Abstract
Health-related quality of life (HRQOL) is an important issue among patients undergoing dialysis treatment. Peritoneal dialysis (PD) is associated with a number of adverse body composition changes. However, whether body composition is associated with HRQOL is uncertain. The purpose of this study was to analyze the effects of body composition on HRQOL in PD patients. We performed a cross-sectional observational study on the association between body composition and HRQOL in PD patients at a single center. Body composition was determined by multifrequency bioimpedance spectroscopy. HRQOL is summarized to three composite scores: kidney disease component summary (KDCS), physical component summary (PCS), and mental component summary (MCS). The relationships between HRQOL and the hydration index, lean tissue index (LTI), and fat tissue index (FTI) were analyzed by regression analysis. One hundred and ninety-seven PD patients were included in the present study. Patients with severe fluid overload showed a lower PCS. The hydration index and FTI showed statistically significant negative associations with PCS. In subgroup analysis, the associations between the hydration index and PCS remained robust after stratifying according to sex, age, and residual urine. Our results indicated that both the hydration index and FTI were negatively associated with HRQOL, especially PCS.
Similar content being viewed by others
Introduction
The concept of health-related quality of life (HRQOL) addresses the effects of individual health on physical, cognitive, and social functioning in daily life1. Patients with end-stage kidney disease (ESKD) who are treated with dialysis have many threats to HRQOL, both from the ESKD-related symptoms per se and from the physical and mental burden of dialysis treatment1,2. The importance of HRQOL to kidney disease patients is obviously not only the basic aspect of the goal itself, but also the association with morbidity and mortality3,4,5,6. The International Society for Peritoneal Dialysis (ISPD) has also recently published practice recommendations on HRQOL to patients undergoing peritoneal dialysis (PD)7. The ISPD recommended that high-quality PD prescriptions should be guided by a number of assessments encompassing the person’s volume and nutritional status.
PD patients encounter complex body composition changes, with overhydration, muscle wasting, and fat gain8,9,10. The assessment of body composition is essential to effectively evaluate nutritional status in PD patients and monitor changes during dialysis11. Body mass index (BMI) is one of the most popular methods for evaluating nutrition status. However, BMI is not able to differentiate between volume overload and obesity and is less associated with muscle wasting in dialysis patients12,13. Therefore, access to specific indicators of body composition to evaluate volume and nutritional status is needed. Multifrequency bioimpedance spectroscopy (BIS) with a body composition model is a validated method to assess hydration and nutritional status in dialysis patients14,15. It has anthropometric parameters to assess both fluid volume, such as tissue hydration, extracellular water (ECW), and intracellular water (ICW), and nutritional status such as lean tissue inde× (LTI) and fat tissue index (FTI).
Whether body composition is associated with HRQOL in PD patients remains unclear. Therefore, the aim of the current study was to evaluate whether body fluid status or nutritional indices assessed by BIS may affect HRQOL in PD patients. To do so, we analyzed the data from patients who are enrolled in the Korean Cohort Study for Outcome in Patients on Peritoneal Dialysis (KNOW-PD) cohort.
Results
Baseline profile of the study population
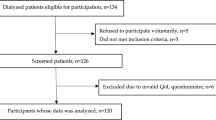
We screened subjects registered in the KNOW-PD cohort, and 197 PD patients passed the inclusion and exclusion criteria and were involved in this study. Table 1 shows the baseline characteristics, laboratory findings, and BIS measurements of the patients. The study population included 54.8% males, and the mean age was 45.8 ± 13.5 years. The median dialysis vintage was 31 months. The assessment of nutritional status revealed 92.4% of subjects having SGA 6–7, which indicates a well-nourished status. 69.5% of subjects were treated with renin-angiotensin aldosterone systems (RAAS) blockade with angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers. 79.2% of subjects were treated with erythropoiesis stimulating agents. According to the BIS parameters, the mean R∆TH of patients was 13.4 ± 10.2%, and the median A∆TH was 2.3 (0.9, 3.5) L, which indicates a fluid overload status. Mean FTI was 7.7 ± 3.8 kg/m2, and the Z-FTI (Z-score of fat tissue index) was − 0.2 ± 1.1. Mean LTI was 14.3 ± 3.0 kg/m2, and the Z-LTI (Z-score of lean tissue index) was − 0.5 ± 1.2.
HRQOL scores according to body composition
Table 2 shows the comparison of KDQOL-SF classified according to R∆TH, ECW/ICW, Z-FTI, and Z-LTI. KDCS and MCS showed no significant differences in the groups classified by R∆TH, ECW/ICW, Z-FTI, and Z-LTI. However, PCS varied significantly between the group with R∆TH ≤ 15% and the group with R∆TH > 15%, as well as between the group with ECW/ICW < 1 and the group with ECW/ICW ≥ 1. The subscales of the KDQOL-SF classified according to R∆TH, ECW/ICW, Z-FTI, and Z-LTI were also compared (Supplement Table S1). The R∆TH > 15% group showed statistically significantly lower scores in two PCS components, physical functioning and general health. The subjects with ECW/ICW ≥ 1 also showed statistically significant lower scores in two PCS components, physical functioning and role-physical.
Correlations between body composition and KDQOL scores
The correlation coefficients between the body composition parameters and each component of KDQOL-SF are shown in Table 3. In brief, we found that KDCS had no correlation with any of the body composition parameters. PCS showed a negative correlation with R∆TH (Spearman’s r = − 0.3, p < 0.001), ECW/ICW (Spearman’s r = − 0.3, p < 0.001), and Z-FTI (Spearman’s r = − 0.2, p = 0.005). MCS was weakly associated with R∆TH (Spearman’s r = − 0.2, p = 0.016), ECW/ICW (Spearman’s r = − 0.2, p = 0.034), and Z-FTI (Spearman’s r = − 0.2, p = 0.036).
To evaluate whether body composition was associated with HRQOL scores, linear regression analysis was performed (Table 4). We adjusted several factors which could affect the HRQOL in several models. Activation of the RAAS in particular increased angiotensin II levels and stimulated growth of myocardial cells, which caused the left ventricular hypertrophy16. Blockade of RAAS in PD patients are likely to preserve residual renal function and reduce left ventricular mass17,18,19, which could affect the body composition of PD patients. Therefore, we performed a linear regression analysis by adding the usage of RAAS blockade as an adjustment variable to model 5. After adjustments for age, sex, dialysis duration, Kt/V, total creatinine clearance, C-reactive protein, body composition parameters, diabetes mellitus, and usage of RAAS blockade (Model 5), R∆TH and ECW/ICW, expressed in the hydration index, showed statistically significant negative associations with PCS, with β = − 1.5 (− 2.8, − 0.1) and β = − 117.2 (− 215.2, − 19.2), respectively. In Model 5, Z-FTI showed a statistically significant negative association with PCS, with β = − 14.3 (− 28.3, − 0.3). FTI showed a similar trend as Z-FTI with PCS (data not shown). Neither LTI (data not shown) nor Z-LTI had a statistically significant association with any component of HRQOL.
Stratified subgroup analysis
After stratifying the subjects according to sex, age, daily urine, and Z-LTI, we performed a subgroup analysis of linear regression to determine the association between the status of fluid volume/nutrition and HRQOL (Table 5). Associations between R∆TH and PCS remained robust after stratifying according to sex, age, and residual urine (Fig. 1). The associations between ECW/ICW and PCS also remained robust after stratifying according to age, residual urine, and Z-LTI. Z-FTI and PCS showed negative associations in the following subgroups: males, age under 50 years, residual daily urine < 1 L/day, and Z-LTI ≥ 0.
Plot of regression coefficients for the association between PCS and R∆TH, ECW/ICW, and Z-FTI. Error bars indicate 95% confidence intervals. R∆TH Relative ∆tissue hydration, ECW extracellular water, ICW intracellular water, Z-FTI Z-score of fat tissue index, Z-LTI Z-score of lean tissue index, PCS physical component summary.
Discussion
The present study demonstrated that an increase in hydration and fat tissue indices was negatively related to HRQOL scores in PD patients. These associations were significant after multivariable adjustment and were consistent throughout subgroup analysis stratified by sex, age, and daily urine. These findings indicate that interventions to improve hydration and fat tissue may potentially improve HRQOL in PD patients.
HRQOL is an important aspect of patient health status and should be considered when monitoring patients with chronic illness20. In particular, kidney diseases have negatively impacted the HRQOL of ESKD patients mainly due to the accompanying impairment or the imposed limitations in almost all aspects of their lives. Poor HRQOL is associated with increased morbidity and mortality6; thus, enhancing HRQOL is a priority in the area of kidney disease research21,22. There have been several studies on HRQOL and its implications among chronic kidney disease23, hemodialysis6, and transplanted patients24,25,26, but fewer studies have focused on PD patients27. In particular, to date, no study has reported the association of body composition and HRQOL in PD patients.
The body composition of PD patients differs from that of the general population28. Protein energy wasting and overhydration, which are common in PD patients, may affect changes in body composition. High glucose in dialysis fluid and a loss of proteins into the dialysate may contribute to a progressive decrease in skeletal muscle mass and increase in adipose tissue29,30. Although adverse changes in body composition are associated with morbidity and mortality in PD patients, there is no consensus on the relationship between body composition and HRQOL in PD patients. Moreover, PD patients with diabetes mellitus are known to have a high burden of both systemic and intraperitoneal inflammation31. In case of PD patients with residual renal function with high proteinuria and inflammatory status, serum albumin would be hard to reflect the nutritional status itself32. With an easy and noninvasive device to measure body composition, we tried to assess the association between body composition and HRQOL in PD patients.
In this study, we showed that R∆TH and ECW/ICW had significant negative associations with PCS after adjustments for multiple variables. The negative association between fluid overload and PCS showed robust relationships in multiple subgroup analyses stratified by sex, age, and dialysis urine. This result was consistent with a previous report27. As PCS is closely related with physical function, several reasons can be postulated. First, fluid overload in PD is not only related with cardiovascular burden but also non-cardiovascular risk factors such as malnutrition and inflammation33,34. Second, target weight may not always be reached due to an increase in intradialytic symptoms in fluid overload status, and fluid overloaded patients require more effort to adjust to their target weight35.
We also showed that FTI had a negative association with PCS after adjustments for multiple variables. PD patients are more susceptible to central obesity due to exposure to glucose-containing dialysate fluid36. However, in this analysis, when comparing subjects with Z-FTI < 0 and those with Z-FTI ≥ 0, the glucose exposure load was not significantly different between both groups (Supplement Table S2). The mechanism of negative association between PCS and Z-FTI has not been completely clarified, although several hypotheses could be inferred by considering the characteristics of the two groups classified as FTI (Supplement Table S2). First, patients with obese or high fat tissue secrete numerous proinflammatory cytokines, including C-reactive protein (CRP), tumor necrosis factor-α, and interleukin (IL)-6. Our study also showed that the lower Z-FTI group showed statistically significantly higher CRP than the higher Z-FTI group. These cytokines modulate lipid and carbohydrate metabolism and orchestrate the inflammatory pathway37. With this mechanism, a previous study showed that elevated CRP and IL-6 levels predict 2-year mortality, cardiovascular events, and technique survival in PD patients30,38. Second, a decline in nutritional status appears to have an impact on the physical domain of HRQOL in higher FTI groups. In our study, groups with Z-FTI ≥ 0 showed statistically significantly lower scores in SGA. Lower SGA has a vulnerability that is characterized by reduced functional reserve and a higher susceptibility to adverse health outcomes39. On the other hand, sarcopenic obesity may develop, where muscles decrease and fat increases in PD patients. In our subgroup analysis, it was confirmed that FTI and PCS had a significant negative association in subjects with higher Z-LTI, but not in those with lower Z-LTI. This association shows that the increased fat tissue itself, independent from the decrease in lean tissue mass, had a negative effect on PCS.
In contrast to the relationship between FTI and PCS, LTI did not show any relationship with HRQOL. This finding was different from a previous study reporting that a reduction of lean body mass was associated with an increased mortality40. Even though LTI did not show a significant association with HRQOL in our study, it should be interpreted with caution. Progressive loss of muscle mass and strength has frequently been observed in ESKD patients. Although this study did not prove any relationship between LTI and HRQOL, it is well known that LTI is an independent predictor of survival in PD41. From a long-term perspective, it is warranted to monitor the overall body composition of PD patients.
There was no association between KDCS and any of the body composition parameters in our study. Because the KDCS combines information from a heterogeneous set of scales42, each component has a different meaning to each of the body components. Even though MCS was weakly related with ECW/ICW and FTI, there was no linear trend between any of the body composition parameters and MCS. This finding was different from a previous study that found that severity of nutritional markers was related not only with PCS, but also with KDCS and MCS43. However, the nutritional status in the above study was evaluated based on a quantitative version of 24-h dietary recall and BMI, which have limitations in the accurate assessment of the body composition.
Our study had several limitations. First, this study was an observational study, and there were inherent limitations such as hidden confounding factors. For example, anemic conditions could affect the body composition and nutrition44. In our study, 79.2% of subjects were treated with erythropoiesis stimulating agents (ESA), and there was no statistically significant difference in the hemoglobin values in the group using ESA and the group not using ESA (11.0 ± 1.3 g/dL vs 10.7 ± 1.5 g/dL, p = 0.431). There are a variety of confounding factors that can affect the HRQOL in PD patients, and anemia in particular can affect the body composition and HRQOL. However, due to the cross-sectional observational study design, there is a possibility of hidden confounders. Second, a causal relationship could not be inferred due to the cross-sectional nature of the study. For example, we could not directly confirm whether the patient's satisfaction would change if the patient's body composition changes due to the influence of therapeutic effects. Even though we performed a linear regression analysis by adding the usage of RAAS blockade as an adjustment variable to model 5, there were inevitable drawbacks in that the causal relationship could not be confirmed. Further longitudinal or interventional studies are needed to better understand and determine the existence of a causal relationship. Third, the sample size of about 200 subjects may be insufficient to verify robust statistical significance. Nevertheless, this is the first study from PD patients evaluating the association between body composition and HRQOL. Moreover, we analyzed the body composition with Z-FTI and Z-LTI from Korean PD patients, using reference values from the Asian general population rather than the absolute values of FTI or LTI itself. This allowed us to investigate more accurately the association between body composition and HRQOL.
Based on the results of this study, it could be suggested that the implementation of strategies for the prevention and management of overhydration and obesity in PD patients could improve quality of life. Multidisciplinary strategies such as nutritional intervention, physical training, and psychological support for the HRQOL would be needed. Lifestyle and therapeutic adherence of PD patients who are overhydrated or obese could be considered in further investigations.
Materials and methods
Patients
This study is a cross-sectional and observational study to analyze the association between the body composition and HRQOL of PD patients enrolled in the KNOW-PD cohort. The KNOW-PD cohort is a single-center prospective cohort aimed at determining the prognosis of PD patients for which monitoring is planned for up to ten years beginning in 2015. In KNOW-PD cohort, patients (age ≥ 18 years) on maintenance PD were enrolled. Exclusion criteria were acute kidney disease, plans to undergo kidney transplant or transition to hemodialysis within 6 months, and pregnancy. Patients registered in the KNOW-PD cohort responded to HRQOL questionnaires at study entry and at 2, 4, and 8 years. The study subjects also underwent body composition measurement annually.
Among 363 patients who were enrolled KNOW-PD cohort, we excluded the subjects who were hybrid dialysis modalities (hemodialysis and PD) (n = 4), unable to undergo BIS because of defibrillators, artificial joints, pins, or limb amputations (n = 28), and those with the different measurement date for BIS and HRQOL (n = 134). Finally, a total of 197 PD patients who were eligible for data collection for both body composition and HRQOL were included.
Measurement of body composition
Body composition was assessed using the BIS device (Body Composition Monitor [BCM]; Fresenius Medical Care, Bad Homburg, Germany). This device measures resistance, reactance, and phase angle from frequencies oscillating between 5 kHz and 1000 kHz45. The BCM was first validated against a gold standard to determine total body water (TBW), ECW, and ICW from the measured impedance data following the model of Moissl et al.46,47. The measurement was obtained by placing electrodes on one hand and one foot in a BCM device and entering current height and weight data into the device. Absolute ∆tissue hydration (A∆TH) represents the difference between the actual amount of ECW in the tissue as detected by the BCM and the amount of water present in tissue as predicted by physiological models under normal physiological conditions48. A∆TH is further normalized to ECW and expressed as a ratio called relative ∆tissue hydration (R∆TH = A∆TH/ECW). As a R∆TH ratio greater than 15% is related to mortality49, this cut-off was used to define “severe fluid overload”45,50. The BCM also provides the values of FTI, calculated as fat tissue mass/height2, and LTI, calculated as lean tissue mass/height2, by estimating the values of ICW, ECW, and TBW. FTI and LTI could be compared to the reference population, which is below the 10th percentile of about 2000 healthy adult subjects aged 18–75 years. We also expressed FTI and LTI as a Z-score, which was calculated by correcting age and sex for a population of 1294 Asian adults.
Health-related quality of life measurement
HRQOL was evaluated using the Kidney Disease Quality of Life Short Form (KDQOL-SF) version 1.351, which has been validated in many previous studies6,24,25,26,27,52. Patient responses to the KDQOL-SF were used to determine the scores of the kidney disease component summary (KDCS), physical component summary (PCS), and mental component summary (MCS). The questions used for the KDCS take into account particular kidney-related concerns of individuals with kidney diseases53. The scale for the KDCS was derived using 11 subscales: (1) symptoms/problems, (2) effects of kidney disease, (3) burden of kidney disease, (4) work status, (5) cognitive function, (6) quality of social interaction, (7) sexual function, (8) sleep, (9) social support, (10) dialysis staff encouragement, and (11) patient satisfaction. The scales for PCS and MCS were derived from eight different subscales, originally developed for the Short-Form Health Survey (SF-36)54. PCS was derived using 4 subscales: (1) physical functioning, (2) role-physical, (3) bodily pain, and (4) general health. MCS was derived using 4 subscales: (1) emotional well-being, (2) role-emotional, (3) social function, and (4) energy/fatigue. In all scales, a score from 0 to 100 was calculated, and a higher score indicates better quality of life.
Data collection and ethical aspects
Baseline data and laboratory parameters were collected from the patient’s medical records. Dialysis adequacy with KT/Vurea per week, creatinine clearance, 24-h urine volume, and peritoneal membrane characteristics based on the results of the peritoneal equilibration test were collected. Information on glucose exposure load and usage of icodextrin was also collected. The Subjective Global Assessment (SGA), which is a tool that assesses nutritional status using medical history and a physical examination, was also administered55. In general, patients with SGA scores of 6–7, 3–5, and 1–2 are considered well nourished, mildly to moderately malnourished, and severely malnourished, respectively56. The Davies score, which is one of the comorbidity indexes, was recorded according to the protocol57. Left ventricular mass index were collected within 1 year from the study period from the echocardiography. The Institutional Review Board of Seoul National University Hospital (Institutional Review Board No. C-1506-097-681) approved the study. This study was conducted in accordance with the Declaration of Helsinki. All patients gave their informed consent to the study protocol before data collection and BIS measurement.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median and interquartile range according to normal or non-normal distribution. The normality of the distribution was assessed using the Kolmogorov–Smirnov test. Categorical variables were expressed as percentage of the total. Differences between groups stratified by body composition were determined using Student’s t-test for variables with normal distribution or Mann–Whitney test for variables with non-normal distribution. Spearman’s correlation analysis was used to determine the correlation between the parameter of body composition and the HRQOL scores. Linear regression testing was used to determine the association between body composition parameters and the HRQOL scores. Multivariate models included the significantly associated parameters according to their weight on univariate testing and clinically relevant parameters. A p value of < 0.05 was considered to indicate statistical significance, and statistical analysis was performed using SPSS software ver. 26.0 (IBM, Armonk, NY, USA).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jaar, B. G., Chang, A. & Plantinga, L. Can we improve quality of life of patients on dialysis?. Clin. J. Am. Soc. Nephrol. 8, 1–4. https://doi.org/10.2215/cjn.11861112 (2013).
Fukuhara, S. et al. Health-related quality of life among dialysis patients on three continents: the dialysis outcomes and practice patterns study. Kidney Int. 64, 1903–1910. https://doi.org/10.1046/j.1523-1755.2003.00289.x (2003).
Grincenkov, F. R. et al. Impact of baseline health-related quality of life scores on survival of incident patients on peritoneal dialysis: A cohort study. Nephron 129, 97–103. https://doi.org/10.1159/000369139 (2015).
Broers, N. J. et al. Quality of life in dialysis patients: A retrospective cohort study. Nephron 130, 105–112. https://doi.org/10.1159/000430814 (2015).
Okubo, R. et al. Health-related quality of life and prognosis in patients with chronic kidney disease: A 3-year follow-up study. Clin. Exp. Nephrol. 18, 697–703. https://doi.org/10.1007/s10157-013-0901-x (2014).
Mapes, D. L. et al. Health-related quality of life as a predictor of mortality and hospitalization: The dialysis outcomes and practice patterns study (DOPPS). Kidney Int. 64, 339–349. https://doi.org/10.1046/j.1523-1755.2003.00072.x (2003).
Brown, E. A. et al. International society for peritoneal dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit. Dial Int. 40, 244–253. https://doi.org/10.1177/0896860819895364 (2020).
Choi, S. J. et al. Changes in body fat mass in patients after starting peritoneal dialysis. Perit. Dial Int. 31, 67–73. https://doi.org/10.3747/pdi.2009.00131 (2011).
Kim, C., Kim, J.-K., Lee, H.-S., Kim, S. G. & Song, Y. R. Longitudinal changes in body composition are associated with all-cause mortality in patients on peritoneal dialysis. Clin. Nutr. 40, 120–126. https://doi.org/10.1016/j.clnu.2020.04.034 (2021).
Ronco, C. et al. Baseline hydration status in incident peritoneal dialysis patients: The initiative of patient outcomes in dialysis (IPOD-PD study). Nephrol. Dial. Transplant. 30, 849–858. https://doi.org/10.1093/ndt/gfv013 (2015).
Fürstenberg, A. & Davenport, A. Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy X-ray absorptiometry. Am. J. Nephrol. 33, 150–156. https://doi.org/10.1159/000324111 (2011).
Sarkar, S. et al. Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int. 70, 1832–1839 (2006).
Valtuille, R., Casos, M. E., Fernandez, E. A., Guinsburg, A. & Marelli, C. Nutritional markers and body composition in hemodialysis patients. Int. Sch. Res. Notices. 2015, 695263. https://doi.org/10.1155/2015/695263 (2015).
Mushnick, R. et al. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dialysis patients. Kidney Int. Suppl. 64, S53-56. https://doi.org/10.1046/j.1523-1755.64.s87.22.x (2003).
Caravaca, F., Martínez del Viejo, C., Villa, J., Martínez Gallardo, R. & Ferreira, F. Hydration status assessment by multi-frequency bioimpedance in patients with advanced chronic kidney disease. Nefrologia. 31, 537–544. https://doi.org/10.3265/Nefrologia.pre2011.Apr.10936 (2011).
Jia, G., Aroor, A. R., Hill, M. A. & Sowers, J. R. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension 72, 537–548. https://doi.org/10.1161/hypertensionaha.118.11065 (2018).
Li, P. K., Chow, K. M., Wong, T. Y., Leung, C. B. & Szeto, C. C. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis: A randomized, controlled study. Ann. Intern. Med. 139, 105–112. https://doi.org/10.7326/0003-4819-139-2-200307150-00010 (2003).
Tai, D. J. et al. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: A meta-analysis. Clin. J. Am. Soc. Nephrol. 5, 623–630. https://doi.org/10.2215/cjn.07831109 (2010).
Io, H. et al. Cardiac function and structure in longitudinal analysis of echocardiography in peritoneal dialysis patients. Perit. Dial. Int. 30, 353–361. https://doi.org/10.3747/pdi.2009.00007 (2010).
Aguiar, R., Pei, M., Qureshi, A. R. & Lindholm, B. Health-related quality of life in peritoneal dialysis patients: A narrative review. Semin. Dial. 32, 452–462. https://doi.org/10.1111/sdi.12770 (2019).
Valderrábano, F., Jofre, R. & López-Gómez, J. M. Quality of life in end-stage renal disease patients. Am. J. Kidney Dis. 38, 443–464. https://doi.org/10.1053/ajkd.2001.26824 (2001).
Finkelstein, F. O., Wuerth, D. & Finkelstein, S. H. Health related quality of life and the CKD patient: Challenges for the nephrology community. Kidney Int. 76, 946–952. https://doi.org/10.1038/ki.2009.307 (2009).
Mujais, S. K. et al. Health-related quality of life in CKD patients: Correlates and evolution over time. Clin. J. Am. Soc. Nephrol. 4, 1293–1301. https://doi.org/10.2215/CJN.05541008 (2009).
von der Lippe, N., Waldum-Grevbo, B., Varberg Reisæter, A. & Os, I. Is HRQOL in dialysis associated with patient survival or graft function after kidney transplantation?. BMC Nephrol. 17, 94. https://doi.org/10.1186/s12882-016-0316-5 (2016).
Molnar-Varga, M. et al. Health-related quality of life and clinical outcomes in kidney transplant recipients. Am. J. Kidney Dis. 58, 444–452. https://doi.org/10.1053/j.ajkd.2011.03.028 (2011).
Ryu, J. H. et al. Better health-related quality of life in kidney transplant patients compared to chronic kidney disease patients with similar renal function. PLoS ONE 16, e0257981. https://doi.org/10.1371/journal.pone.0257981 (2021).
Yoon, H. E. et al. Overhydration negatively affects quality of life in peritoneal dialysis patients: Evidence from a prospective observational study. Int. J. Med. Sci. 13, 686–695. https://doi.org/10.7150/ijms.16372 (2016).
Pellicano, R., Strauss, B. J., Polkinghorne, K. R. & Kerr, P. G. Longitudinal body composition changes due to dialysis. Clin. J. Am. Soc. Nephrol. 6, 1668–1675. https://doi.org/10.2215/cjn.06790810 (2011).
Kittiskulnam, P. et al. Protein-energy wasting and mortality risk prediction among peritoneal dialysis patients. J. Ren. Nutr. 31, 679–686. https://doi.org/10.1053/j.jrn.2020.11.007 (2021).
Shu, Y. et al. The effect of overhydration on mortality and technique failure among peritoneal dialysis patients: A systematic review and meta-analysis. Blood Purif. 46, 350–358. https://doi.org/10.1159/000492148 (2018).
Chang, D. C., Xu, X., Ferrante, A. W. & Krakoff, J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol Metab. Syndr. 11, 14. https://doi.org/10.1186/s13098-019-0409-y (2019).
Kang, S. H., Cho, K. H., Park, J. W., Yoon, K. W. & Do, J. Y. Impact of heavy proteinuria on clinical outcomes in patients on incident peritoneal dialysis. BMC Nephrol. 13, 171. https://doi.org/10.1186/1471-2369-13-171 (2012).
Dekker, M. J. E. et al. Interactions between malnutrition, inflammation, and fluid overload and their associations with survival in prevalent hemodialysis patients. J. Ren. Nutr. 28, 435–444. https://doi.org/10.1053/j.jrn.2018.06.005 (2018).
Krediet, R. T. & Balafa, O. Cardiovascular risk in the peritoneal dialysis patient. Nat. Rev. Nephrol. 6, 451–460. https://doi.org/10.1038/nrneph.2010.68 (2010).
van der Sande, F. M., van de Wal-Visscher, E. R., Stuard, S., Moissl, U. & Kooman, J. P. Using bioimpedance spectroscopy to assess volume status in dialysis patients. Blood Purif. 49, 178–184. https://doi.org/10.1159/000504079 (2020).
Kim, J. K. et al. Effects of excessive body fat accumulation on long-term outcomes during peritoneal dialysis. Perit. Dial. Int. 39, 268–275. https://doi.org/10.3747/pdi.2018.00164 (2019).
Rincón Bello, A. et al. Fat tissue and inflammation in patients undergoing peritoneal dialysis. Clin. Kidney J. 9, 374–380. https://doi.org/10.1093/ckj/sfw007 (2016).
Avesani, C. M. et al. Inflammation and wasting in chronic kidney disease: Partners in crime. Kidney Int. 70, S8–S13. https://doi.org/10.1038/sj.ki.5001969 (2006).
Walters, B. A., Hays, R. D., Spritzer, K. L., Fridman, M. & Carter, W. B. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am. J. Kidney Dis. 40, 1185–1194. https://doi.org/10.1053/ajkd.2002.36879 (2002).
Vega, A. et al. Low lean tissue mass is an independent risk factor for mortality in patients with stages 4 and 5 non-dialysis chronic kidney disease. Clin. Kidney J. 10, 170–175. https://doi.org/10.1093/ckj/sfw126 (2017).
Parthasarathy, R., Oei, E. & Fan, S. L. Clinical value of body composition monitor to evaluate lean and fat tissue mass in peritoneal dialysis. Eur. J. Clin. Nutr. 73, 1520–1528. https://doi.org/10.1038/s41430-019-0391-3 (2019).
Peipert, J. D., Nair, D., Klicko, K., Schatell, D. R. & Hays, R. D. Kidney disease quality of life 36-item short form survey (KDQOL-36) normative values for the United States dialysis population and new single summary score. J. Am. Soc. Nephrol. 30, 654–663. https://doi.org/10.1681/asn.2018100994 (2019).
Orozco-González, C. N. et al. Severity of protein-energy wasting and obesity are independently related with poor quality of life in peritoneal dialysis patients. Nefrologia https://doi.org/10.1016/j.nefro.2021.04.003 (2021).
van Haalen, H., Jackson, J., Spinowitz, B., Milligan, G. & Moon, R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: Analysis of multinational real-world data. BMC Nephrol. 21, 88. https://doi.org/10.1186/s12882-020-01746-4 (2020).
Van Biesen, W. et al. Fluid status in peritoneal dialysis patients: The European body composition monitoring (EuroBCM) study cohort. PLoS ONE 6, e17148. https://doi.org/10.1371/journal.pone.0017148 (2011).
Moissl, U. M. et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol. Meas. 27, 921–933. https://doi.org/10.1088/0967-3334/27/9/012 (2006).
Wabel, P., Chamney, P., Moissl, U. & Jirka, T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 27, 75–80. https://doi.org/10.1159/000167013 (2009).
Wang, J. & Pierson, R. N. Jr. Disparate hydration of adipose and lean tissue require a new model for body water distribution in man. J. Nutr. 106, 1687–1693. https://doi.org/10.1093/jn/106.12.1687 (1976).
Chazot, C. et al. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol. Dial. Transplant. 27, 2404–2410. https://doi.org/10.1093/ndt/gfr678 (2012).
Tabinor, M. et al. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): Systematic review and subgroup meta-analysis. Sci. Rep. 8, 4441–4441. https://doi.org/10.1038/s41598-018-21226-y (2018).
Hays, R. D. et al. Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3: A Manual for Use and Scoring (Rand, 1997).
Joshi, V. D., Mooppil, N. & Lim, J. F. Validation of the kidney disease quality of life-short form: A cross-sectional study of a dialysis-targeted health measure in Singapore. BMC Nephrol. 11, 36–36. https://doi.org/10.1186/1471-2369-11-36 (2010).
Hays, R. D., Kallich, J. D., Mapes, D. L., Coons, S. J. & Carter, W. B. Development of the kidney disease quality of life (KDQOL) instrument. Qual. Life Res. 3, 329–338. https://doi.org/10.1007/bf00451725 (1994).
Ware, J. E. SF-36 Physical and Mental Health Summary Scales: A User’s Manual (Health Institute New England Medical Center, 1994).
Steiber, A. L. et al. Subjective global assessment in chronic kidney disease: A review. J. Ren. Nutr. 14, 191–200 (2004).
Kwon, Y. E. et al. Change of nutritional status assessed using subjective global assessment is associated with all-cause mortality in incident dialysis patients. Medicine 95, e2714. https://doi.org/10.1097/MD.0000000000002714 (2016).
Davies, S. J., Russell, L., Bryan, J., Phillips, L. & Russell, G. I. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: Their interrelationship and prediction of survival. Am. J. Kidney Dis. 26, 353–361. https://doi.org/10.1016/0272-6386(95)90657-6 (1995).
Acknowledgements
This work was funded by Fresenius Medical Care, Korea (06-2016-1040). We thank Fresenius Medical Care for providing reference BCM data for the Asian population. This study was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF), and funded by the Korean Government (MSIT) (2017M3A9E4044649).
Author information
Authors and Affiliations
Contributions
Research conception and study design: S.-M.K., K.-H.O.; analysis of data: S.-M.K., K.-H.O.; drafting the article: S.-M.K.; revise the article: S.-M.K., K.-H.O.; providing intellectual content of critical importance of the work; S.-M.K., M.K., E.K., J.H.K., Y.K., H.R., S.S.H., H.L., Y.C.K., K.-O.H.; and final approval of the version to be published: K.-O.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, SM., Kang, M., Kang, E. et al. Associations among body composition parameters and quality of life in peritoneal dialysis patients. Sci Rep 12, 19192 (2022). https://doi.org/10.1038/s41598-022-19715-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19715-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.