 8 h to evaluate carbon acquisition by autotrophy (DI13C or DI12C) or heterotrophy (13C-rotifers or 12C-rotifers). Allocation of 13C was measured during 7 days of a chase phase in symbiont cells, host tissue, and eggs. (c) Mean daily sea surface temperature (SST) from NOAA Station MOKH1 (21° 25′ 59" N 157° 47′ 23" W) for 2008–2009, a non-bleaching year when egg size for Monitpora capitata was previously reported42, and 2015–2016, when bleaching occurred. SST is compared to egg size (mean ± standard error) for M. capitata42 and Porites compressa (Padilla-Gamiño, unpublished data).
Methods
Coral species
We examined carbon acquisition and allocation in two Hawaiian reef-building corals, Montipora capitata and Porites compressa. Both coral species are dominant in Kāne'ohe Bay (O'ahu, Hawai'i, USA), where the study was performed. Montipora capitata is a simultaneous hermaphrodite with buoyant gamete (i.e., egg-sperm) bundles and surface water fertilization37,38. Porites compressa is a gonochoric spawner with neutrally buoyant eggs and fertilization within the water column39. To assess parental provisioning to eggs in both species, we focused analyses on female colonies of P. compressa in this study. Both of these species spawn during the summer, although P. compressa has a more extended (summer through fall) and less synchronized spawning period than M. capitata40. Montipora capitata has higher settlement rates than P. compressa but early settlers have higher mortality and slow growth compared to P. compressa40.
Experimental design
Our study was designed to follow the timing of both thermal stress (a naturally occurring bleaching event41) and the coral reproductive cycle (development of gametes and release during the spawning season42). Our study site, reef K4 (21°26′ 36.6″ N, 157° 48′ 21.6″ W), is a fringing reef located in the central part of Kāne'ohe Bay where water has a residence time of 10–20 days43. Flow is wave driven and influenced by wind driven long-shore currents originating in the southern portion of the bay43,44,45. Between 1 August and 25 September 2015 there was a bleaching event in Kāne'ohe Bay when seawater temperatures ranged from 28.4 to 29.8 °C41. During this period, corals from multiple reefs throughout Kāne'ohe Bay were impacted41, including those at reef K4. Throughout Kāne'ohe Bay, 16.6% ± 4.7 of Montipora capitata and 19.7% ± 0.7 of Porites compressa colonies bleached in 2015, with most colonies visibly recovered within 3–4 months of the event46.
In October 2015 when eggs started to develop42 and approximately one month after peak seawater temperatures41, we tagged visibly bleached and non-bleached colonies of both species at ~ 2 m depth at reef K4. Every 2–3 months colonies were monitored, and tags were cleaned to ensure the identification of the colonies for future collection prior to the spawning season when gametes were fully developed (Fig. 1). Both M. capitata and P. compressa have long gametogenic cycles that can last 9–10 months42 and coincide with the recovery of other physiological parameters of adult corals after a bleaching event26.
On 27 May 2016 (approximately eight months after the bleaching event), we collected fragments from four previously bleached and four previously non-bleached tagged colonies each of M. capitata and P. compressa. Colony fragments were approximately 10 cm3 each in size and contained at least eight fingers; similarly sized fragments were used from both species. At the time of collection, all colonies of both species appeared visibly non-bleached (Fig. 1); this was confirmed with no significant difference in chlorophyll a (µg/g) concentrations (method described in26) between previously bleached and non-bleached adult colonies (Student’s t-test for M. capitata: df = 3, F = 0.88, p = 0.4477; P. compressa: df = 7, F = 1.51, p = 0.2656). Coral fragments were transported to outdoor flow-through seawater tanks at the Hawai'i Institute of Marine Biology; the seawater was unfiltered, temperature ranged from 25.2 to 26.4 °C, and light availability ranged from 584 to 1249 µmol photons/m2/s. Each fragment was further divided into four nubbins for genetic replication across the following four treatments: autotrophy pulse-chase labeled; autotrophy unlabeled; heterotrophy pulse-chase labeled; and heterotrophy unlabeled.
On 1 June 2016 we began the autotrophy pulse-chase experiment. Autotrophy pulse-chase labeled and autotrophy unlabeled fragments were isolated in 2 L chambers filled with 0.2 µm-filtered and sterilized seawater to remove all plankton and limit heterotrophy of the nubbins. Chambers were placed in flow-through seawater tanks to maintain a constant ambient temperature through the experiment. One hour after sunrise, the pulse phase of the autotrophy experiment began with the introduction of 0.117 M of 98 at.% 13C NaHCO3 was added to each treatment chamber for a final concentration of dissolved inorganic carbon of approximately 26 µmol/l25. The same volume of unlabeled 0.2 µm-filtered and sterilized seawater was added to each control chamber. After 9 h, the isolation chambers were flushed with unlabeled 0.2 µm-filtered seawater to begin the chase phase. On day 1 (2 June 2016) and day 7 (8 June 2016) of the chase phase, we collected small pieces from each labeled and unlabeled colony for isotopic analyses. Water was exchanged within each chamber every 6 h during the chase period.
To prepare 13C-labelled rotifers for the heterotrophy pulse-chase experiment, we obtained cultures of native Hawaiian phytoplankton (Nannochloropsis oculata) and rotifers (Brachionus plicatilis). Cultures were maintained in 0.2 µm-filtered and sterilized seawater. The phytoplankton culture was grown with 0.117 M of 98 at.% 13C NaHCO325 for at least two days and fed to the rotifer culture three times daily for three days prior to their use in the heterotrophy experiment. Resulting δ13C values for labeled and unlabeled phytoplankton were 75.82‰ and − 15.87‰, respectively; δ13C values of labeled and unlabeled rotifers were 19.32‰ and − 16.98‰, respectively. On 2 June 2016, heterotrophy pulse-chase labeled and heterotrophy unlabeled fragments were isolated in 2 L chambers filled with 0.2 µm-filtered and sterilized seawater to remove any non-labeled plankton from the chambers. One hour after sunset, the pulse phase of the heterotrophy experiment began with the introduction of 150 ml of 13C-labelled rotifers at a concentration of 2–4 rotifers per ml to each treatment chamber25. The same volume and concentration of unlabeled rotifers was added to each control chamber. Coral polyps were active and displayed extended tentacles. After eight hours, the isolation chambers were flushed with unlabeled unfiltered seawater to begin the chase phase. On day 1 (3 June 2016) and day 7 (9 June 2016) of the chase phase, we collected small pieces from each labeled and unlabeled colony for isotopic analyses. Coral samples from both pulse-chase experiments were collected and immediately frozen at -80 °C before being transported to Villanova University for further processing.
Coral tissue was removed from the skeleton using deionized water and an airbrush. Symbiont cells and host tissue were separated with a tissue grinder and centrifugation47. Separated cells and tissue were pipetted into different tin capsules (EA Consumables, LLC, Marlton, NJ), dried at 60 °C for at least 24 h. We visually checked all samples of symbiont cells and host tissues under a microscope prior to ensure that no skeletal pieces were in the capsules. Then, all capsules were folded into small, uniform pellets in preparation for isotopic analyses.
On the evenings of 5 and 6 June 2016, some M. capitata colonies released gamete bundles during a natural spawning event (1–2 days after the new moon). These dates coincided with days 3–4 of the chase phase for colonies in the heterotrophic experiment and days 4–5 of the chase phase for colonies in the autotrophic experiment (Fig. 1). Gamete bundles were collected from the surface of isolation chambers using pipettes for M. capitata18. We were unable to collect spawned eggs from P. compressa. This species has been sporadically observed to spawn during and after the full moon38,48 and the timing for gamete release is not as predictable as for M. capitata. To assess the isotopic signature of in situ developing eggs from both species, we preserved an additional fragment from each colony in 1.85% formaldehyde on day 7 of the chase. These fragments were decalcified using Cal-Ex II Fixative/Decalcifier, rinsed in 70% ethanol and developing eggs were dissected from the coral tissue. There was no significant difference in the isotopic values of gamete bundles released during the spawning event and eggs dissected from the same colony (paired t-test: df = 8, t = 0.25, p = 0.8090). Whether released or dissected, gametes bundles/eggs were pipetted into tin capsules, dried at 60 °C for at least 24 h, and folded into small, uniform pellets in preparation for isotopic analyses.
Stable isotope analyses
All tin capsules were combusted in an Elementar Pyrocube and the resulting CO2 gas was analyzed with an Elementar Isoprime100 isotope ratio mass spectrometer at The Academy of Natural Sciences at Drexel University. δ13C values are reported relative to Vienna Peedee Belemnite Limestone Standard (vPDB) (δ13C = per mil deviation of the ratio of stable carbon 13C:12C relative to vPDB). Samples were analyzed in duplicate. Standards, B2150 (EA Consumables, LLC, Marlton, NJ), internal elk tissue, DORM (fish muscle) and bird feather standards had a precision of ± 0.14‰ for δ13C.
Statistical analyses
Statistically significant differences in δ13C values were determined separately for each species and trophic pulse-chase experiment with mixed effects modeling (Supplementary Table 1). These compared the effects of prior bleaching status (bleached, non-bleached), pulse period treatment (13C-labeled, unlabeled), and tissue type (symbiont, host, eggs/bundles), and the repeated effect of time during the chase period (day 1, day 7). Each model included a random effect of genotype and tissue type (symbiont cells, host tissue and egg/bundle) was nested within prior bleaching status. The random and repeated effects were compared with covariance parameter estimates and the fit statistic, -2res log likelihood. Post-hoc Tukey–Kramer tests determined the factors that were significantly different from each other within significant interactions of the main model effects (Supplementary Tables 2–5). p ≤ 0.05 was considered statistically significant. We calculated percent enrichment values to compare average labeled values to their respective controls for each pulse-chase experiment and species (Supplementary Table 6). All statistical analyses were generated using SAS statistical software Version 9.4 of the SAS System for Windows.
Results and discussion
Pathways for autotrophic acquisition of carbon
Our results provide the first direct evidence of resource allocation of carbon from adult coral colonies to their eggs (Fig. 2). Both species relied on autotrophy for carbon allocation to eggs. By day 1 of the chase for Montipora capitata, there was significant incorporation of 13C from autotrophy in symbiont cells (Fig. 2a) and host tissue (Fig. 2b) in labeled compared to control corals of both bleached and non-bleached adult colonies. Similarly, by days 4–5 there was significant 13C enrichment in labeled compared to control gamete bundles released from both bleached and non-bleached adult colonies (Fig. 2c). In M. capitata, the location of 13C allocation and its retention time within adults during the chase phase was different based on prior bleaching status. In bleached colonies, significantly more 13C was allocated to symbiont cells than host tissue, and significantly more 13C was allocated to host tissue than gamete bundles beginning on day 1 of the chase (Fig. 2a–c; Supplementary Table 2). By day 7, 13C was depleted (i.e., lower δ13C) in both symbiont cells and host tissue of bleached corals with no significant difference between labeled and control colonies (Fig. 2a, b). However, eggs of bleached colonies continued to be a significant storage site (i.e., higher δ13C) for 13C allocation at day 7 (Fig. 2c). In contrast, non-bleached colonies retained 13C-label in symbiont cells, host tissue, and eggs through day 7 of the chase (Fig. 2a–c). Despite this, there was significantly more 13C translocated to developing eggs of bleached than non-bleached colonies by day 7 (Fig. 2c; Supplementary Table 2). Altogether these patterns indicate significant prioritization on gamete development over adult tissue maintenance in M. capitata when carbon is autotrophically-acquired, particularly when bleaching has occurred.
Carbon acquisition for gamete development is predominantly autotrophic. (a–l) Acquisition of carbon by autotrophy or heterotrophy in Montipora capitata (a–c, g–i) and Porites compressa (d–f, j–l) colonies. Allocation occurred during the chase phase to symbiont cells (a, d, g, j), host tissue (b, e, h, k), and eggs (c, f, i, l) for each species. All stable carbon isotopic values (δ13C) are shown; with control colonies (DI12C or 12C-rotifers) in white symbols; labeled colonies (DI13C or 13C-rotifers) in colored symbionts. Green, blue, or pink symbols indicate statistically significant differences compared to control colonies; and labeled colonies in grey symbols indicate no significant difference compared to control colonies. Complete statistical analyses can be found in Supplementary Tables 1–5.
For both bleached and non-bleached Porites compressa, patterns of autotrophic allocation of 13C were similar to that of bleached M. capitata. In P. compressa on day 1 of the chase, there was significantly greater incorporation of 13C in symbiont cells (Fig. 2d) and host tissue (Fig. 2e) in labeled compared to control corals of both bleached and non-bleached adult colonies (Supplementary Table 3). There was more 13C allocated to the symbiont than the host regardless of prior bleaching status. By day 7, 13C-label was significantly depleted in symbiont cells and host tissue of both bleached and non-bleached adult colonies (Fig. 2d, e; Supplementary Table 3). Although we were unable to assess the gametes of P. compressa before day 7 of the chase, eggs dissected from both bleached and non-bleached colonies were significantly more enriched in 13C in labeled compared to control corals (Fig. 2f). In contrast to M. capitata, there was greater allocation of 13C to eggs of non-bleached than bleached colonies in P. compressa (Fig. 2f; Supplementary Table 3). Adults that did not bleach were able to allocate more autotrophically-acquired carbon to their eggs than those that did bleach.
Pathways for heterotrophic acquisition of carbon
While prior biochemical studies of adult coral colonies hypothesized that heterotrophy would be critical to egg development in M. capitata especially following bleaching and less so in P. compressa26,28, we find that neither species relied heavily on heterotrophy. Approximately 8 months after a natural bleaching event, resource allocation of both species to their eggs relied predominantly on autotrophically-acquired carbon (Fig. 2). In M. capitata, there was little acquisition of heterotrophic 13C by symbiont cells (Fig. 2g) and host tissue (Fig. 2h) of adult colonies and developing eggs or released gamete bundles (Fig. 2i), except by symbiont cells from bleached colonies at day 7 (Fig. 2g; Supplementary Table 4). Although M. capitata has been shown to feed more heterotrophically while bleached and in the early months of recovery from bleaching47,49,50, it is more reliant on autotrophy than other conspecifics when non-bleached28. More recently, δ13C analyses indicated a limited influence of heterotrophy on M. capitata during post-bleaching recovery51. Our data indicate that carbon from heterotrophy was not incorporated by gametes in M. capitata, suggesting that this source is less critical to gametogenesis than previously hypothesized.
In contrast, heterotrophic carbon was incorporated into the developing eggs of some non-bleached colonies of P. compressa, although not statistically significant as a group (Fig. 2l). Similarly, P. compressa adults showed little to no acquisition of heterotrophic 13C, except by day 1 when there was significant enrichment of 13C by symbiont cells from non-bleached colonies (Fig. 2j; Supplementary Table 5). This indicates that if feeding occurred during the experiment, any associated carbon was metabolized quickly (within hours) of the chase. By day 7, the developing eggs of non-bleached labeled P. compressa were on average 40% more enriched with 13C than non-bleached control colonies (Fig. 2l). Like autotrophic acquisition, there was greater allocation of 13C to eggs from non-bleached to bleached colonies of P. compressa following heterotrophy (Fig. 2l; Supplementary Table 5). Accumulation of heterotrophic carbon in P. compressa eggs of some colonies at day 7 (Fig. 2l), while not statistically significant, may have been translocated via the host tissue in less than 24 h. Transfer of carbon is known to occur within 24 h between cellular organelles of adult tissues52 and among tissue layers of larvae53, therefore translocation from parent to gametes may also occur under similar time periods. Heterotrophic acquisition of carbon to eggs may occur in P. compressa and deserves further investigation.
Low enrichment values indicate limited evidence of carbon allocation by heterotrophy. This may be due to loss of carbon via respiration of the coral holobiont25 and/or mucus production54 and subsequent uptake of carbon by microbes in coral mucus55. The timing of the experiment with respect to spawning periods of each species, may have further impacted carbon uptake by heterotrophy. For M. capitata, spawning occurred in the midst of the experiment, while for P. compressa spawning would have occurred approximately two weeks later 38,48. It is important to note that some colonies of P. compressa contained eggs with heterotrophically-acquired carbon, suggesting that coral feeding can contribute to egg development in this species. To date we know little about how or if coral feeding behavior changes as eggs grow and occupy more space in the gastrovascular cavity or how feeding is impacted by the process of bundle formation and preparation for gamete release. Further research is needed to better understand the role of heterotrophy in coral reproduction34,35.
Parental provisioning of carbon
Parental provisioning in corals with different reproductive strategies (brooders vs spawners) may occur at different rates and stages during gametogenesis and/or embryogenesis. In our study we found that broadcast spawners transferred carbon mostly autotrophically to eggs within days before their release. Similarly, gametes of M. capitata56 and P. compressa48 both receive symbionts from their parents prior to their release (i.e., vertical transmission), not from the environment (i.e., horizontal transmission). In the brooding coral, Stylophora pistillata translocation of carbon preferentially occurred at the planula (post-fertilization) stage36, while planulae of S. pistillata are known to receive symbionts both by vertical and horizontal transmission57. Regardless of trophic source, carbon allocation from parents to gametes may coincide with vertical transmission of symbionts to more effectively supply offspring with resources required immediately upon release.
Pulse-chase experiments conducted on planulae support this hypothesis. For example, planulae of Pocillopora damicornis relied on carbon supplied by the parent for at least 24 h after being released, then shifted to carbon acquired autotrophically from their own symbionts at 48 h53. Progressively older larvae relied more on autotrophy for their carbon supply than on parental reserves. Pocillopora damicornis planulae < 5 days old received 16–27% of fixed carbon from their symbionts58, while nearly one-month old planulae acquired approximately 70–85% of carbon from symbionts59,60. Similarly-aged Montipora digitata larvae acquired up to 90% of fixed carbon from photosynthate of their symbionts60. Although it is not known when or by what trophic pathway parental provisioning occurs in P. damicornis (a brooding species with vertical transmission) or M. digitata (a spawning species with vertical transmission), parental sources of carbon are relied upon immediately once offspring reach the water column and for several subsequent days. The initial lack of photosynthate production by symbionts in gametes/larvae of these species may prevent oxidative damage during the first 24 h of release as they drift near the surface and may be at greater risk of heat stress61. Thus, further emphasizing the importance of parental provisioning of carbon to offspring.
Parental trade-offs
Trade-off theory indicates that reproduction will generally interfere with maintenance and/or growth of the parent, with metabolic trade-offs becoming more complex when symbiosis is involved62. For example, in damaged or dislodged coral colonies, metabolic costs associated with regeneration resulted in decreased reproduction63,64. In our study, we found that coral parents translocated carbon resources to their gametes prior to release, but this occurred at a detriment to carbon storage in adult tissue. Adult colonies of both species experienced a physiological trade-off when supplying their eggs with carbon, especially among those colonies that had previously bleached. Gametes enriched in 13C coincided with depletion of 13C in both symbiont cells and host tissue, evidence of a resource trade-off for parents. Loss of carbon from the parent tissue due to reproduction is expected and has been calculated as part of carbon budgets36,65, or implicated from field observations and energy reserve analyses15. This is the first direct measure of that metabolic trade-off as carbon depletion in parental tissues.
In our study, we cannot assess how much 13C, if any, was lost by adult tissues through respiration. However, no change in 13C between day 1 and 7 in non-bleached symbiont cells (Fig. 2a) or host tissue (Fig. 2b) of M. capitata (Supplementary Table 2), suggests that respiration of newly acquired carbon was minimal. A trade-off in carbon allocation occurred within just 7 days of acquisition in our study, suggesting that maintenance of symbiont cells and host tissue are being impacted by carbon requirements for developing gametes over relatively short time periods. In the case of M. capitata this trade-off occurred for carbon acquired autotrophically by previously bleached colonies only, while for P. compressa autotrophically-acquired carbon was translocated from adults to developing eggs by both bleached and non-bleached colonies. Reallocation of carbon away from adults and for egg development occurred in both species.
Other studies have shown long-term trade-offs for adult colonies that reproduce. For example, reproducing colonies of P. damicornis had half the annual linear extension rate of non-reproducing colonies65. Long-term trade-offs have also been associated with reproduction following a bleaching event. In Orbicella annularis (previously Montastraea annularis), gametogenesis occurred after bleaching in colonies that had visibly recovered, while colonies that remained visibly bleached consumed their own structural material for maintenance and did not reproduce15,17. In Acropora spp. reproductive output was proportional to bleaching susceptibility, but growth rates were independent of bleaching severity66. While bleaching is known to reduce calcification in some Hawaiian species9,47, reproduction continued despite bleaching18. To date, long-term trade-offs associated with growth and reproduction after a bleaching event remain unclear67 and warrant further investigation.
Trophic dynamics and coral metabolism
Heterotrophy in bleached corals is hypothesized to be indicative of a colony under stress and/or a resilience mechanism to survive bleaching50,68. Adult reliance on heterotrophic acquisition of 13C was lower in our study (i.e., eight months after natural bleaching) compared to eleven months after experimental bleaching for the same species50. Differences in adult response were probably due to differences in temperature severity as the experimental bleaching was ~ 3 °C warmer than the 2015 natural bleaching event of our study25,69. In addition, shading, cloud cover, rain events, and flow may all moderate conditions on a reef during some natural bleaching events, allowing affected corals to recover faster69 than with more controlled temperature treatments during experimental bleaching. Our findings suggest that the duration of heterotrophic reliance post-bleaching depends on the severity of the stress.
While heterotrophic acquisition of carbon seems indicative of bleaching severity in some coral species50,70, autotrophic acquisition and allocation may be more indicative of the physiological stress on the adult colony. In previously bleached colonies of M. capitata and all colonies of P. compressa, autotrophically-acquired 13C was catabolized, not stored, as it decreased from day 1 to day 7 of the chase (Fig. 2a–f). Conversely, newly acquired carbon was stored and maintained in colonies of M. capitata that did not bleach in the 2015 event. Our results show that even if bleached M. capitata colonies recovered (i.e., acquired symbionts and developed gametes), there was still a fundamental difference in carbon requirements between bleached and non-bleached colonies eight months later.
Species-specific differences in how carbon is allocated following bleaching may reflect differences in the timing of their gametogenic cycles and spawning periods. In Hawai'i, the timing between the bleaching season (Sept-Oct) and the spawning season (May–June) is at least seven months41,71. Recovery of bleached colonies and the development of gametes can occur within this timeframe. In M. capitata, egg development starts in Aug-Sept, takes approximately eight to ten months and spawning occurs during May-Aug, two to four days after the new moon18,42. The length of the gametogenic cycle in P. compressa is unknown, but sporadic observations indicate that this species releases gametes during and after the full moon in summer months38,48. In our experiments, M. capitata had already spawned on days 3–5 of the chase, while P. compressa may have spawned ~ 11–12 days after the conclusion of our study. Significant translocation of 13C from adults to in situ eggs may indicate that P. compressa was allocating resources to eggs and preparing for spawning (Fig. 2f, l). Although we were unable to observe spawning in P. compressa, our data clearly show that this species has the capacity to produce gametes after a bleaching event and that carbon was supplied to eggs in both previously bleached and non-bleached colonies. For both species, long-term recovery of bleached colonies occurred in parallel to the development of gametes42. As thermal stress events become more frequent, intense and longer in duration, the window to recover and develop gametes may shorten, impacting reproductive life history strategies.
Strategies for reproduction following coral bleaching
A comparison of previously bleached and non-bleached colonies provides evidence of the physiological impacts that occur eight months after a natural bleaching event in adults and developing gametes. Previously bleached M. capitata depleted carbon storage within symbiont cells (by 80%) and host tissue (by 50%) compared to non-bleached colonies (Fig. 2a, b). Yet, translocating carbon to eggs was an energetic priority for both bleached and non-bleached colonies (Fig. 2c). Carbon translocation to eggs occurred at the detriment of bleached adult colonies, while carbon storage was maintained in non-bleached adult colonies. Furthermore, previously bleached colonies supplied more 13C to eggs (by 10%) at day 7 than non-bleached colonies (Fig. 2c, Supplementary Table 2). These findings show that the M. capitata prioritized gamete development and that the amount of energy supplied by the parent for this physiological process is not limited by prior bleaching. Previously bleached colonies were able to meet the energy demand for eggs by depleting stored carbon reserves in adults. These results support those by Cox18 and provide a mechanism for continued gametogenesis in M. capitata despite bleaching. Additionally, similar amounts of carbon were translocated to developing eggs despite differences in bleaching susceptibility (Supplementary Table 2). This supports previous findings that identified low phenotypic and biochemical variability of eggs in M. capitata from parents with distinct morphology, physiology, and exposure to environmental stress72.
In P. compressa, gametogenesis continued after bleaching and developing eggs were provisioned with newly acquired carbon in previously bleached and non-bleached colonies. Although 13C was incorporated autotrophically to eggs in P. compressa (Fig. 2f, l), there was no difference in incorporation of carbon by symbiont cells or host tissue in bleached compared to non-bleached colonies via either trophic pathway (Supplementary Tables 3, 5). This suggests that P. compressa adults had physiologically recovered from the bleaching event26. Yet, we observed at least one remaining impact of the bleaching event in their developing eggs, as 45–80% less carbon was translocated to eggs of previously bleached than non-bleached colonies (Fig. 2f, l). Porites compressa may only supply carbon to eggs when there is surplus available and after the adult has recovered. While bleached P. compressa produced eggs and may spawn after a bleaching event, non-bleached colonies likely spawn earlier or produce more, or larger eggs compared to bleached colonies.
Our study highlights two different strategies of coral parental provisioning with important consequences for the survival of adults and offspring in the context of future and repeated bleaching events. By increasing carbon allocated to eggs, bleached M. capitata prioritized gametogenesis at the expense of the adult colony. Higher investment in reproduction may help compensate for the low survival of early recruits of this species40. For P. compressa, there was no difference in the allocation of carbon to the host tissues of bleached and non-bleached adult colonies. However, less carbon was transferred to eggs of bleached colonies, and this may lead to smaller and/or fewer eggs in bleached colonies. Since P. compressa has less evolutionary pressure to produce large amounts of gametes40, this strategy for carbon allocation may maintain reproductive potential after a bleaching event.
Data availability
All data are provided in the main manuscript and in the supplemental material.
References
Stearns, S. C. Trade-offs. In The Evolution of Life Histories 72–90 (Oxford University Press, 1992).
Madin, J. S. et al. A trait-based approach to advance coral reef science. Trends Ecol. Evol. 31, 419–428 (2016).
Mousseau, T. A. & Fox, C. W. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (1998).
Marshall, D. J. Transgenerational plasticity in the sea: Context-dependent maternal effects across the life history. Ecology 89, 418–427 (2008).
Torda, G. et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Change 7, 627–636 (2017).
Calosi, P., Putnam, H. M., Twitchett, R. J. & Vermandele, F. Marine metazoan modern mass extinction: Improving predictions by integrating fossil, modern, and physiological data. Ann. Rev. Mar. Sci. 11, 369–390 (2019).
Thibault, C. et al. Within- and trans-generational responses to combined global changes are highly divergent in two congeneric species of marine annelids. Mar. Biol. 167, 1–17 (2020).
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science (80-). 318, 1737–1742 (2007).
Coles, S. L. et al. Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ 2018, 1–24 (2018).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 50, 839–866 (1999).
van Hooidonk, R., Maynard, J. & Planes, S. Temporary refugia for coral reefs in a warming world. Nat. Clim. Change 3, 508–511 (2013).
Donner, S., Heron, S. F. & Skirving, W. J. Future scenarios: A review of modelling efforts to predict the future of coral reefs in an era of climate change. In Coral Bleaching. Ecological Studies (Analysis and Synthesis) (eds van Oppen, M. & Lough, J.) https://doi.org/10.1007/978-3-319-75393-5_13 (Springer Cham, 2018).
Donner, S. D. Coping with commitment: Projected thermal stress on coral reefs under different future scenarios. PLoS ONE 4, e5712 (2009).
Jokiel, P. L. Lunar periodicity of planula release in the reef coral Pocillopora damicornis in relation to various environmental factors. In Proceedings of the 5th International Coral Reef Congress, Vol. 4 307–312 (1985).
Szmant, A. M. & Gassman, N. J. The effects of prolonged ‘bleaching’ on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8, 217–224 (1990).
Ward, S., Harrison, P. & Hoegh-Guldberg, O. Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In Proceedings of the 9th International Coral Reef Congress, Vol. 2 1123–1128 (2000).
Mendes, J. M. & Woodley, J. D. Effect of the 1995–1996 bleaching event on polyp tissue depth, growth, reproduction and skeletal band formation in Montastraea annularis. Mar. Ecol. Prog. Ser. 235, 93–102 (2002).
Cox, E. F. Continuation of sexual reproduction in Montipora capitata following bleaching. Coral Reefs 26, 721–724 (2007).
Negri, A. P., Marshall, P. A. & Heyward, A. J. Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species. Coral Reefs 26, 759–763 (2007).
Sudek, M., Aeby, G. S. & Davy, S. K. Localized bleaching in Hawaii causes tissue loss and a reduction in the number of gametes in Porites compressa. Coral Reefs 31, 351–355 (2012).
Airi, V. et al. Reproductive efficiency of a mediterranean endemic zooxanthellate coral decreases with increasing temperature along a wide latitudinal gradient. PLoS ONE 9, 1–8 (2014).
Levitan, D. R., Boudreau, W., Jara, J. & Knowlton, N. Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar. Ecol. Prog. Ser. 515, 1–10 (2014).
Padilla-Gamiño, J. L. & Gates, R. D. Spawning dynamics in the Hawaiian reef-building coral Montipora capitata. Mar. Ecol. Prog. Ser. 449, 145–160 (2012).
Armoza-Zvuloni, R., Segal, R., Kramarsky-Winter, E. & Loya, Y. Repeated bleaching events may result in high tolerance and notable gametogenesis in stony corals: Oculina patagonica as a model. Mar. Ecol. Prog. Ser. 426, 149–159 (2011).
Hughes, A. D., Grottoli, A. G., Pease, T. K. & Matsui, Y. Acquisition and assimilation of carbon in non-bleached and bleached corals. Mar. Ecol. Prog. Ser. 420, 91–101 (2010).
Rodrigues, L. J. & Grottoli, A. G. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 52, 1874–1882 (2007).
Grottoli, A. G., Tchernov, D. & Winters, G. Physiological and biogeochemical responses of super-corals to thermal stress from the northern gulf of Aqaba, Red Sea. Front. Mar. Sci. 4, 1–12 (2017).
Grottoli, A. G., Rodrigues, L. J. & Palardy, J. E. Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189 (2006).
Sangmanee, K. et al. Influence of thermal stress and bleaching on heterotrophic feeding of two scleractinian corals on pico-nanoplankton. Mar. Pollut. Bull. 158, 111405 (2020).
Conti-Jerpe, I. E. et al. Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 6, eaaz5443 (2020).
Arai, T., Kato, M., Heyward, A. J., Ikeda, Y. & Maruyama, T. Lipid composition of positively buoyant eggs of reef building corals. Coral Reefs 12, 71–75 (1993).
Figueiredo, J. et al. Ontogenetic change in the lipid and fatty acid composition of scleractinian coral larvae. Coral Reefs 31, 613–619 (2012).
Baumann, J., Grottoli, A. G., Hughes, A. D. & Matsui, Y. Photoautotrophic and heterotrophic carbon in bleached and non-bleached coral lipid acquisition and storage. J. Exp. Mar. Biol. Ecol. 461, 469–478 (2014).
Séré, M. G., Massé, L. M., Perissinotto, R. & Schleyer, M. H. Influence of heterotrophic feeding on the sexual reproduction of Pocillopora verrucosa in aquaria. J. Exp. Mar. Biol. Ecol. 395, 63–71 (2010).
Gori, A. et al. Effects of food availability on the sexual reproduction and biochemical composition of the Mediterranean gorgonian Paramuricea clavata. J. Exp. Mar. Biol. Ecol. 444, 38–45 (2013).
Rinkevich, B. The contribution of photosynthetic products to coral reproduction. Mar. Biol. 101, 259–263 (1989).
Heyward, A. J. Sexual reproduction in five species of the coral Montipora. Hawaii Inst. Mar. Biol. Tech. Rep. 37, 170–178 (1986).
Kolinski, S. P. & Cox, E. F. An update on modes and timing of gamete and planula release in Hawaiian scleractinian corals with implications for conservation and management. Pac. Sci. 57, 17–27 (2003).
Hunter, C. L. Genotypic Diversity and Population Structure of the Hawaiian Reef Coral, Porites compressa (University of Hawaii, 1988).
Kolinski, S. P. Sexual Reproduction and the Early Life History of Montipora capitata in Kaneohe Bay, Oahu, Hawaii (University of Hawaii, 2004).
Bahr, K. D., Rodgers, K. S. & Jokiel, P. L. Impact of three bleaching events on the reef resiliency of Kāne’ohe Bay, Hawai’i. Front. Mar. Sci. 4, 398 (2017).
Padilla-Gamiño, J. L. et al. Sedimentation and the reproductive biology of the Hawaiian reef-building coral Montipora capitata. Biol. Bull. 226, 8–18 (2014).
Lowe, R. J., Falter, J. L., Monismith, S. G. & Atkinson, M. J. A numerical study of circulation in a coastal reef-lagoon system. J. Geophys. Res. Ocean. 114, 1–18 (2009).
Bathen, K. H. A descriptive study of the physical oceanography of Kaneohe Bay, Oahu, Hawaii. Honolulu (HI): Hawai’i Institute of Marine Biology, University of Hawai’i. 14 (1968).
Smith, S. V., Kimmerer, W. J., Laws, E. A., Brock, R. E. & Walsh, T. W. Kaneohe Bay sewage diversion experiment: Perspectives on ecosystem responses to nutritional perturbation. Pac. Sci. 35, 279–396 (1981).
Ritson-Williams, R. & Gates, R. D. Coral community resilience to successive years of bleaching in Kāne‘ohe Bay, Hawai‘i. Coral Reefs 39, 757–769 (2020).
Rodrigues, L. J. & Grottoli, A. G. Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 70, 2781–2789 (2006).
Neves, E. G. Histological analysis of reproductive trends of three Porites species from Kane’ohe Bay, Hawai’i. Pac. Sci. 54, 195–200 (2000).
Palardy, J. E., Rodrigues, L. J. & Grottoli, A. G. The importance of zooplankton to the daily metabolic carbon requirements of healthy and bleached corals at two depths. J. Exp. Mar. Biol. Ecol. 367, 180–188 (2008).
Hughes, A. D. & Grottoli, A. G. Heterotrophic compensation: A possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress?. PLoS ONE 8, 1–10 (2013).
Wall, C. B., Ritson-Williams, R., Popp, B. N. & Gates, R. D. Spatial variation in the biochemical and isotopic composition of corals during bleaching and recovery. Limnol. Oceanogr. 64, 2011–2028 (2019).
Kopp, C. et al. Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. MBio 6, 1–9 (2015).
Kopp, C., Domart-Coulon, I., Barthelemy, D. & Meibom, A. Nutritional input from dinoflagellate symbionts in reef-building corals is minimal during planula larval life stage. Sci. Adv. 2, e1500681 (2016).
Wild, C., Naumann, M., Niggl, W. & Haas, A. Carbohydrate composition of mucus released by scleractinian warm- and cold-water reef corals. Aquat. Biol. 10, 41–45 (2010).
Silveira, C. B. et al. Microbial processes driving coral reef organic carbon flow. FEMS Microbiol. Rev. 41, 575–595 (2017).
Padilla-Gamiño, J. L., Weatherby, T. M., Waller, R. G. & Gates, R. D. Formation and structural organization of the egg-sperm bundle of the scleractinian coral Montipora capitata. Coral Reefs 30, 371–380 (2011).
Byler, K. A., Carmi-Veal, M., Fine, M. & Goulet, T. L. Multiple Symbiont Acquisition Strategies as an Adaptive Mechanism in the Coral Stylophora pistillata. PLoS ONE 8, 1–7 (2013).
Richmond, R. H. Energetic considerations in the dispersal of Pocillopora damicornis (Linnaeus) planulae. In Proceedings of the 4th International Coral Reef Symposium, Vol. 2 153–156 (1981).
Gaither, M. R. & Rowan, R. Zooxanthellar symbiosis in planula larvae of the coral Pocillopora damicornis. J. Exp. Mar. Biol. Ecol. 386, 45–53 (2010).
Harii, S., Yamamoto, M. & Hoegh-Guldberg, O. The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Mar. Biol. 157, 1215–1224 (2010).
Yakovleva, I. M. et al. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar. Ecol. Prog. Ser. 378, 105–112 (2009).
van Woesik, R. & Jordán-Garza, A. G. Coral populations in a rapidly changing environment. J. Exp. Mar. Biol. Ecol. 408, 11–20 (2011).
Ward, S. The effect of damage on the growth, reproduction and storage of lipids in the scleractinian coral Pocillopora damicornis (Linnaeus). J. Exp. Mar. Biol. Ecol. 187, 193–206 (1995).
Rinkevich, B. Do reproduction and regeneration in damaged corals compete for energy allocation?. Mar. Ecol. Prog. Ser. 143, 297–302 (1996).
Richmond, R. H. Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar. Biol. 93, 527–533 (1987).
Baird, A. H. & Marshall, P. A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 237, 133–141 (2002).
Precoda, K., Hardt, M. J., Baird, A. H. & Madin, J. S. Tissue biomass trades off with growth but not reproduction in corals. Coral Reefs 39, 1027–1037 (2020).
Anthony, K. R. N., Hoogenboom, M. O., Maynard, J. A., Grottoli, A. G. & Middlebrook, R. Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Funct. Ecol. 23, 539–550 (2009).
Bahr, K. D., Jokiel, P. L. & Rodgers, K. S. Seasonal and annual calcification rates of the Hawaiian reef coral, Montipora capitata, under present and future climate change scenarios. ICES J. Mar. Sci. 74, 1083–1091 (2017).
Anthony, K. R. N. & Fabricius, K. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 252, 221–253 (2000).
Stimson, J. S. Mode and timing of reproduction in some common hermatypic corals of Hawaii and Enewetak. Mar. Biol. 48, 173–184 (1978).
Padilla-Gamiño, J. L. et al. Are all eggs created equal? A case study from the Hawaiian reef-building coral Montipora capitata. Coral Reefs 32, 137–152 (2013).
Acknowledgements
We thank the faculty and staff of the Hawai'i Institute of Marine Biology (HIMB), especially Dr. Ruth Gates; assistance in the field and laboratory was provided by E. Lenz, M. McMahon, J. Lopez, A. Trujillo, M. Jaffe, and J. Axworthy; isotopic analyses by D. Velinsky and P. Zelanko at the Academy of Natural Sciences of Drexel University; and P. Bernhardt for statistical assistance. This research was conducted under Hawai'i Department of Land and Natural Resources scientific permit 2017-13 to JPG. This research was supported by a Villanova University College of Liberal Arts and Sciences Faculty Development Grant and the National Science Foundation’s Division of Integrated Organismal Systems, Integrated Ecological Physiology Program (NSF IOS-IEP) 1655888 to LJR and NSF IOS-IEP 1655682 and Sloan Research Fellowship to JPG. This is HIMB contribution #1906 and School of Ocean and Earth Science and Technology (SOEST) contribution #11579.
Author information
Authors and Affiliations
Contributions
L.J.R. and J.L.P.G. designed, performed the research, and wrote the paper; L.J.R. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodrigues, L.J., Padilla-Gamiño, J.L. Trophic provisioning and parental trade-offs lead to successful reproductive performance in corals after a bleaching event. Sci Rep 12, 18702 (2022). https://doi.org/10.1038/s41598-022-21998-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21998-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.