Abstract
An accurate prediction of cardiovascular (CV) risk in patients with Axial Spondyloarthritis (axSpA) is a strong unmet need, as CV risk algorithms poorly perform in these subjects. The aim of this study was to establish whether the persistence of high C-reactive protein (CRP) and high disease activity may be considered predictive factors of CVD in axSpA. 295 patients without personal history of CVD, were consecutively enrolled in this study. To evaluate the relationship between CV events occurrence (fatal and non-fatal) and the persistence of increased CRP levels, ASDAS (Ankylosing Spondylitis Disease Activity Score) > 2.1, and BASDAI (Bath Ankylosing Spondylitis Disease Activity) > 4 during the follow-up, univariable and multivariable Cox Proportional Hazard Models have been performed. During follow-up (we analyzed 10-years retrospective data), 23 patients had a CV event. Multivariable Cox Proportional Hazard Models showed a strong association between CV event and the persistency of increased CRP levels (namely, percentage of visits in which CRP levels were increased) (HR = 1.03; 95%CI 1.015–1.045; p < 0.001), of ASDAS > 2.1 (HR = 1.014, 95%CI 1.000–1.028, p = 0.047), and of BASDAI > 4 (HR 1.019, 95%CI 1.006–1.033, p = 0.006) during follow-up, after adjustment for age, sex, and diabetes. This study suggests that persistence of increased CRP levels and high disease activity may be considered biomarkers to identify those axSpA patients at higher risk of CVD. Innovative axSpA-specific CV risk score, including these variables, have to be developed.
Similar content being viewed by others
Introduction
Spondyloarthritis (SpA) is a heterogeneous group of chronic inflammatory arthropathies, mainly affecting the spine (axial SpA, axSpA) but also involving peripheral joints, entheses and extra-articular sites1. AxSpA group includes both non-radiographic axSpA (nr-axSpa) and ankylosing spondylitis (AS)2. AS is characterized by spinal inflammation, resulting in a limitation of spinal mobility associated with radiological evidence of structural damage of the sacroiliac (SI) joints and spine3. AxSpA deeply affects both physical function and quality of life of patients; moreover, it is well known that the presence of different comorbidities may significantly influence the prognosis of this disease4,5.
In axSpA, as well as in other types of inflammatory arthritis, an increased cardiovascular (CV) risk has been reported6,7; specifically, axSpA patients show a 20–40% increase of CV mortality, when compared to general population. An increased prevalence of traditional CV risk factors, inflammation, and potential adverse effects of drugs, especially nonsteroidal anti- inflammatory drugs (NSAIDs), also contribute to CV comorbidity8,9,10,11.
As far as AS is concerned, European League Against Rheumatism (EULAR) recommends that physicians carry out an annual assessment of CV risk12. An accurate prediction of CV risk is still a strong unmet need, and the early identification of high-risk patients is mandatory to improve the outcome13,14. Several CV risk algorithms have been proposed. The performance and calibration of these algorithms for calculating CV risk in Rheumatoid Arthritis (RA) is still matter of debate15. Different scores, including Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (HeartScore), Reynold’s Risk Score (RRS), and QRISK2, under-estimate the CV risk in RA patients15,16,17. For this reason, EULAR recommended a multiplication factor of 1.5, in RA and other inflammatory arthritis, except for QRISK2, QRISK3, and ASSIGN, already including an intrinsic multiplication factor for RA. Adapting the CV risk algorithms, according to EULAR recommendations, does not provide a significant improvement in discriminative ability, both in Psoriatic Arthritis (PsA) and in AS, when compared to general population18,19,20, showing the huge limitations of EULAR-adapted traditional CV risk algorithms, in patients with AS. In this setting, a Machine-Learning approach showed that the most important variable for assessing CVD risk was the baseline CRP levels19. It is well known that CRP is 1 out of the 5 variables included in the ASDAS (Ankylosing Spondylitis Disease Activity Score)21 activity score, reflecting the role that systemic inflammation plays in maintaining an active disease. On this basis, the aim of this study was to understand whether the persistence of higher CRP levels during the follow-up, ASDAS > 2.1 (active disease) and/or BASDAI (Bath Ankylosing Spondylitis Disease Activity Index)21 > 4 was significantly correlated with the increased CV risk of these patients, independently of other traditional risk factors, to confirm that an optimal control of disease activity may improve the outcome of these patients.
Results
Data from 295 AxSpA patients were analyzed. During follow-up, 23 patients experienced a CV event: 10 cases of myocardial infarction, 4 cases of stable angina, 3 cases of stroke, 3 cases of TIA, 2 cases of HF; 1 fatal CV event was reported. Patient’s demography and characteristics are summarized in Table 1.
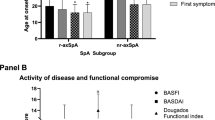
All the patients were Caucasian, with a large preponderance of male (65.4%) and a median age of 47 (40–56) years.
At baseline, patients showed a median BASDAI value of 4.04 (2.1–6) and ASDAS-CRP value of 2.1 (1.3–3). Total cholesterol median value was 189 (168–212) mg/dL, HDL cholesterol median value was 53 (45–63) mg/dL, LDL cholesterol median value was 110 (92–130) mg/dL, and BMI median value of 25.7 (23.4–30.9); finally, 6.4% of patients were diabetic.
Patients experiencing CV events during follow-up were significantly older than patients without CV involvement (58 (52–66) vs. 46 (39–55.5) respectively, p < 0.0001). Furthermore, comparing patients experiencing CV events with the other patients, we observed that the former, at baseline, showed: i. higher prevalence of CVD family history (38.1% vs. 19%, respectively, p = 0.042), ii. higher prevalence of diabetes (17.4% vs. 5.5%, respectively, p = 0.026), iii. higher prevalence of enthesitis (36.4% vs. 18.4%, respectively, p = 0.04), iv. higher CRP levels (7.6 (5.04–13) vs. 3 (1–5.09) mg/dL respectively, p < 0.0001), v. higher diastolic blood pressure (82 (80–87.5) vs. 80 (80–85) mmHg, respectively, p = 0.049), vi. higher prevalence of anti-hypertensive treatment (60.9% vs. 22.4%, respectively, p < 0.0001), vii. higher prevalence of anti-platelets treatment (39.1% vs. 3.7%, respectively, p < 0.0001), viii. higher prevalence of statin treatment (21.7% vs. 5.5%, respectively, p = 0.003).
Finally, we must point out that patients experiencing CV events were affected from a more severe AxSpA, as showed by their higher BASDAI score and ASDAS-CRP score, when compared to the other patients (mean BASDAI 5.9 (4–6.8) vs. 4 (2–6), respectively, p = 0.01; mean ASDAS-CRP:3.2 (3–3.7) vs. 2.1 (1.1–3), respectively, p = 0.0004).
A close correlation between the occurrence of CV events and the increased CV risk prediction scores, assessed according to the original algorithms and according to the EULAR-adapted algorithms, was observed, as reported in Table 2.
Table 3 shows Univariable Cox Proportional Hazard Model. We observed a significant association between the incidence of CV events and these variables at baseline: i. older age (HZ = 1.084, 95% CI 1.04–1.129; p < 0.001), ii. prevalence of diabetes (HZ = 3.137, 95% CI 1.053–9.344; p = 0.04), iii. baseline HDL cholesterol values (HZ = 1.035; 95% CI 1.006–1.064; p = 0.016), iv. presence of enthesitis (HZ = 2.901, 95% CI 1.167–7.21; p = 0.022), v. presence of uveitis (HZ = 6.596, 95% CI 1.902–22.879, p = 0.003). vi. hypertension treatment (HZ = 5.093, 95% CI 2.048–12.66; p < 0.001), vii. anti-platelet treatment (HZ = 10.135, 95% CI 4.265–24.083; p < 0.001), viii. statin treatment (HZ = 3.229, 95% CI 1.176–8.866; p = 0.023). Of interest, as far as the variables regarding the activity of the disease are concerned, a significant association between the occurrence of CV events and: i. CRP value at baseline (HZ = 4.666, 95% CI 1.702–12.788; p = 0.003); ii. the persistence of CRP levels above the normal values (> 5 mg/L) (HZ = 1.033, 95% CI 1.019–1.047, p < 0.001); iii.the percentage of time, during follow-up, in which ASDAS > 2.1 (HZ = 1.016, 95% CI 1.003–1.028, p = 0.012); iv. ASDAS-CRP score at baseline (HZ = 1.624, 95% CI 1.053–2.506, p = 0.028) was observed.
Due to the low number of CV events reported during follow-up, a non-parsimonious approach in multivariable statistical analysis was not possible. Nevertheless, different Multivariable Cox Proportional Hazard Models confirmed a strong association between CV events and: i. the persistency of increased CRP levels during the follow-up; ii. the persistency of high or very high disease activity, as shown by ASDAS-CRP scores; iii. the persistency of high disease activity according to BASDAI, as reported, in detail in Table 4. Due the substantial collinearity between the duration of elevated CRP and the duration of elevated ASDAS-CRP, only a multivariable model comprising age, gender, duration of elevated CRP, and duration of BASDAI > 4 was performed, showing that the duration of elevated CRP and the duration of BASDAI > 4 were significantly associated with CV events (HZ 1.032, 95%CI 1.019–1.046, p < 0.001 and HZ 1.013, 95%CI 1.001–1.026, p = 0.04, respectively).
Discussion
CVD repreents one of the more important causes of morbidity and mortality in patients with axSpA22. An accurate prediction of CV risk may lead to the development of preventive strategies to improve the overall outcome23.
In this study, we analyzed 10-years retrospective data of a large multicenter cohort of axSpA patients, to provide a real-life estimation of the CV burden. At the best of our knowledge, our cohort is one the largest cohorts published in available literature, and this large number of patients partially overcomes the limitations of a retrospective design. Here, we showed that both a persistent high CRP values and/or persistent high disease activity may be considered very sensitive biomarkers of CV events, in patients with axSpA.
Patients experiencing CV events during follow-up significantly differ from the patients without CV complications. CV events mainly affected older patients and patients with higher prevalence of traditional CV risk factors, such as higher prevalence of CVD family history, diabetes, anti-hypertensive, acetylsalicylic acid and statin treatment, diastolic blood pressure, compared to patients without CV events.
Despite of the well-known role of traditional CV risk factors, to induce CV events in general population, our analysis failed to show any possible association among some of these factors and the development of CV events, in axSpA24,25. As far as hypercholesterolemia and BMI are concerned, we did not find any association with the insurgence of new CV events. It is well known that in inflammatory arthritis the so called “lipid paradox”26, modifying the composition of lipids, during inflammation, impairs the predictive role of BMI and lipids profile on CVD. although this paradox has been largely studied in RA, it is a matter of debate if the systemic inflammatory process, observed in the other inflammatory joint diseases, may induce similar effects27. Our study, mirroring what observed in RA, suggest a negative predictive role of lipids concentration on CVD, also in axSpA, whereas this result needs to be confirmed in studies specifically designed. We must point out that axSpA patients experiencing CV events during follow-up showed higher disease activity at the first visit, as confirmed by higher CRP, BASDAI and ASDAS-CRP levels. These data confirm the strong association between systemic chronic inflammation and CV comorbidity and mortality. We may also suggest that the occurrence of fatal or non-fatal CV events may derive from a synergy between both axSpA-related factors and traditional CV risk factors, confirming the need of a strict management of systemic inflammation to improve the long-term outcome of axSpA.
These data confirm the results of our previous work, showing the lower effect of the traditional CV risk factors, used by FRS and other traditional CV risk algorithms, in predicting the development of CV events in patients with axSpA and suggesting the huge limitations of both traditional and EULAR-adapted CV risk algorithms in these patients18.
Furthermore, we were able to show that not only increased CRP and higher disease activity scores at the first visit but also their persistence during the follow-up may be predictive markers of CV events in our patients. In fact, as confirmed by the univariable model, patients experiencing fatal or non-fatal CV events had persistently both higher CRP value and higher disease activity scores.
These data were furtherly confirmed by the multivariable Cox Proportional Hazard Model, highlighting that a persistent high CRP value, as well as a persistent high disease activity score (ASDAS > 2.1 or BASDAI > 4), independent of the patient's age, gender, and main metabolic comorbidities, are strongly correlated with the occurrence of a new fatal or non-fatal CV event.
Taken together, our data strongly suggest the role of the inflammatory process in those axSpA patients with a persistent, poorly controlled, active disease in increasing the risk of CV events.
We are aware of some possible limitations of our study, which did not allow us to fully capture the great heterogeneity of CV risk in axSpA population, despite of the large number of patients enrolled, considering the relative rarity of the disease. First, a non-parsimonious approach to multivariable analysis has been used due to the relatively low number of CV events reported during follow-up. Moreover, due to the observational design of this study, it could be subjected to a number of possible biases. We tried to minimize the main methodological problems by a careful definition of each variable to be assessed. Furthermore, patients with significant missing data, which were considered meaningful for the analyses, were removed. Specifically, patients with missing data in the main outcomes were removed from the analyses. On the other hand, our study would provide a “real-life” estimation of the occurrence of CV events in consecutive patients with axSpA, admitted in 8 Italian Rheumatology Units. Moreover, analyzing a real-life cohort, in which the treatments of axSpA patients were not randomized, we did not analyze any possible association between the effect of anti-rheumatic treatments and the CV outcomes, thus avoiding the risk of a “confounding by indication” bias, a bias deriving when physicians decided to prescribe a more intensive treatment to those patients that, in their opinion, are affected by a more aggressive disease [22]. In this context, the lack of RCTs, specifically designed to evaluate the effect of different drugs in controlling the insurgence of CVD, strongly limits the possibility to reach robust conclusions.
On the other hand, despite the reported limitations, this study strongly confirms, in a very large cohort of patients, that persistent elevation of CRP levels and disease activity scores during follow-up may be considered the most important biomarkers to identify axSpA patients at higher risk of CVD. These results could lead the rheumatologic scientific community to develop a CV risk prediction algorithm in which CRP and disease activity scores may have a more relevant weight, to earlier identify those patients with an increased CV risk, thus implementing preventive strategies to modify the overall outcome of these patients.
Methods
A retrospective analysis of data from AxSpA cohorts of 8 Italian Rheumatology Units has been performed, in accordance with the STROBE guidelines. The observational time frame was 2010–2020, and 295 patients, fulfilling the 2009 ASAS (Assessment of Spondyloarthritis International Society) Criteria28, without a history of CVD before 2010, were consecutively included. Although unequal follow-up time was allowed [the median duration in our cohort was 42 months (24–84)], two visits/year had to be done.
The study was approved by the Ethics Committee of University of Rome “Campus Bio-Medico" (approval number: 60/18 OSS), and conducted according to the Declaration of Helsinki and its amendments. Written informed consent was obtained from all patients. All patients received one visit/semester. Baseline characteristics included: age, gender, weight (kg), height, CRP (mg/L), erythrocyte sedimentation rate (ESR) (mm/h), axial arthritis (grade of radiographic sacroiliitis: 0, I, II, III, IV; non-radiographic sacroiliitis), peripheral arthritis, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI)21, enthesitis, dactylitis, psoriasis, history of inflammatory bowel diseases (IBD), history of uveitis, family history of CVD, smoking status, hypertension, use of antihypertensive medication, use of statins and aspirin, diabetes mellitus, atrial fibrillation, chronic kidney disease stage IV–V, angina or heart attack in a 1st-degree-relative\60 years, systolic blood pressure (SBP), total cholesterol, and high-density lipoprotein cholesterol (HDL-C).
CV events (fatal and non-fatal) included in our database were: sudden cardiac death, coronary artery diseases (CAD) (stable and unstable angina, myocardial infarction), cerebral vascular accident (CVA), transient ischemic attack (TIA), peripheral artery disease (PAD) and heart failure (HF). The baseline 10-year general FRS for CVD, QRISK2, QRISK3, CUORE, RRS, and ASSIGN were calculated using already-published algorithms15,16,17,18,29,30. SCORE algorithm for low-risk countries was used18,31. The default median value 15.89 for the Scottish Index of Multiple Deprivation (SIMD) was used to calculate the ASSIGN score. Two patients were excluded from the analysis due to missing data regarding the main outcomes.
Continuous variables are reported as median (25th-75th percentile), while categorical variables are reported as percentage. Chi2 test was used for analysis of contingency tables, while Mann–Whitney test was used to compare ranks; to evaluate the relationship between CV events and time duration of increased CRP levels (percentage of follow-up visits with CRP levels > 5 mg/L), high or very high disease activity according to ASDAS-CRP (percentage of follow-up visits with ASDAS-CRP > 2.1), and/or high disease activity according to BASDAI (percentage of follow-up visits with BASDAI > 4) univariable and multivariable Cox Proportional Hazard Models were performed. Stata V.14 was used for statistical analysis.
References
Sieper, J., Braun, J., Dougados, M. & Baeten, D. Axial spondyloarthritis. Nat. Rev. Dis. Prim. 11(1), 1–16 (2015).
Chimenti, M. S. et al. Tackling the autoimmune side in Spondyloarthritis: A systematic review. Autoimmun. Rev. 19, 102648 (2020).
Van Der Heijde, D. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991 (2017).
Kelly, K. Ankylosing spondylitis and undifferentiated spondyloarthritis: The relationship between living with these diseases and psychological well-being. Musculoskeletal Care 19, 158–164 (2021).
Hoepken, B., Serrano, D., Harris, K., Hwang, M. C. & Reveille, J. Validation of the ankylosing spondylitis quality of life assessment tool in patients with non-radiographic axial spondyloarthritis. Qual. Life Res. 30, 945–954 (2021).
Ruscitti, P. et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One 12, (2017).
Romano, S. et al. Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr. Rheumatol. Rep. 20, (2018).
Eder, L. & Harvey, P. Cardiovascular morbidity in psoriatic arthritis: What is the effect of inflammation?. J. Rheumatol. 44, 1295–1297 (2017).
England, B. R., Thiele, G. M., Anderson, D. R. & Mikuls, T. R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 361, (2018).
Caso, F. et al. Mediterranean diet and Psoriatic Arthritis activity: A multicenter cross-sectional study. Rheumatol. Int. https://doi.org/10.1007/s00296-019-04458-7 (2019).
Navarini, L. et al. Experimental and investigational pharmacotherapy for psoriatic arthritis: Drugs of the future. J. Exp. Pharmacol. 12, 487–502 (2020).
Agca, R. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76, 17–28 (2016).
Pletcher, M. J. & Moran, A. E. Cardiovascular risk assessment. Med. Clin. North Am. 101, 673–688 (2017).
Ruscitti, P. et al. Subclinical and clinical atherosclerosis in rheumatoid arthritis: Results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res. Ther. 21, (2019).
Arts, E. E. A. et al. Prediction of cardiovascular risk in rheumatoid arthritis: Performance of original and adapted SCORE algorithms. Ann. Rheum. Dis. 75, 674–680 (2016).
D’Agostino, R. B. et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation 117, 743–753 (2008).
Hippisley-Cox, J. et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ 336, 1475–1482 (2008).
Navarini, L. et al. Cardiovascular risk prediction in ankylosing spondylitis: From traditional scores to machine learning assessment. Rheumatol. Ther. https://doi.org/10.1007/s40744-020-00233-4 (2020).
Navarini, L. et al. A machine-learning approach to cardiovascular risk prediction in psoriatic arthritis. Rheumatology (Oxford) https://doi.org/10.1093/rheumatology/kez677 (2020).
Conforti, C. et al. Moderate-to-severe plaque psoriasis, described by PASI ≥10%, can be associated with higher cardiovascular risk according to seven risk algorithms: Results of a 10-year single-center retrospective study and clinical management of psoriatic patients with ca. Dermatol. Ther. https://doi.org/10.1111/dth.14451 (2020).
Zochling, J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing spondylitis disease activity score (ASDAS), ankylosing spondylitis quality of life scale (ASQoL), bath ankylosing spondylitis disease activity index (BASDAI), bath ankylosing spondylitis functional index (BASFI), bath ankylosing spondylitis global score (BAS-G), bath ankylosing spondylitis metrology index (BASMI), dougados functional index (DFI), and health assessment questionnaire for the spondylarthropathies (HAQ-S). Arthritis Care Res. (Hoboken) 63(Suppl 11), S47–S58 (2011).
Shen, J., Shang, Q. & Tam, L. S. Targeting inflammation in the prevention of cardiovascular disease in patients with inflammatory arthritis. Transl. Res. 167, 138–151 (2016).
Manolis, A. S. & Tzioufas, A. G. Cardio-rheumatology: Two collaborating disciplines to deal with the enhanced cardiovascular risk in autoimmune rheumatic diseases. Curr. Vasc. Pharmacol. 18, 533–537 (2020).
Caso, F. et al. Metabolic syndrome and psoriatic arthritis: Considerations for the clinician. Expert Rev. Clin. Immunol. 16, 409–420 (2020).
Peluso, R. et al. Biomarkers of subclinical atherosclerosis in patients with psoriatic arthritis. Open Access Rheumatol. Res. Rev. 11, 143–156 (2019).
Venetsanopoulou, A. I., Pelechas, E., Voulgari, P. V. & Drosos, A. A. The lipid paradox in rheumatoid arthritis: The dark horse of the augmented cardiovascular risk. Rheumatol. Int. 40, 1181–1191 (2020).
Rollefstad, S. et al. Systemic inflammation in patients with inflammatory joint diseases does not influence statin dose needed to obtain LDL cholesterol goal in cardiovascular prevention. Ann. Rheum. Dis. 74, 1544–1550 (2015).
Rudwaleit, M. et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Hippisley-Cox, J., Coupland, C. & Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 357, (2017).
Van Dis, I. et al. Effect of including nonfatal events in cardiovascular risk estimation, illustrated with data from the Netherlands. Eur. J. Prev. Cardiol. 21, 377–383 (2014).
Conroy, R. M. et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 24, 987–1003 (2003).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work, the acquisition, and interpretation of data. All authors contributed to the critical review and revision of the manuscript and approved the final version. All the authors agreed to be accountable for all aspects of the work. L.N. study design, data acquisition, statistical analysis, interpretation of data, writing of the first draft of the paper; D.C. study design, data acquisition, statistical analysis, interpretation of data, writing of the first draft of the paper; A.M. study design, data acquisition, interpretation of data; S.D.D. study design, data acquisition, interpretation of data; A.B. study design, data acquisition, interpretation of data; F.C. study design, data acquisition, interpretation of data; L.C. study design, data acquisition, interpretation of data; M.T. study design, data acquisition, interpretation of data; PR study design, data acquisition, interpretation of data; V.P. study design, data acquisition, interpretation of data; O.B. study design, data acquisition, interpretation of data; A.C. study design, data acquisition, interpretation of data; I.P. study design, data acquisition, interpretation of data; F.C. study design, data acquisition, interpretation of data; M.S.C. study design, data acquisition, interpretation of data; A.D. study design, data acquisition, interpretation of data; F.U. study design, data acquisition, interpretation of data; A.C. study design, data acquisition, interpretation of data; F.P.C. study design, data acquisition, interpretation of data; RP study design, data acquisition, interpretation of data; G.G. study design, data acquisition, interpretation of data; F.C. study design, data acquisition, interpretation of data; PC study design, data acquisition, interpretation of data; R.S. study design, data acquisition, interpretation of data; A.A. study design, data acquisition, interpretation of data; R.G. study design, data acquisition, interpretation of data, interpretation of data, writing of the first draft of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Navarini, L., Currado, D., Marino, A. et al. Persistence of C-reactive protein increased levels and high disease activity are predictors of cardiovascular disease in patients with axial spondyloarthritis. Sci Rep 12, 7498 (2022). https://doi.org/10.1038/s41598-022-11640-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11640-8
This article is cited by
-
Cardiovascular risk in axial spondyloarthritis—a systematic review
Clinical Rheumatology (2023)
-
Cardiovascular comorbidities in spondyloarthritis
Clinical Rheumatology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.