Abstract
The increasing widespread use of lithium, which is preferred as an energy source in batteries produced for electric vehicles and in many electronic vehicles such as computers and mobile phones, has made it an important environmental pollutant. In this study, the toxicity profile of lithium carbonate (Li2CO3) was investigated with the Allium test, which is a bio-indicator test. Dose-related toxic effects were investigated using Li2CO3 at doses of 25 mg/L, 50 mg/L, and 100 mg/L. The toxicity profile was determined by examining physiological, cytotoxic, genotoxic, biochemical and anatomical effects. Physiological effects of Li2CO3 were determined by root length, injury rate, germination percentage and weight gain while cytotoxic effects were determined by mitotic index (MI) ratio and genotoxic effects were determined by micronucleus (MN) and chromosomal aberrations (CAs). The effect of Li2CO3 on antioxidant and oxidant dynamics was determined by examining glutathione (GSH), malondialdehyde (MDA), catalase (CAT) and superoxide dismutase (SOD) levels, and anatomical changes were investigated in the sections of root meristematic tissues. As a result, Li2CO3 exhibited a dose-dependent regression in germination-related parameters. This regression is directly related to the MI and 100 mg/L Li2CO3 reduced MI by 38% compared to the control group. MN and CAs were observed at high rates in the groups treated with Li2CO3. Fragments were found with the highest rate among CAs. Other damages were bridge, unequal distribution of chromatin, sticky chromosome, vagrant chromosome, irregular mitosis, reverse polarization and multipolar anaphase. The genotoxic effects were associated with Li2CO3-DNA interactions determined by molecular docking. The toxic effects of Li2CO3 are directly related to the deterioration of the antioxidant/oxidant balance in the cells. While MDA, an indicator of lipid peroxidation, increased by 59.1% in the group administered 100 mg/L Li2CO3, GSH, which has an important role in cell defense, decreased by 60.8%. Significant changes were also detected in the activities of SOD and CAT, two important enzymes in antioxidant defense, compared to the control. These toxic effects, which developed in the cells belonging to the lithium-treated groups, were also reflected in the tissue anatomy, and anatomical changes such as epidermis cell damage, cortex cell damage, flattened cell nucleus, thickening of the cortex cell wall and unclear vascular tissue were observed in the anatomical sections. The frequency of these changes also increased depending on the Li2CO3 dose. As a result, Li2CO3, which is one of the lithium compounds, and has become an important contaminant in the environment with increasing technological developments, caused a combined and versatile toxicity in Allium cepa L. meristematic cells, especially by causing deterioration in antioxidant/oxidant dynamics.
Similar content being viewed by others
Introduction
Industrialization and advances in technology have not only made people's lives easier, but also have caused various problems to arise. With the technological developments, the use of electronic products has increased day by day, and the rapid production and consumption of these products has brought many problems. Electronic waste, called e-waste is unused electrical and electronic devices. E-waste contains many materials such as plastic, metal and glass, and when they degrade, dangerous substances are released into the environment. E-waste contains more than a thousand substances in its structure and includes metals such as lead, mercury, cadmium, chromium and lithium1,2. Contamination of these metals to the environment adversely affects both environmental safety and all organisms. Lithium, which is used as an energy source in electric vehicles batteries and many electronic vehicles such as computers and mobile phones, is an important pollutant that contaminates the environment through e-waste. On-site disposal of lithium-hydride and lithium-deuterium material, waste from the electronics, fabric, ceramics and cosmetics industries are the main sources of environmental lithium contamination3. Lithium, which is used for industrial purposes in colorants, batteries and metal alloys, contaminates water resources and soil and causes serious pollution4.
It has been reported that lithium compounds, which are widely distributed in nature, are found at rates of 30 mg/kg in the earth's crust, 25 mg/kg in the soil, 2 mg/kg in drinking water, 170–190 mg/L in sea water and 2 ng/m3 in the atmosphere3. Due to its wide distribution, lithium is easily taken up by plants and reaches other organisms through the food chain. Lithium uptake and accumulation differ among plant species. Some plants are hyper- or bio- accumulators of lithium, while others keep their lithium intake below the threshold level5. Some metals are toxic to plants, and increasing their concentration from the optimum level delays plant growth and yield. Although the toxic effects of lithium on plants are not yet clear, it is reported that lithium salts are highly toxic and cause a significant reduction in plant growth by triggering the formation of necrotic zones. However, different plant species exhibit different behaviors in terms of sensitivity and tolerance to lithium toxicity. Although studies have reported that low concentrations of lithium can stimulate growth in some plant species, it is reported that high concentrations of lithium reduce or completely inhibit growth. It has been suggested that some harmful effects of lithium, whose toxicity mechanism is still unclear, may be related to oxidative stress3,4,5. It is reported that the production of free radicals and oxidative stress occur in severe lithium exposure. Lithium induces the formation of free oxygen radicals with Fenton-type reactions and can cause oxidative damage in the cell. Oxidative stress formation under lithium stress also reduces plant growth6. In the presence of oxidative stress, macromolecules such as DNA, lipid and protein are damaged, and the physiological and biochemical pathways in the cell are disrupted. Free radicals mainly attack the unsaturated lipids acids of cell membranes, causing lipid peroxidation and cell membrane damage. Proteins can also be oxidized as a result of oxidative stress, and amino acids in the protein structure are converted into various intermediates by oxidation. These changes in protein structure cause loss of function. It is also known that DNA damages occur in the presence of oxidative stress in cells. Oxidative damage, especially in purine bases, causes abnormalities in DNA7,8. Oxidative stress induced by lithium can cause various damages to cellular macromolecules and may also cause regression in plant growth. Some of the other potential effects of high concentrations of lithium are decreased chlorophyll content and photosynthesis, DNA condensation, inhibition of protein and amide biosynthesis, and conformational changes in DNA9. In this study, the toxic effects of lithium on meristematic cells, whose toxicity mechanism and possible effects in higher plants have not yet been revealed in detail, were investigated. This toxicity risk monitoring study was performed using the Allium test. Higher plants such as A. cepa are important indicator organisms used to investigate the cytotoxic and mutagenic effects of chemical agents. Compared to other tests, the Allium test is an easy to apply, fast and cost-effective method. The results of Allium test show important compatibility with other toxicity tests. Cytotoxicity tests with human lymphocyte cells and algal cells show high compatibility with the results of the Allium test10. In this context, the Allium test is the first alternative test system in determining the possible multifaceted toxicity caused by environmental toxic agents. A. cepa has an oxidase-enzyme system that exhibits similar activity to detoxifying mechanisms in mammals. This similarity provides a high correlation between the Allium test and the toxicity tests performed in mammals. With the Allium test, substances that cause toxic effects in eukaryotes can be detected and the results obtained can be used as a preliminary assessment in all animal and plant biodiversity. The Allium test allows the investigation of not only the cytological or genetic effects of various natural or synthetic components, but also the physiological, clastogenic, aneugenic, anatomical and biochemical effects11,12.
In this study, physiological, cytotoxic, genotoxic, biochemical and anatomical effects of lithium carbonate (Li2CO3) compound on meristematic cells were investigated using the Allium test. Different analysis methods were used within the scope of the study. In this way, it is aimed to determine the multiple toxicity of lithium. The data obtained in each analysis were correlated with each other and the mechanism of toxicity was tried to be clarified. The potential of lithium to induce oxidative stress was evaluated by investigating changes in the antioxidant/oxidant balance. For this purpose, glutathione (GSH) and malondialdehyde (MDA) levels, catalase (CAT) and superoxide dismutase (SOD) activities were measured in meristematic cells. Mitotic index (MI) rates were used to determine the cytotoxic effects and the micronucleus (MN) and chromosomal aberrations (CAs) frequencies were investigated to determine the genotoxic effects. In particular, the effect of Li2CO3 on DNA fragmentation was investigated in order to evaluate its genotoxic mechanism of action, and the interaction between Li2CO3-DNA was investigated by molecular docking. The possible physiological effects of Li2CO3 were investigated using closely related parameters such as germination rate, root length, weight gain and relative injury rate analyses. Anatomical changes as a result of Li2CO3 exposure were also determined by root tip sections. The potential toxicity mechanism was interpreted by correlating all the obtained data with each other.
Material and methods
Test material and chemical
A. cepa bulbs (2n = 16) were purchased from a commercial market in Giresun (Turkiye), and Li2CO3 (CAS No: 554-13-2), carmine (CAS No: 1390-65-4), low melting agarose (CAS No: 39346-81-1), ethidium bromide (CAS No: 1239-45-8) were purchased from Merck and Sigma-Aldrich.
Experimental process
Allium test was used to examine Li2CO3 toxicity and for this purpose bulbs were divided into four groups as Control (Group I), 25 mg/L Li2CO3 (Group II), 50 mg/L Li2CO3 (Group III), 100 mg/L Li2CO3 (Group IV).
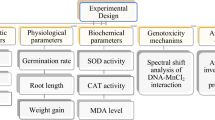
A preliminary study was conducted to determine the EC50 value, based on the dose ranges known to inhibit growth in various plants in the literature13. The EC50 value was investigated in dose ranges of 0–120 mg/L and was determined as 50 mg/L. Three different doses were used in the study, EC50 value (50 mg/L), half (25 mg/L) and double (100 mg/L) of this value. The bulbs in the control group were germinated with tap water, and the bulbs in the treatment groups were germinated with three different doses of Li2CO3. The germination process was continued at 24 °C for 72 h without interruption. The beakers were checked every twenty-four h and the decreasing solution were added. At the end of the period, the germinated root tips were washed with distilled water, cut into approximately 1 cm length, and prepared for spectrophotometric measurements and microscopic observations by applying routine homogenization and crushing preparation processes14. Toxicity profile was obtained by using physiological, cytogenetic, biochemical and anatomical parameters in root tips obtained from bulbs germinating in solutions containing Li2CO3, and all parameters tested in the study are given in Fig. 1.
Physiological parameters
The effects of Li2CO3 doses on root elongation were determined by measuring with a millimetric ruler of the radicle length, which is the structure in the plant embryo and forming the root. The effects on the weight gain were determined by weighing the bulb weights before and after the application with the help of precision scales. Relative injury rate and the effects on germination rate (GR) were determined with the help of Eqs. (1) and (2), respectively12.
Cytotoxic and genotoxic effects
Cytotoxic effects of Li2CO3 were determined by MI rates and genotoxic effects were investigated with MN and CAs frequencies in meristematic cells. Acetocarmine crushing technique was used for the detection of CAs and MN. Root tips were cut about 1 cm long, fixed in “Clarke” solution for 2 h, washed in ethyl alcohol (96%) for 15 min, hydrolyzed in 1 N HCl (at 60 °C) for 17 min, and kept in glacial acetic acid (45%) for 30 min. In the last stage, root tips were stained with acetocarmine for 24 h, placed on a slide and crushed with a coverslip. CAs and MN observations were performed under the Irmeco IM-450 TI model research microscope and photographed at × 500 magnification15. Three criteria suggested by Fenech et al.16 were taken into account in the detection of MN. MI, which shows the ratio of cells undergoing mitosis in a cell population, was determined with the help of Eq. (3).
Comet assay
The method of Chakraborty et al.17 was followed for comet assay. The procedures were carried out in low light to minimize DNA degradation and examined using a fluorescence microscope. Comets were evaluated using Comet Assay software (CASP) version 1.2.3b using tail DNA length parameters18. A total of 1.000 cells were examined for DNA damage in each group, with 100 cells examined in each bulb. The degree of DNA damage was graded on a scale of 0 to 4 based on the severity of DNA damage. The cells were divided into five groups depending on the length of their tail DNA, which ranged from zero to four.
The total DNA damage per group, expressed as arbitrary units, was calculated using Eq. (4).
i degree of damage (0, 1, 2, 3, 4), Ni the number of cells in i degree.
Antioxidant/oxidant dynamics
The effects of Li2CO3 on antioxidant/oxidant balance were investigated with GSH, MDA, SOD and CAT levels in root meristematic cells. Root MDA levels were measured by applying the method suggested by Unyayar et al.19 and the MDA levels are shown as µM/g FW. GSH levels were analyzed by sulfhydryl level measurement as described by Kurt et al.20 and expressed as µmol/g. For enzyme activity measurements, enzyme extraction procedure was carried out at + 4 °C. SOD and CAT activities were measured using the method proposed by Çavuşoğlu et al.21. SOD activity and CAT activities were shown as U/mg FW and OD240nmmin/g, respectively. MDA, GSH, SOD and CAT analysis were performed in triplicate.
Anatomical alterations
Root tips were cut 1 cm long, washed with distilled water, placed between styrofoam material and cross-sectioned with a sterile razor blade. Sections were placed on a slide, stained with methylene blue (5%) for 2 min and covered with a coverslip. Anatomical observations were made under a research microscope and photographed at × 200 magnification22.
Molecular docking
Molecular docking analysis was used for interactions of Li2CO3 with different DNA molecules. The 3D structures of, B-DNA dodecamer (PDB ID: 195d)23, DNA (PDB ID: 1cp8)24 and B-DNA dodecamer (PDB ID: 1bna)25 molecules were obtained from the protein data bank. The 3D structure of Li2CO3 (DB Accesion Number: DB14509) was retrieved from the Drugbank. Energy minimization of DNA molecules was done with Gromos 43B1 using Swiss-PdbViewer26 (v.4.1.0) software whereas energy minimization of the 3D structure of Li2CO3 was accomplished with the Universal Force Field (UFF) employing Open Babel v.2.4.0 software27. The molecular docking process was carried out with the grid box containing the entire structure of DNA molecules. Since the atomic parameters and charge of the lithium element are not available by default in the AutoDock software, the parameters of the lithium atom have been added to the AD4_parameters.dat file. The docking and 3D visualizations were obtained with Biovia Discovery Studio 2020 Client.
Statistical analysis
Statistical analyzes were performed by using SPSS Statistics 22 (IBM SPSS, Turkey) program. All data are shown as mean ± standard deviation (SD). The statistical significance between the means was determined with the help of one-way analysis of variance, “One-way ANOVA” and “Duncan” tests. Obtained values were considered statistically significant when p < 0.05.
Results and discussion
Li2CO3 effects on germination related parameters
The effects of Li2CO3 application on some physiological parameters are given in Table 1. In the control group, root length, germination rate and weight gain were 4.20 ± 0.95 cm, 98% and 5.16 g, respectively. Important decreases in physiological parameters were detected in Li2CO3 applied groups compared to the control. As the Li2CO3 dose increased, the decrease in all parameters also enhanced. 100 mg/L Li2CO3 application decreased germination rate, root length and weight gain by 39.7%, 54.7% and 27%, respectively. The relative injury rate was calculated in the groups and the highest injury rate was 0.39 in Group IV, which was administered 100 mg/L Li2CO3. These results showed that Li2CO3 caused physiological damages in A. cepa. These changes observed in physiological parameters in A. cepa can be explained by the inhibitory properties of Li2CO3 on photosynthesis reactions. Lithium reduces photosynthetic activity by damaging the chloroplast ultrastructure, inhibiting electron chain reactions, or replacing with Mg in chlorophyll3. Inhibition of photosynthetic reactions in plants causes a decrease in weight gain, germination percentage and root elongation, resulting in a slowdown in physiological development. Root elongation and weight gain also occur in plants with germination. Delays in germination cause disruptions in other physiological processes. The decrease in all three parameters tested in the lithium-administered groups confirms this hypothesis. There are similar studies in the literature that support our findings. Kalinowska et al.28 stated that increasing lithium doses in lettuce plant decreased root and shoot biomass. Hawrylak-Nowak et al.9 determined that exposure to 25 mg/dm3 lithium in sunflower caused a 16% decrease in carotenoid content, and the appearance of necrotic spots on leaves in corn, a decrease of approximately 45% in chlorophyll a and b content and a 67% decrease in carotenoid content. Gayathri et al.29 reported that the germination rate of A. cepa decreased depending on the lithium concentration dose and determined a germination rate of 73% at 50 ppm, 57% at 75 ppm and 41% at 100 ppm.
Li2CO3 effects on antioxidant/oxidant dynamics
The effects of Li2CO3 application on antioxidant/oxidant balance in A. cepa are given in Fig. 2. To determine this effect, GSH and MDA levels, CAT and SOD activities were examined. 25, 50 and 100 mg/L Li2CO3 administration increased MDA levels by 29.5%, 46.4% and 59.1%, respectively, compared to the control. In the groups treated with Li2CO3, a decrease in GSH level was observed and the most significant decrease was 60.8% in the group treated with 100 mg/L Li2CO3. The severity of all these changes in MDA and GSH levels increased depending on the dose, and accordingly, the antioxidant/oxidant balance was impaired. This effect of Li2CO3 application is closely related to oxidative stress induction in cells. Lithium provides the emergence of free radicals from Fenton-type reactions and causes oxidative damage3. It has also been reported that lithium reduces chlorophyll content and photosynthesis, which is due to increased free radical production. Lithium toxicity and excessive free radical production cause oxidative stress in plants9. As a result of lipid peroxidation, toxic and mutagenic intermediates such as MDA are formed, cell membrane structure is deteriorated and cell integrity is damaged30. The endogenous antioxidant defense system of the cell provides protection against free radicals formed in the cell. GSH has an important place in the endogenous defense system and is widely found in plant organelles such as the endoplasmic reticulum, cytosol, mitochondria, chloroplast, vacuole and peroxisome. Decreased GSH level and increased MDA level in cells causes increased oxidation and disruption of antioxidant/oxidant balance19. It has been reported in many studies in the literature that lithium compounds cause the antioxidant/oxidant balance to deteriorate. Hawrylak-Nowak et al.9 reported that the lipid peroxidation level and MDA production in sunflower and corn plants increased in the presence of 50 mg/dm3 of lithium, and this situation disrupted the membrane integrity. Another evidence Li2CO3 administration disrupts the antioxidant/oxidant balance is the changes in SOD and CAT activities. Li2CO3 administration caused significant changes in enzyme activities compared to the control. 25 mg/L and 50 mg/L Li2CO3 treatment caused an increase in activities by inducing SOD and CAT enzymes. 50 mg/L Li2CO3 increased SOD activity by 53% and CAT activity by 54% compared to the control. Administration of 100 mg/L Li2CO3, the highest dose used in this study, caused a regression in both enzyme levels, but despite this regression, the enzyme levels remained above the control levels. Plants have different mechanisms to cope with oxidative stress and induction of antioxidant enzyme activities such as CAT and SOD is one of these mechanisms. SOD and CAT enzymes are two enzymes that play an important role in the scavenging free radicals. It has been reported by many studies that these two enzymes are induced in the presence of oxidative stress. Nciri et al.31 found that 1 mM lithium application increased SOD activity while decreasing glutathione peroxidase (GPx) activity In another study, it was reported that lithium application increased SOD, CAT, ascorbate peroxidase (APx) enzyme activities in spinach shoots32. While antioxidant enzyme activities are induced to cope with oxidative stress in cells, excessive stress can also cause denaturation of these enzymes. In this study, the decrease in SOD and CAT activities in the group administered 100 mg/L Li2CO3 as the highest dose can be explained by potential denaturation. Oxidative stress induced by free radicals triggers deterioration in the structure of proteins, oxidation of amino acids and formation of carbonyl groups. Such changes in protein structure cause loss of function and disruptions in various biochemical processes in the cell33. The decrease in SOD and CAT activities can be explained by these effects of oxidative stress. Similarly, it is reported in the literature that high-dose lithium applications inhibit antioxidant enzymes30.
Cytotoxic effects of Li2CO3
Cytotoxic effects Li2CO3 application on A. cepa bulbs were investigated by MI analyses. The effects of Li2CO3 on MI in A. cepa are given in Fig. 3. While 866 cells were divided within 10.000 cells in the control, this number decreased to 537 in the 100 mg/L Li2CO3 administered group. Briefly, Li2CO3 application triggered a remarkable decrease in MI compared to the control. MI is a parameter used to determine the cytotoxicity of chemicals as an indicator of cell proliferation34. 25, 50 and 100 mg/L Li2CO3 administration reduced MI rates by 8.2%, 19.4%, and 38%, respectively, compared to the control. Decreased MI rates compared to control are indicative of slower mitotic cell divisions in the meristem cells. In addition, the decrease in MI is consistent with the reduction of root elongation, germination percentage and weight gain, which are associated with growth parameters. In similar studies it was reported that different metal ions have a regressive effect on MI. Yalçın et al.35 reported that HgCl2 application reduced MI in A. cepa bulbs. Likewise, Macar et al.36 determined that Co(NO3)2 stress reduced MI and showed a cytotoxic effect in A. cepa. Kikuda et al.37 reported significant changes in MI rates of A. cepa germinated with lithium-containing Buritis Lake water.
Genotoxic effects of Li2CO3
The effects of Li2CO3 on CAs and MN frequencies, which indicate genotoxic effects, are given in Table 2 and Figs. 4, 5. While a few statistically insignificant MN, sticky chromosome and unequal distribution of chromatin were found in the control group, the frequency of abnormalities increased depending on the dose of Li2CO3. Different types of aberrations such as bridge, sticky chromosome, fragment, unequal distribution of chromatin, vagrant chromosome, reverse polarization, irregular mitosis and multipolar anaphase were detected in Li2CO3 applied groups. Essentially, all CAs types represent aberrant mitotic divisions. Toxic effects induced by Li2CO3 exposure occur through many mechanisms. One of the mechanisms of action of lithium stress is disruptions in nucleic acid metabolism. High concentrations of lithium can inhibit biosynthesis of protein, DNA condensation, produce toxic effects on nucleic acid metabolism or induce conformational change in DNA3. It has been reported in the literature that many metals such as Li2CO3 reach the environment and organisms and cause various CA formations. Sticky chromosome, highly induced by lithium application, is the result of increased chromosomal condensation, de-polymerization of DNA and partial dissolution of nucleoproteins. Sticky chromosomes, often irreversible and possibly leading to cell death, indicate highly toxic effects. Fragment, another type of CA detected at high frequency as a result of lithium application, shows that it causes breaks in DNA. These fragments transform into MN at later stages of cell division38. In parallel with our study, Yalçın et al.35 reported that HgCl2 caused an increase in MN and various types of CAs in A. cepa. Similarly, Macar et al.36 reported that Co stress showed a remarkable increase in terms of CAs and MN in A. cepa. Liu et al.39 showed that different metal ions can cause varying degrees of nucleus, nucleolus and chromosome irregularities in A. cepa. It has been reported in the literature that DNA integrity changes and chromosomal damage occur in A. cepa germinating with water samples containing lithium37.
CAs induced by Li2CO3. MN in interphase (a), sticky chromosome in prophase (b), fragment in anaphase (c), unequal distribution of chromatin in anaphase (d), bridge in anaphase (e), vagrant chromosome in telophase (f), reverse polarization in telophase (g), irregular mitosis in metaphase (h), multipolar anaphase (ı).
DNA fragmentation
DNA strand breaks due to Li2CO3 application in the nucleus of A. cepa root tip cells were evaluated by comet assay. Figure 6 demonstrates the effects of Li2CO3 treatment on DNA fragmentation in A. cepa. The obtained comet assay results showed that Li2CO3 application caused DNA strand breaks. While the average DNA damage score was 10.83 ± 3.48 in Group I (control), a sharp increase occurred in Group II, which was administered 25 mg/L Li2CO3, and the average DNA damage score was 176.50 ± 19.35. In Group III, where the Li2CO3 dose increased to 50 mg/L, the DNA damage score increased to 232.17 ± 16.29. The DNA damage score was determined as 275.67 ± 18.84 in Group IV treated with 100 mg/L dose of Li2CO3. The DNA damage score increased with increasing Li2CO3 doses, demonstrating that the occurrence of DNA fragmentation increased as Li2CO3 doses increased. Our findings are also confirmed by the results of other studies. Although there is no study in the literature investigating DNA fragmentation in plants with Comet, it is reported that lithium compounds cause DNA breaks in some cells40.
The effect of Li2CO3 treatment on A. cepa root tip cell nucleus (0: no damage, 1: low damage, 2: moderate damage, 3: high damage, 4: extreme damage. A total of 1.000 cells were examined for DNA damage in each group. Data with different letters(a-d) in the columns are statically significant at P < 0.05.
Potential interactions of Li2CO3 with DNA molecules
Lithium binding affinities on DNA were investigated to support the genotoxic mechanism of action of lithium. Table 3 and Fig. 7 show evidence of lithium interactions with DNA sequences. Lithium interacted with B-DNA dodecamer (1BNA) with a binding energy of − 3.24 kcal/mol. Lithium showed molecular interactions with bases G10 and C11 in the A chain and with A18 in the B chain. The interaction of lithium in DNA (1CP8) occurred with a binding energy of − 2.75 kcal/mol. It showed interactions with G4 and C5 bases in the A chain and with C6 and A7 bases in the B chain. Lithium interacted with bases A7 and A8 in the chain A of B-DNA Dodecamer D (195D), with bases T18 and A19 in the chain B with a binding energy of − 3.20 kcal/mol. The findings of molecular docking studies between lithium and various DNA molecules confirmed lithium's capacity to intercalate by engaging with same and distinct strands in DNA. It also shows that lithium may influence DNA structure by binding to areas rich in G-C, C-A, A-A, and T-A nucleotides.
Li2CO3 effects on anatomic alterations
The anatomical changes caused by Li2CO3 application in A. cepa are given in Table 4 and Fig. 8. As a result of 100 mg/L Li2CO3 application, severe epidermis cell damage and cortex cell damage, moderate flattened cell nuclei, thickening of the cortex cell wall and unclear vascular tissue were observed. In 25 mg/L Li2CO3 application, epidermis cell damage, cortex cell damage, flattened cell nucleus, thickening in the cortex cell wall, and unclear vascular tissue were little, while these parameters were moderate in 50 mg/L Li2CO3 application. The response of roots to heavy metals is very important because the roots are the main input of metal ions in plants. Plant roots are the first organ to come into contact with lithium in the soil, and excess lithium has been reported to alter the root gravitropic growth of plants41. Deformation in epidermis cells and thickening of the cortex cell wall may be a possible defense mechanism of the plant to prevent excess lithium uptake. These results are consistent with the previous results (genotoxicity, oxidative stress) of this study. Considering the increase in MDA induced by Li2CO3, the structural deformations in the meristematic tissue may be due to oxidative stress-induced damage to the cell membranes. In the literature, there are studies reporting anatomical changes in plants as a result of metal contamination. Yalcin et al.35 reported that administration of HgCl2 in A. cepa caused meristematic cell damage and different anatomical aberrations.
Li2CO3 induced meristematic cell damages. Epidermis cells in control (a), epidermis cell damage (b), appearance of cell nucleus in control-oval (c), flattened cell nucleus (d), cortex cells in control (e), cortex cell damage (f), thickening of the cortex cell wall (g), vascular tissue in control (h), unclear vascular tissue (ı).
Conclusion
In this study, the in vivo toxicity profile of Li2CO3, which is used for various purposes in many fields, especially in the energy sector, and released to the environment as waste, was investigated using meristematic cells. Li2CO3 caused a cytotoxic effect by causing a regression in MI rates, and a genotoxic effect by inducing MN and CAs. Genotoxicity mechanism of Li2CO3, which was determined to cause DNA fragmentation by comet test, was examined by in silico analysis and it was determined that DNA interaction through intercalation was the main cause of genotoxicity. Root elongation and regression in germination indicate that Li2CO3 negatively affects physiological growth. The possible reason for this effect was related to the antioxidant/oxidant balance, which was disturbed by the abnormalities in MDA and GSH levels and the changes in antioxidant enzyme activities. The contamination levels of lithium to the environment vary between 3.74 and 169.5 mg/kg in soil and 1.58–1700 µg/L in aquatic environments42. Industrial contaminations can cause these levels to increase. In this study, it was determined that lithium doses of 25–100 mg/L caused toxic effects in A. cepa. It is clear that the use of lithium-containing wetlands in agricultural applications would be dangerous due to the toxic effects on eukaryotic organisms such as A. cepa. The toxic effects of Li2CO3 in A. cepa, a eukaryotic and bio-indicator organism can be used as a preliminary assessment for effects in other eukaryotic organisms. The high compatibility of Allium test results with other toxicity tests and the fact that it has an oxidase system indicates that the toxic effects determined by this test may also occur in other living things, even mammals. Considering that it is an important environmental contaminant, the detected toxic effects of Li2CO3, are quite thought-provoking. The rapid spread of technology and consumption-based society increases the interest in electronic products day by day, and a huge amount of e-waste is generated every minute. Unless regular recycling of e-waste is ensured, it also complicates the supply of raw materials used in electronic devices. Extracting the lithium called as clean energy from its ore and converting it into a commercially usable form such as Li2CO3 or lithium hydroxide (LiOH) accelerates the contamination of the environment. Serious environmental problems occur as a result of both non-recyclable e-waste and the acceleration in lithium mining. Lithium compounds, which reach many organisms through the food chain, also exhibit various toxic effects. For this reason, recycling of lithium-containing e-waste will protect living beings, the environment, natural resources, save energy and prevent fertile lands from being filled with waste.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Herat, S. Sustainable management of electronic waste (e-waste). Clean-Soil Air Water 35, 305–310 (2007).
Widmer, R., Oswald-Krapf, H., Sinha-Khetriwal, D., Schnellmann, M. & Böni, H. Global perspectives on e-waste. Environ. Impact Assess. Rev. 25, 436–458 (2005).
Shahzad, B. et al. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities–a review. Plant Physiol. Biochem. 107, 104–115 (2016).
Schrauzer, G. Lithium: Occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 21, 14–21 (2002).
Kabata-Pendias, A. & Mukherjee, A. Trace elements of group 12 (Previously group IIb) in Trace Elements from Soil to Human 283–319 (New York, 2007).
Tanveer, M., Hasanuzzaman, M. & Wang, L. Lithium in environment and potential targets to reduce lithium toxicity in plants. J. Plant Growth Regul. 38, 1574–1586 (2019).
VanderVeen, L. A., Hashim, M. F., Shyr, Y. & Marnett, L. J. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc. Natl. Acad. Sci. 100, 14247–14252 (2003).
Weidinger, A. & Kozlov, A. V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 5(2), 472–484 (2015).
Hawrylak-Nowak, B., Kalinowska, M. & Szymańska, M. A. Study on selected physiological parameters of plants grown under lithium supplementation. Biol. Trace Elem. Res. 149, 425–430 (2012).
Solange, B. T. & Haywood, D. L. Bioindicator of genotoxicity: The Allium cepa test in Environmental contamination (ed. Srivastava, J. K.) 138 (IntechOpen, 2012)
Demirtaş, G., Çavuşoğlu, K. & Yalçin, E. Aneugenic, clastogenic, and multi-toxic effects of diethyl phthalate exposure. Environ. Sci. Pollut. Res. 27, 5503–5510 (2020).
Çavuşoğlu, D. et al. Molecular docking and toxicity assessment of spirodiclofen: Protective role of lycopene. Environ. Sci. Pollut. Res. 28, 57372–57385 (2021).
Hayyat, M. U. et al. Investigation of lithium application and effect of organic matter on soil health. Sustainability 13, 1705 (2021).
Yalçın, E., Uzun, A. & Çavuşoğlu, K. In vivo epiclorohidrine toxicity: Cytogenetic, biochemical, physiological, and anatomical evidences. Environ. Sci. Pollut. Res. 26, 22400–22406 (2019).
Staykova, T. A., Ivanova, E. N. & Velcheva, D. Cytogenetic effect of heavy-metal and cyanide in contaminated waters from the region of southwest Bulgaria. J. Mol. Cell Biol. 4, 41–46 (2005).
Fenech, M. et al. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 534, 65–75 (2003).
Chakraborty, R., Mukherjee, A. K. & Mukherjee, A. Evaluation of genotoxicity of coal fly ash in Allium cepa root cells by combining comet assay with the Allium test. Environ. Monit. Assess. 153, 351–357 (2009).
Końca, K. et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 534, 15–20 (2003).
Unyayar, S., Celik, A., Çekiç, F. Ö. & Gözel, A. Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis 21, 77–81 (2006).
Kurt, D. et al. Genotoxic effects and molecular docking of 1, 4-dioxane: Combined protective effects of trans-resveratrol. Environ. Sci. Pollut. Res. 28, 54922–54935 (2021).
Çavuşoğlu, K., Kalefetoğlu, M. T., Macar, O., Çavuşoğlu, D. & Yalçın, E. Comparative investigation of toxicity induced by UV-A and UV-C radiation using Allium test. Environ. Sci. Pollut. Res. 29(23), 33988–33998 (2022).
Çavuşoğlu, K., Kurt, D. & Yalçın, E. A versatile model for investigating the protective effects of Ceratonia siliqua pod extract against 1, 4-dioxane toxicity. Environ. Sci. Pollut. Res. 27, 27885–27892 (2020).
Balendiran, K., Rao, S., Sekharudu, C., Zon, G. & Sundaralingam, M. X-ray structures of the B-DNA dodecamer d (CGCGTTAACGCG) with an inverted central tetranucleotide and its netropsin complex. Acta Crystallogr. D. 51, 190–198 (1995).
Katahira, R. et al. Solution structure of the novel antitumor drug UCH9 complexed with d (TTGGCCAA) 2 as determined by NMR. Nucleic Acids Res. 26, 744–755 (1998).
Drew, H. R. et al. Structure of a B-DNA dodecamer: Conformation and dynamics. PNAS 78, 2179–2183 (1981).
Guex, N. & Peitsch, M. Swis-model and the Swiss-Pdb viewer: An environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
O’Boyle, N. M. et al. Open Babel: An open chemical toolbox. J. Cheminform. 3, 1–14 (2011).
Kalinowska, M., Hawrylak-Nowak, B. & Szymańska, M. The influence of two lithium forms on the growth, L-ascorbic acid content and lithium accumulation in lettuce plants. Biol. Trace Elem. Res. 152, 251–257 (2013).
Gayathri, N., Sailesh, A. R. & Srinivas, N. Effect of lithium on seed germination and plant growth of Amaranthus viridis. J. Appl. Nat. Sci. 14(1), 133–139 (2022).
Eraković, V., Župan, G., Varljen, J., Laginja, J. & Simonić, A. Lithium plus pilocarpine induced status epilepticus—biochemical changes. Neurosci. Res. 36, 157–166 (2000).
Nciri, R. et al. Lipid peroxidation, antioxidant activities and stress protein (HSP72/73, GRP94) expression in kidney and liver of rats under lithium treatment. J. Physiol. Biochem. 68, 11–18 (2012).
Bakhat, H. F. et al. Growth and physiological response of spinach to various lithium concentrations in soil. Environ. Sci. Pollut. Res. 27, 39717–39725 (2020).
Dalle-Donne, I., Rossi, R., Giustarini, D., Milzani, A. & Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 329, 23–38 (2003).
Leme, D. M. & Marin-Morales, M. A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. Rev. Mutat. Res. 682, 71–81 (2009).
Yalçın, E. et al. Multi-protective role of Echinacea purpurea L. water extract in Allium cepa L. against mercury (II) chloride. Environ. Sci. Pollut. Res. 28, 62868–62876 (2021).
Macar, O., Kalefetoğlu, M. T., Çavuşoğlu, K. & Yalçın, E. Determination of protective effect of carob (Ceratonia siliqua L.) extract against cobalt (II) nitrate-induced toxicity. Environ. Sci. Pollut. Res. 27, 40253–40261 (2020).
Kikuda, R. et al. Evaluation of water quality of Buritis Lake. Water 14(9), 1414 (2022).
Khanna, N. & Sharma, S. Allium cepa root chromosomal aberration assay: A review. Indian J. Pharm. Biol. Res. 1(3), 105–111 (2013).
Liu, D., Jiang, W., Wang, W. & Zhai, L. Evaluation of metal ion toxicity on root tip cells by the Allium test. Isr. J. Plant Sci. 43, 125–133 (1995).
Sironval, V. et al. LiCoO2 particles used in Li-ion batteries induce primary mutagenicity in lung cells via their capacity to generate hydroxyl radicals. Part. Fibre Toxicol. 17, 1–9 (2020).
Mulkey, T. J. Alteration of growth and gravitropic response of maize roots by lithium. Gravit. Cosmol. 18, 552 (2007).
Bolan, N. et al. From mine to mind and mobiles–Lithium contamination and its risk management. Environ. Pollut. 290, 118067 (2021).
Acknowledgements
This study has not been financially supported by any institution.
Author information
Authors and Affiliations
Contributions
S.S.K: investigation; methodology; visualization; writing-review and editing. E.Y.: conceptualization; methodology; data curation; software; visualization; writing-review and editing. K.Ç.: conceptualization; data curation; investigation; methodology; visualization; writing-review. A.A.: software; data curation; visualization; writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuloğlu, S.S., Yalçin, E., Çavuşoğlu, K. et al. Dose-dependent toxicity profile and genotoxicity mechanism of lithium carbonate. Sci Rep 12, 13504 (2022). https://doi.org/10.1038/s41598-022-17838-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17838-0
This article is cited by
-
LC–MS/MS, GC–MS and molecular docking analysis for phytochemical fingerprint and bioactivity of Beta vulgaris L.
Scientific Reports (2024)
-
DNA fragmentation and multifaceted toxicity induced by high-dose vanadium exposure determined by the bioindicator Allium test
Scientific Reports (2023)
-
Detection of heavy metal contamination in Batlama Stream (Turkiye) and the potential toxicity profile
Scientific Reports (2023)
-
Potential toxicity assessment of mycotoxin fusaric acid with the spectral shift profile on DNA
Environmental Science and Pollution Research (2023)
-
Cytogenotoxic effects of 3-epicaryoptin in Allium cepa L. root apical meristem cells
Protoplasma (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.