Abstract
Myocardial injury reduction and recovery under acute cardiac stress are adversely impacted by insulin resistance (IR). We previously demonstrated that Rhodiola improved cardiac anti-stress capacity in mice. Thus, this study focuses on the preventive efficacy of Rhodiola on exhaustive exercise (EE)-induced myocardial injury of IR mice. An 8-week high-fat diet (HFD) model of IR mice was established. Rhodiola was administrated by garaging. After the 8-week intervention, half of the mice performed EE to simulate acute cardiac stress, and determine myocardial injury; The remaining mice were sacrificed following fasting to assess metabolic disorder. We found myocardial injury induced by EE in IR mice was worse and was alleviated by Rhodiola pre-conditioning. Further, the nuclear factor erythroid 2-related factor 2 (Nrf2)-related antioxidant system was impaired by HFD, while mitochondrial dynamic fusion and fission were activated by HFD as a physiological protective compensation. The Rhodiola administration rescued Nrf2 impairment and further facilitated mitochondrial fusion and fission. All these results indicate that Rhodiola is a potential treatment for the prevention of cardiac events in type 2 diabetes mellitus and metabolic syndrome patients, and the Nrf2-related antioxidant activity and mitochondrial dynamics are the proposed mechanisms.
Similar content being viewed by others
Introduction
Organ adaptability, or anti-stress capacity, is the damage reduction and functional recovery from an acute pathological stimulus. Insulin resistance (IR), as the basis of cardiovascular disease, was found to adversely impact cardiac anti-stress capacity. For IR or diabetic patients, the survival rate and prognosis of acute stress including myocardial infarction, are poor1,2, which indicates that cardiac anti-stress capacity is impaired by IR. In our previous research, we found that autophagy, heat shock protein 70 (HSP70), and mitochondrial quality control were found to be vital in anti-fatal stress capacity in healthy mice3,4,5.
Due to the huge energy consumption in the myocardium, myocardial cells contain abundant mitochondria indicating the fundamental role of the mitochondrion in cardiac anti-stress capacity6. Moreover, mitochondrion produces an important by-product, reactive oxygen species (ROS), which influences almost all physiological functions, and even damages the mitochondrion in return7. The mitochondrial dynamic, which involves the fission and fusion of mitochondrial membranes, is an important component of the quality control system, especially in cardiac cells8. Mitochondrial fission participates in the disposal of damaged mitochondria, while fusion is related to the preservation of ATP production and ROS dissipation9. It is the Fission and fusion, that determine the structural and functional status of mitochondria10.
Evidence has reported that mitochondrial dynamic is associated with ROS production and scavenging11. The cellular oxidizing conditions determine mitochondrial fusion and fission by modulating the activity and expression of dynamic proteins, including OPA1 and MFNs12,13. Previous studies have shown that defects in mitochondrial dynamic lead to increased ROS, thereafter resulting in reduced ATP production, and exacerbated diabetic nephropathy14. All these indicate the correlation between mitochondrial dynamic and ROS is critical in maintaining mitochondrial hemostasis.
In the myocardium of diabetic mice, mitochondrial dynamic disorder, as well as increased oxidative stress has been observed15, which could be one of the reasons behind poor cardiac anti-stress capacity in IR patients. However, further research is needed to explore its mechanisms and to develop efficient therapies.
Rhodiola (RS), a natural herb historically used to improve body hypoxia resistance at high altitudes16, has been shown to be a mitochondrial protectant17,18. Our previous study found Rhodiola enhances mitochondrial quality including mitochondrial dynamic, to increase cardiac anti-stress capacity in healthy mice5. However, whether this improvement can be extended under IR conditions and its underlying mechanism remains unclear. Recently, Rhodiola was found to be a Nuclear factor erythroid 2-related factor 2 (Nrf2) activator, which enhances the antioxidant system to exert neuroprotective effects19 or relieve hypoxia-induced liver injury20. Thus, we hypothesize that Rhodiola activates Nrf2 and the downstream antioxidant system to regulate mitochondrial dynamic homeostasis, finally strengthening cardiac anti-stress capacity under IR conditions.
In this study, a high-fat diet (HFD) mice model was established to simulate the vulnerability to cardiac stress in patients with IR, and exhaustive swimming exercise was used to simulate acute cardiac stress to induce myocardial injury. Then, we investigate Rhodiola pre-conditioning’s effect on the cardiac anti-stress capacity of HFD mice and explore the role of the Nrf2 signaling pathway.
Results
Rhodiola reduces exhaustive exercise-induced myocardial mitochondrial injury of HFD Mice
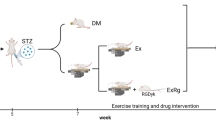
After adaptive feeding, the mice were fed a normal diet (ND) or HFD with/without Rhodiola administration, then the mice underwent exhaustive swimming or not. The animal protocol is further described in Fig. 1A. We found the forced swimming time had decreased in the HFD group compared to ND. However, when accompanied by Rhodiola treatment, the mice following HFD demonstrated a comparable swimming time to that of the ND group (Fig. 1B). Following exhaustive swimming, myocardium damage was subsequently evaluated by serum creatine kinase (CK), blood urea nitrogen (BUN), and electron microscopy. We found that serum CK and BUN were increased in HFD mice (Fig. 1C,D). In parallel, damaged mitochondrial with edema and ruptured cristae (yellow arrows) in the myocardium were observed with greater abundance in HFD mice (Fig. 1E,F). However, neither the morphologic alteration in myocardium nor CK and BUN release occurred in HFD + RS groups (Fig. 1C–F), suggesting that Rhodiola prevents EE-induced cardiac mitochondrial injury in HFD mice.
Rhodiola reduces exhaustive exercise-induced myocardial injury of HFD Mice. (A) After a 1-week adaptive diet, the mice were fed a normal diet (ND) or high-fat diet (HFD) with either Rhodiola (RS) for 8 weeks, then mice underwent exhaustive swimming (EE) or not. (B) The forced swimming time was recorded and analyzed; (C,D) Blood urea nitrogen (BUN), and serum creatine kinase (CK) of mice were measured after EE; (E) The morphology of myocardium after EE was observed by transmission electron microscope (TEM), intact mitochondria (the letter m), as well as damaged mitochondrion (yellow arrows), were indicated in images, scale bar = 2 μm; (F) The ratio of damaged mitochondria number, which have ruptured cristae and edema, to total mitochondrial number per field-of-view was analyzed. Data are expressed as Mean ± SD, n = 6, *, ** represent P < 0.05, P < 0.01 in comparison with ND; #, ## represent P < 0.05, P < 0.01 in comparison with HFD.
Rhodiola relieves the insulin resistance of HFD mice
The HFD mouse model was characterized by dyslipidemia, which indicates lipotoxicity and energy oversupply. An 8-week HFD led to increased weight gain compared to ND mice, while Rhodiola administration restrained this tendency in HFD mice even comparable to that in ND (Fig. 2A). In-keeping with the bodyweight result, visceral fat weight in HFD mice also increased, while Rhodiola prevented this change (Fig. 2B). As for glucolipid metabolism, we found serum cholesterol, triglyceride, low-density lipoprotein (LDL), blood glucose, and insulin increased in HFD mice, in which the HOMA-IR index also increased. However, Rhodiola administration prevented these changes in HFD mice (Fig. 2C–H).
Rhodiola relieves the insulin resistance of HFD mice. The mice were fed a normal diet (ND) or a high-fat diet (HFD) with either Rhodiola (RS) for 8 weeks, after 5-h fasting they were sacrificed to assess pre-adaptive changes. (A) Bodyweight of mice before and after intervention was monitored, (B) visceral adipose weight of mice was recorded; (C–G) Serum cholesterol, triglyceride, LDL-cholesterol, blood glucose, and blood insulin were assessed; (H) HOMA-IR index was calculated. Data are expressed as Mean ± SD, n = 6, *, ** represent P < 0.05, P < 0.01 in comparison with ND; #, ## represent P < 0.05, P < 0.01 in comparison with HFD.
Rhodiola activates Nrf2-related antioxidants in HFD mice
Considering the close correlation between oxidative stress and acute exercise stress in the myocardium, we evaluated oxidative stress and Nrf2-related antioxidant signaling. As shown in Fig. 3A,B, the activity of SOD2 decreased in HFD mice, while the content of malondialdehyde (MDA) increased, indicating oxidative stress. Rhodiola administration alleviated HFD-induced oxidative stress. To investigate the role of Nrf2 signaling, we assessed the protein expression of Nrf2 and its downstream HO-1. Our results showed that Rhodiola improved inhibited Nrf2/HO-1 pathway in HFD mice (Fig. 3C,D). Protein expression of GPX4 and SOD2 exhibited a similar tendency (Fig. 3E).
Rhodiola activates Nrf2-related antioxidants in high-fat diet mice. After an 8-week intervention and 5-h fasting, the mice were sacrificed to assess the pre-adaptive changes. (A,B) The myocardium samples were obtained, MnSOD activity and MDA content were assessed; (C,D) And relative protein expression of Nrf2, HO-1, GPX4, and SOD2 were assessed, and the original blots are presented in Supplementary Figure 3. Data are expressed as Mean ± SD, n = 3, *, ** represent P < 0.05, P < 0.01 in comparison with ND; #, ## represent P < 0.05, P < 0.01 in comparison with HFD.
Rhodiola prevents mitochondrial dynamics disorder in high-fat diet mice
Mitochondrial dynamic plays a vital role in maintaining the mitochondrial function, which supplies energy for cardiac contractile function. To evaluate the degree of fusion and fission in the myocardium, we assessed fusion factors MFN1, MFN2, and OPA1, as well as fission factors DRP1 and FIS1 expression. As shown in Fig. 4A–C, mitochondrial fusion and fission were both activated in HFD mice. This might be a stress cellular condition due to HFD. However, Rhodiola administration changed this: mitochondrial fusion was further enhanced in HFD + RS mice, while fission was prevented. Moreover, the ratio of mitochondrial DNA and ATP content was assessed to evaluate mitochondrial content and function. We found that mitochondrial DNA and ATP content decreased in the myocardium of HFD mice when compared with ND mice, Rhodiola treatment reversed this (Fig. 4D).
Rhodiola prevents mitochondrial dynamics disorder in high-fat diet mice. After an 8-week intervention and 5-h fasting, the mice were sacrificed to assess the pre-adaptive changes. (A–C) The myocardium samples were obtained, relative protein expression of mitochondrial fusion marker MFN1, MFN2, OPA1 as well as fission marker DRP1 and FIS1 were determined, and the original blots are presented in Supplementary Figure 4; (D) The ratio between mitochondrial DNA (mtDNA) and nuclear DNA (nucDNA) determined, and the ATP content of myocardium was detected. Data are expressed as Mean ± SD, n = 3, *, ** represent P < 0.05, P < 0.01 in comparison with ND; #, ## represent P < 0.05, P < 0.01 in comparison with HFD.
Discussion
This study aimed to illustrate the mechanism through which Rhodiola improves cardiac anti-stress capacity impaired by HFD in an IR mice model. Our results showed that an HFD increases EE-induced cardiac injury in mice, inhibits Nrf2 signaling regulated antioxidant enzyme, and triggers mitochondrial dynamic disorder. However, all these were alleviated by Rhodiola pre-conditioning. These findings suggest that Rhodiola is a potential clinical therapy for IR patients to improve cardiac anti-stress capacity.
There are several methods to stimulate cardiac stress, including myocardial infarction, ischemia/reperfusion, chemical drugs, and EE. In comparison with other models, EE would better represent a complicated and changeable condition in clinical settings. Thus, we investigated anti-stress capacity in an EE model. Research by Behringer and colleagues has demonstrated that mitochondrial dysfunction due to EE leads to muscle injury characterized by increased oxidative stress, cytoskeletal damage, and various markers21. In addition, it has been demonstrated that EE regulates the Bcl-2 family via PI3K/AKT signaling pathway to decrease the openness level of mPTP, consequently resulting in myocardial injury22. Similar results were observed in our previous study, where we found Rhodiola increased weight-load swimming time and reduce cardiac injury after EE in normal mice, indicating that Rhodiola enhanced cardiac anti-stress capacity5. We previously demonstrated that oxidative stress remission and mitochondrial quality control adaptation, including fusion and fission, activated by Rhodiola, were the target mechanisms.
Clinical research has demonstrated that the exercise capacity of patients with IR and type 2 diabetes mellitus (T2DM) is limited23, while further evidence has established poor exercise capacity as an independent and strong predictor for all-cause mortality24. In this study, HFD mice presented decreased exercise capacity and worsened cardiac injury post-EE, indicating worse cardiac anti-stress capacity, which was alleviated following Rhodiola treatment. In skeletal muscle, another important target organ of IR, HFD mice have also been found to be worse affected than normal mice25. Noticeably, the inhibitor of NADPH oxidase, which is an important resource of ROS, relieved HFD-impaired mitochondrion function and exercise capacity. However, the role of disorder in the physiological antioxidant system is not fully known.
Rhodiola and its active constituent salidroside were found to be a natural antioxidant that protects mitochondria against injury in multiple diseases18,26. Nrf2, one of the targets of Rhodiola, is a key regulator of the cellular antioxidant response19. Besides oxidative stress, the activity of Nrf2 is also regulated by energy-based stimuli, thus, HFD inhibits Nrf2 and impairs the antioxidant system27. In this study, we found the expression of Nrf2 and downstream HO-1 were inhibited in HFD mice, while the antioxidant enzyme SOD2 and GPX4 were also inhibited, and the lipid peroxidation product MDA had increased. This implies the presence of a disorder of the antioxidant system in the myocardium of HFD mice which may contribute to the impaired cardiac anti-stress capacity. As expected, we found Rhodiola treatment redeemed the Nrf2-regulated antioxidant system and reduced oxidative stress. Future studies may focus their attention on structure optimization of salidroside to improve the targeting ability or pharmaceutical effect28.
In consideration of the coadjustment of oxidative and mitochondrial dynamics, we assessed the mitochondrial fusion and fission levels to further illustrate the mechanism behind Rhodiola’s protective effect. Fusion is a vital process preserving ATP production and ROS dissipation. Muscle from obese or T2DM patients show a reduced degree of fusion29. However, our results show that mitochondrial fusion marker MFN1, MFN2, and OPA1 increased in HFD mice after EE. Concerning the fission caused by damaged mitochondria, it was enhanced in response to HFD and EE stress in this study. We presume this discrepancy is a result of the difference in tissue type or pathological staging, as the fusion and fission level in this study was activated as physiological compensation for mitochondrial quality control and cardioprotection30. Futhermore, we found Rhodiola treatment, which improved ATP content in HFD mice myocardium, further facilitated mitochondrial fusion while inhibiting fission. This indicates that 8-week Rhodiola pre-intervened mice did not exhibit such severe injury in mitochondrial after EE or/and this injury was relieved by the self-protective quality control process including fusion. However, further studies with specific inhibitor administration or gene conditional knockout mice are needed to explore this issue.
In summary, this study used an EE mice model to simulate cardiac stress and explore Rhodiola's effect on the HFD-impaired cardiac anti-stress capacity in mice: Rhodiola activates Nrf2 signal and downstream antioxidant system and improves cardiac anti-stress capacity via regulating mitochondrial dynamic disorder, including fusion and fission (Fig. 5). The results conclude that Nrf2-mediated antioxidant enzyme and mitochondrial dynamic are the targets of Rhodiola, which may be a potential treatment to prevent cardiac event for T2DM and MS patients.
Proposed pathway of Rhodiola pre-conditioning enhances cardiac anti-stress capacity of Insulin Resistant Mice. Reactive oxygen species (ROS) production in cellular was closely associated with mitochondrial dynamics including fusion and fission. And mitochondrial dysfunction is critically involved in the acute stress-induced myocardial injury for mice. We found high fat diet (HFD) inhibited the antioxidant pathway Nrf2/HO-1 and aggravated the myocardial injury, while Rhodiola activated Nrf2/HO-1 pathway, reduced ROS production, regulated mitochondrial dynamics, and relieved the myocardial injury after exhaustive exercise.
Methods
Ethics statement animal experimentation
8-week-old male C57BL/6J mice (20 ± 2 g) were bought from the Laboratory Animal Centre, Xiangya Medical School (Changsha, China). They were housed in temperature-controlled (22 °C ± 2 °C) quarters with a 12:12-h light–dark cycle and given free access to water and food. All animal procedures followed the guidelines for the use of live animals of the National Institute of Health and were approved by the Medicine Animal Welfare Committee of Xiangya Medical School, Central South University (Changsha, China). The initial approval ID (SYXK 2015-0017) was obtained in December 2015 and the re-approval ID (SYXK 2020-0019) was obtained in December 2020.
After 1-week adaptive feeding, mice were randomly divided into three groups (n = 16 for each): normal diet (ND), high-fat diet (HFD), and HFD + Rhodiola (HFD + RS). In HFD feeding 45% of total calories came from fat, while in ND feeding this figure is 18%. Mice were administrated with the same quantity of Rhodiola solution (5 mg/0.1 mL/10 g body weight) or normal saline (0.1 mL/10 g body weight) as a placebo by gavage every day for 8 consecutive weeks.
After 8 weeks, mice in each group were further divided into two subgroups: with or without an exhaustive exercise (EE) test (n = 8). Mice in the non-EE group were anesthetized via 1% pentobarbital sodium (150 mg/kg, i.p.) and then sacrificed via exsanguination after fasting, and collect blood samples from the inferior vena cava for lipid profile assays, visceral fat including mesenteric, epididymal, and perirenal fat tissue were dissected and weighted; Mice in EE subgroups underwent an exhaustive exercise as previously described5, while the researchers are blind to the groups. 12 h after the experiments, mice were anesthetized with 150 mg/kg pentobarbitone by intraperitoneal injection and then sacrificed, blood and myocardium samples were then collected. This study was conducted under the ARRIVE guidelines and any unnecessary animal suffering was avoided. The protocol has been described in Fig. 1A.
Rhodiola
The extract from the root of Rhodiola chrysanthemifolia subsp. sacra (Raym. -Hamet) H. Ohba (Rhodiola, RS) was provided by the Tibet Rhodiola Pharmaceutical Holding Company. The extract complied with The Chinese Pharmacopeia 2015 (inspection report number C1051612067). The extraction method and the HPLC–MS analysis were described previously31. Briefly, the root of R.sacra was decocted twice for 1.5 h and 1 h respectively. The decoction was collected and dehydrated to powder after filtration. HPLC–MS analysis showed the proportion of main effective components, salidroside (C14H20O7, 2.62%) and flavone (C27H30O16, 3.27%). This powder was dissolved in distilled water at 50 mg/mL.
Lipid profile assays
Serum was isolated from fasting blood by centrifugation at 4000 rpm for 10 min. The supernatants were collected and stored at -80 °C. Serum levels of total cholesterol, triglycerides, and low-density lipoprotein cholesterol were measured by the enzymatic method on an automatic biochemical analyzer (Beckman, IN, USA).
HOMA-IR index
Blood glucose and insulin were respectively determined by glucometer (Accu-Chek Performa, Roche Diagnostics, Indianapolis, USA) and ELISA (CSB-E05071m, Wuhan, Hubei China). The HOMA-IR index was calculated as follows: HOMA-IR = fasting glucose (mmol/L) × fasting insulin (mU/L)/22.5.
Exhaustive exercise test
After the 8-week intervention, forced weight-loaded swimming was performed as an exhaustive exercise (EE) test to assess cardiac anti-stress capacity5,31. Briefly, mice were forced to swim with a lead sheath of 5% of body weight till exhaustion, defined as the failure to rise above the surface of the water for 7 s.
Exercise capacity
EE was performed in mice of the EE subgroup and the swimming time was recorded, which is regarded as exercise capacity as previously described5.
Creatine kinase (CK), and blood urea nitrogen (BUN)
Blood samples were collected 12 h after the EE test. Serum was isolated from the blood sample by centrifugation. The CK concentration was determined by a colorimetric kit (Jiancheng, Nanjing, China), and the BUN was determined by a urease methods kit (Jiancheng), procedures are performed according to the manufacturer’s instructions.
Transmission Electron Microscope (TEM)
Myocardium samples were collected after the EE test and resized to 1 × 1 × 3 mm3, then fixed and dehydrated. Next, we performed the embedding and solidifying of the tissue. After being sliced, tissues were observed by a transmission electron microscope (Tecnai G2 Spirit, FEI, United States).
Western blot
Myocardium tissues were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor (Beyotime). The protein concentrations of the lysates were measured by a BCA Protein Assay kit (Beyotime). After SDS-PAGE, the proteins were transferred to PVDF membranes with 0.45 μm pores (Millipore, MA, USA). After blocking with 5% non-fat dry milk for 2 h, the membranes were cropped to the kDa around the target proteins and then treated with primary antibodies against mitofusin1 (MFN1, 1:1000), MFN2 (1:2000), Dynamin-like 120 kDa protein (OPA1, 1:2000), dynamin-related protein 1 (DRP1, 1:1000) Mitochondrial fission 1 protein (FIS1, 1:1000), Nuclear factor erythroid 2-related factor 2 (Nrf2, 1:1000), Heme Oxygenase-1 (HO-1, 1:1000), Glutathione peroxidase 4 (GPX4, 1:1000), Superoxide dismutase 2 (SOD2, 1:4000), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:3000), respectively. Following HRP-labeled goat anti-rabbit IgG or goat anti-mice IgG (1:5000, Proteintech), the membranes were incubated with ECL chemiluminescence solution (Thermo, Waltham, USA) and exposed to X-ray film for seconds to minutes. After developing and flushing, scan the film and analyzed the bands were analyzed using a gel documentation system (Bio-Rad, Hercules, CA, USA).
Mitochondrial DNA ratio
The protocol was described previously31. Total DNA was isolated from myocardium tissue using a DNeasy Kit (Qiagen, GER). The succinate dehydrogenase complex subunit A (SDHA) and MtCO3 oligos were analyzed to evaluate the quantification of nuclear and mitochondrial genomes. The primers’ sequences are listed in Table 1.
Adenosine triphosphate (ATP) content
The ATP content in the myocardium was determined via the phosphomolybdic acid colorimetric method (Jiancheng). Procedures were performed according to the manufacturer’s instructions.
SOD2 and Malondialdehyde (MDA) assay
The xanthine oxidase method was used to determine the activity of SOD2 in myocardium tissues, and the thiobarbituric acid method was used to detect the content of MDA according to the manufacturer’s instructions respectively (Jiancheng).
Statistical analysis
Statistical analyses were performed by Prism 6 software (GraphPad, Inc.).The results are expressed as Mean ± SD. One-way ANOVA plus the Student–Newman–Keuls test was used for statistical analysis. P < 0.05 represents statistical significance.
Data availability
The original contributions presented in the study are included in the article/ “Supplementary Material”, further inquiries can be directed to the corresponding author and S.L.
References
Granger, C. B. et al. Outcome of patients with diabetes mellitus and acute myocardial infarction treated with thrombolytic agents. The Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. J. Am. Coll. Cardiol. 21, 920–925. https://doi.org/10.1016/0735-1097(93)90348-5 (1993).
Kapur, A. & De Palma, R. Mortality after myocardial infarction in patients with diabetes mellitus. Heart 93, 1504–1506. https://doi.org/10.1136/hrt.2006.112656 (2007).
Jiang, L. et al. Exercise combined with trimetazidine improves anti-fatal stress capacity through enhancing autophagy and heat shock protein 70 of myocardium in mice. Int. J. Med. Sci. 18, 1680–1686. https://doi.org/10.7150/ijms.53899 (2021).
Xie, M., Jiang, L., Dun, Y., Zhang, W. & Liu, S. Trimetazidine combined with exercise improves exercise capacity and anti-fatal stress ability through enhancing mitochondrial quality control. Life Sci. 224, 157–168. https://doi.org/10.1016/j.lfs.2019.03.027 (2019).
Dun, Y., Liu, S., Zhang, W., Xie, M. & Qiu, L. Exercise combined with Rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid. Med Cell Longev. 2017, 8024857. https://doi.org/10.1155/2017/8024857 (2017).
Anzell, A. R., Maizy, R., Przyklenk, K. & Sanderson, T. H. Mitochondrial quality control and disease: Insights into ischemia-reperfusion injury. Mol. Neurobiol. 55, 2547–2564. https://doi.org/10.1007/s12035-017-0503-9 (2018).
Andreyev, A. Y., Kushnareva, Y. E., Murphy, A. N. & Starkov, A. A. Mitochondrial ROS metabolism: 10 years later. Biochemistry (Mosc). 80, 517–531. https://doi.org/10.1134/S0006297915050028 (2015).
Tahrir, F. G., Langford, D., Amini, S., Mohseni Ahooyi, T. & Khalili, K. Mitochondrial quality control in cardiac cells: Mechanisms and role in cardiac cell injury and disease. J. Cell Physiol. 234, 8122–8133. https://doi.org/10.1002/jcp.27597 (2019).
Pernas, L. & Scorrano, L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 78, 505–531. https://doi.org/10.1146/annurev-physiol-021115-105011 (2016).
Santel, A. & Frank, S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 60, 448–455. https://doi.org/10.1002/iub.71 (2008).
Millet, A. M. et al. Loss of functional OPA1 unbalances redox state: implications in dominant optic atrophy pathogenesis. Ann. Clin. Transl. Neurol. 3, 408–421. https://doi.org/10.1002/acn3.305 (2016).
Norton, M. et al. ROMO1 is an essential redox-dependent regulator of mitochondrial dynamics. Sci. Signal. 7, ra10. https://doi.org/10.1126/scisignal.2004374 (2014).
Shutt, T., Geoffrion, M., Milne, R. & McBride, H. M. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 13, 909–915. https://doi.org/10.1038/embor.2012.128 (2012).
Guo, K. et al. Protective role of PGC-1alpha in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS ONE 10, e0125176. https://doi.org/10.1371/journal.pone.0125176 (2015).
Ding, M. et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1alpha pathway. J. Pineal Res. 65, e12491. https://doi.org/10.1111/jpi.12491 (2018).
Kelly, G. S. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 6, 293–302 (2001).
Xue, H. et al. Salidroside stimulates the Sirt1/PGC-1alpha axis and ameliorates diabetic nephropathy in mice. Phytomedicine 54, 240–247. https://doi.org/10.1016/j.phymed.2018.10.031 (2019).
Bai, X. L., Deng, X. L., Wu, G. J., Li, W. J. & Jin, S. Rhodiola and salidroside in the treatment of metabolic disorders. Mini. Rev. Med. Chem. 19, 1611–1626. https://doi.org/10.2174/1389557519666190903115424 (2019).
Wu, Y., Wang, Y., Wu, Y., Li, T. & Wang, W. Salidroside shows anticonvulsant and neuroprotective effects by activating the Nrf2-ARE pathway in a pentylenetetrazol-kindling epileptic model. Brain Res. Bull. 164, 14–20. https://doi.org/10.1016/j.brainresbull.2020.08.009 (2020).
Xiong, Y., Wang, Y., Xiong, Y. & Teng, L. Protective effect of Salidroside on hypoxia-related liver oxidative stress and inflammation via Nrf2 and JAK2/STAT3 signaling pathways. Food Sci. Nutr. 9, 5060–5069. https://doi.org/10.1002/fsn3.2459 (2021).
Behringer, M. et al. Exhaustive exercise–a near death experience for skeletal muscle cells?. Med. Hypotheses 83, 758–765. https://doi.org/10.1016/j.mehy.2014.10.005 (2014).
Li, J. et al. Exercise preconditioning plays a protective role in exhaustive rats by activating the PI3K-Akt signaling pathway. Evid. Based Complement Alternat. Med. 2020, 3598932. https://doi.org/10.1155/2020/3598932 (2020).
Grundy, S. M. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 47, 1093–1100. https://doi.org/10.1016/j.jacc.2005.11.046 (2006).
Kodama, S. et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 301, 2024–2035. https://doi.org/10.1001/jama.2009.681 (2009).
Yokota, T. et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 297, H1069–H1077. https://doi.org/10.1152/ajpheart.00267.2009 (2009).
Xu, F., Xu, J., Xiong, X. & Deng, Y. Salidroside inhibits MAPK, NF-kappaB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 24, 70–74. https://doi.org/10.1080/13510002.2019.1658377 (2019).
Vasconcelos, A. R., Dos Santos, N. B., Scavone, C. & Munhoz, C. D. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front Pharmacol. 10, 33. https://doi.org/10.3389/fphar.2019.00033 (2019).
Liu, C. et al. Discovery of salidroside-derivated glycoside analogues as novel angiogenesis agents to treat diabetic hind limb ischemia. J. Med. Chem. 65, 135–162. https://doi.org/10.1021/acs.jmedchem.1c00947 (2022).
Zorzano, A., Liesa, M. & Palacin, M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch. Physiol. Biochem. 115, 1–12. https://doi.org/10.1080/13813450802676335 (2009).
Hernandez-Resendiz, S. et al. Targeting mitochondrial fusion and fission proteins for cardioprotection. J. Cell Mol. Med. 24, 6571–6585. https://doi.org/10.1111/jcmm.15384 (2020).
You, B. et al. The treatment of Rhodiola mimics exercise to resist high-fat diet-induced muscle dysfunction via sirtuin1-dependent mechanisms. Front. Pharmacol. 12, 646489. https://doi.org/10.3389/fphar.2021.646489 (2021).
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Number: 82002403 to YSD), and Natural Science Foundation of Hunan Province (Grant Number: 2021JJ60072 to JL).
Author information
Authors and Affiliations
Contributions
B.Y. and J.C. performed part of the study and wrote the manuscript, they contribute equally to this manuscript; Y.D. analyzed and interpreted the data, and fund this study; J.W.R.-G. critically revised and edited the manuscript; J.L. performed part of the study and fund this study; D.L. performed part of the study; C.H. and S.L. designed and supervised this study, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
You, B., Cheng, J., Dun, Y. et al. Rhodiola pre-conditioning reduces exhaustive exercise-induced myocardial injury of insulin resistant mice. Sci Rep 12, 20277 (2022). https://doi.org/10.1038/s41598-022-20376-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20376-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.