Abstract
The clinical data on the biologic disease-modifying antirheumatic drug (bDMARD) use in late-onset ankylosing spondylitis (LOAS) is limited. Thus, this study aimed to evaluate the drug efficacy and retention rate of bDMARDs in LOAS and compare it to young-onset ankylosing spondylitis (YOAS). Data of patients with AS receiving bDMARDs were extracted from the Korean College of Rheumatology Biologics and Targeted Therapy registry. Patients whose age of onset was > 50 years and ≤ 50 years were classified as having LOAS and YOAS, respectively. Their baseline characteristics and disease-associated parameters were evaluated. Drug efficacy [Ankylosing Spondylitis Disease Activity Score (ASDAS)-clinically important improvement (CII), ASDAS-major improvement (MI), Assessment of SpondyloArthritis International Society (ASAS) 20, and ASAS 40] at 1-year follow-up and drug retention rates were assessed. A total of 1708 patients (comprising 1472 patients with YOAS and 236 patients with LOAS) were included in this analysis. The LOAS group had a lower prevalence among males, lower HLA-B27 positivity and a higher prevalence of peripheral arthritis. Patients with LOAS were more likely to have higher disease-associated parameters (inflammatory reactants, patient global assessment, ASDAS-erythrocyte sedimentation rate, and ASDAS-C-reactive protein). LOAS was negatively associated with achieving ASDAS-CII, ASAS 20, and ASAS 40. The drug retention rate was lower in LOAS; however, the propensity score-matched and covariate-adjusted hazard ratios for bDMARD discontinuation were comparable to YOAS. There were no differences in the drug retention rates based on the type of bDMARD used in LOAS. Inferior clinical efficacy and shorter drug retention time were found in patients with LOAS receiving bDMARDs using real-world nationwide data. There were no differences among each bDMARD type.
Similar content being viewed by others
Introduction
Ankylosing spondylitis (AS) is a prototype of axial spondyloarthritis (axSpA), which is a chronic and systemic inflammatory arthritis of the axial skeleton, associated with radiographic changes1. Symptoms usually begin before the age of 30 years, and patients are often diagnosed before the age of 40 years. Patients with human leukocyte antigen (HLA)-B27 are more likely to develop axSpA2.
Although the Assessment of SpondyloArthritis International Society (ASAS) classification has criteria for inflammatory back pain starting at < 45 years of age3, some patients are diagnosed at an older age due to late symptom onset4. The issue of patients with late-onset AS (LOAS) raises concerns for revising the definition of inflammatory back pain. Unlike young-onset AS (YOAS), LOAS is less likely to be associated with HLA-B27 positivity, inflammatory back pain, alternating buttock pain, radiographic sacroiliitis, and hip involvement4,5,6,7,8. More frequently observed features in LOAS are cervical spine involvement and peripheral arthritis as first manifestations, dactylitis, articular synovitis on power Doppler ultrasound, tenosynovitis or peritendinitis of digit and wrist flexors, and enthesitis of digital collateral ligaments. Additionally, LOAS is associated with female sex, higher levels of acute-phase reactants, nail involvement, and psoriasis4,5,6,9.
AS is usually an early onset disease and is rarely diagnosed in older people. Late-onset disease (symptom onset > 50 years of age) can mimic other rheumatic diseases. This results in a delayed diagnosis of axSpA. Physicians often misdiagnose LOAS as rheumatoid arthritis, polymyalgia rheumatica, and osteoarthritis, as these are more common in the middle age group10. The current entry criterion for inflammatory back pain onset < 45 years in axSpA makes diagnosing LOAS challenging and increases the chances of delayed diagnoses and misdiagnoses.
Biologic disease-modifying antirheumatic drugs (bDMARDs) are emerging as promising treatments for AS. Current ASAS/European League Against Rheumatism (EULAR)11 and American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network guidelines12 recommend their use in patients who do not respond to non-steroidal anti-inflammatory drugs (NSAIDs). The demand for bDMARD prescription would increase if it was shown to have a higher retention rate. However, there is insufficient data on the long-term drug efficacy and retention rate in patients with LOAS10.
This study aimed to identify the efficacy and retention rate of bDMARDs in patients with AS > 50 years of age (LOAS) and compare them with the YOAS group in the Korean population. In addition, we also aimed to evaluate the impact of LOAS on drug efficacy and retention rate.
Methods
Patient population and data collection
Data were extracted from the Korean College of Rheumatology Biologics and Targeted Therapy (KOBIO) Registry. This is a nationwide prospective cohort that collects data on adverse events and efficacy of biologic and targeted synthetic DMARDs (tsDMARDs) in patients with AS, rheumatoid arthritis, and psoriatic arthritis13. A total of 58 tertiary hospital rheumatologic clinics participate in this registry that has been established in 2012. The inclusion criteria for this study were age > 18 years; patients fulfilling the 1984 modified New York (mNY) criteria for AS14; and patients who were initiated on, switched to, or re-started on bDMARDs. Patients with non-radiographic axSpA are not enrolled in the KOBIO registry. The patients with AS were divided into LOAS (symptom onset at age > 50 years) and YOAS (symptom onset at age ≤ 50 years) as per previous studies4,10,15. Data of patients from the beginning of the registry until October 2020 were included in this study and 2023 patients were initially screened. Exclusion criteria were as follows: (1) patients who lacked follow up data and (2) patients who withdraw informed consent. Informed consent was obtained from all participants before enrolment at each centre. This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Konkuk University Medical Centre (KUMC 2020-12-001).
Data analysis
Baseline demographics assessed the features of AS and the laboratory information according to the ASAS classification criteria for axSpA , which are inflammatory back pain, arthritis, enthesitis, uveitis, dactylitis, psoriasis, Crohn’s colitis, good response to NSAIDs, family history of SpA, HLA-B27 positivity, and elevated C-reactive protein (CRP). Disease-associated parameters such as Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Patient’s Global Assessment (PGA), Bath Ankylosing Spondylitis Functional Index (BASFI), Ankylosing Spondylitis Disease Activity Score (ASDAS)-Erythrocyte Sediment Rate (ESR), and ASDAS-CRP were calculated. They were assessed at the 1-year follow-up and changes from baseline were used to assess drug efficacy. This assessment schedule accounted for the fact that longer administration of bDMARDs could precipitate the production of anti-drug antibodies, which could consequently reduce drug efficacy16,17. The total bDMARD retention rate was also evaluated. The ASDAS criteria for improvement were ≥ 1.1 units for clinically important improvement (CII) and ≥ 2.0 units for major improvement (MI)18. The treatment response was assessed using ASAS 20 and ASAS 40. ASAS 20 was defined as an improvement of least 20% and at least 1 unit in 3 out of 4 domains (PGA, pain assessment, BASFI, and inflammation) on a scale of 0 to 10, without worsening of the remaining domain. ASAS 40 was defined as an improvement of at least 40% and at least 2 units in 3 domains on a scale of 0 to 10, without worsening of the remaining domain19. All results of LOAS were compared with those of YOAS.
Statistical analysis
Continuous variables were initially assessed using the Kolmogorov–Smirnov test to define the normality of distribution. Then Student’s t-test and Mann–Whitney U test were used to compare the continuous variables and present them as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Categorical variables were presented as frequencies and percentages. The χ2-test (Mantel–Haenszel χ2-test for more than 2 × 2 categorical data) or Fisher’s exact test were used for comparing categorical variables. Logistic regression identified predictors of ASDAS-CII, ASDAS-MI, ASAS 20, and ASAS 40 achievement at 1-year follow-up, and all known covariates potentially influencing drug efficacy were adjusted for. These include sex, body mass index (BMI), HLA-B27 positivity, ASDAS, BASFI, presence of peripheral arthritis, history of bDMARD use (a dichotomous variable; bDMARDs naïve versus bDMARDs exposed), type of bDMARD, and smoking status20. The results of the logistic regression analysis were presented as odds ratio (OR) with 95% confidence intervals (CI). Drug retention was analysed using Kaplan–Meier plots and the log-rank test. Cox regression analysis was used to determine the associated variates of bDMARD discontinuation. Propensity score (PS) matching was performed as a 1:1 ratio via imputing variables known to influence drug response. These variables include sex, BMI, smoking status, HLA-B27 positivity, ASDAS, BASFI, peripheral arthritis, and history of bDMARD use. A value of p < 0.05 was considered statistically significant. All analyses were performed using SPSS ver. 25 (version 25.0 for Windows, Chicago, IL, USA).
Results
Baseline characteristics
A total of 1708 patients with 1-year follow-up data were included in this study, 1472 of whom belonged to the YOAS group and 236 to the LOAS group. The median age was 29.0 years (22.1–37.1 years) and 57.0 years (IQR 54.0–61.1 years) for the YOAS and LOAS groups, respectively. Male predominance was lower in the LOAS group than in the YOAS group (144 patients [61.0%] vs 1170 [79.5%], p < 0.001). More patients in the LOAS group had never smoked (140 patients [59.3%] vs 719 patients [48.8%], p < 0.001). Patients with LOAS were less likely to be HLA-B27 positive and more likely to have peripheral arthritis. They were also more likely to have higher ESR, CRP, PGA, and ASDAS-ESR/CRP than those with YOAS. In addition, combined co-morbidities (hypertension, ischemic heart disease, hyperlipidemia, osteoporosis, diabetes without complication, hypothyroidism, renal failure, and anemia) were more likely to be present in the LOAS group (Table 1).
Drug efficacy at 1-year follow-up
ASDAS-CII, ASDAS-MI, ASAS 20, and ASAS 40 achievement rates at 1-year follow-up for every type of bDMARD are summarised in Table 2. Overall, ASDAS-CII and ASDAS-MI were not different between the two groups, whereas ASAS 20 and ASAS 40 were higher in the YOAS group than in the LOAS group. In the subgroup analysis of specific bDMARDs, there was no significant difference in terms of ASDAS-CII, ASDAS-MI, ASAS 20, and ASAS 40 between the two groups.
The logistic regression analysis showed that LOAS, female sex, and secukinumab were negatively associated with achieving ASDAS-CII. Higher ASDAS-ESR and biologics naïve was positively associated with achieving ASDAS-CII (OR 2.882 and OR 2.004, respectively, Table 3). Higher ASDAS-ESR, higher BASFI, and biologics naïve were positively associated with achieving ASDAS-MI (Supplementary Table S1). LOAS, female sex, BMI < 18.5 kg/m2, ex-smoker and current smoker statuses, and HLA-B27 negativity were negatively associated with ASAS 20 response. The same variates (except for smoking status and BMI) were also negatively associated with ASAS 40 response. Higher ASDAS-ESR, higher BASFI, and biologics naïve were positively associated with ASAS 20 and ASAS 40 responses (Supplementary Tables S2 & S3).
AS-associated parameters were evaluated using BASDAI, PGA, ESR, CRP, ASDAS-ESR/CRP, and BASFI. The overall scores of disease activity were higher in the LOAS group than in the YOAS group at baseline and 1-year follow-up except for ESR. BASDAI was associated with a lesser decline in the LOAS group than the YOAS group, whereas ESR and CRP were associated with a greater decline in LOAS group. The changes of PGA, ASDAS-ESR/CRP, and BASFI between LOAS and YOAS were not significantly different (Table 4).
Drug retention
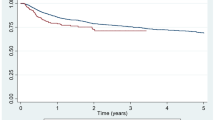
The overall bDMARD retention rates of YOAS and LOAS with median durations of 13.5 months and 11.0 months, respectively are summarised in Fig. 1A. In the LOAS group, the drug retention time was significantly shorter than YOAS (Log-rank test p = 0.003). The drug retention rate of LOAS at 1-year follow-up was 72.5%, 57.9% at 3-year, 48.0% at 5-year, and 36% at 7-year follow-up. In YOAS, the drug retention rate at 1-year follow-up was 81.2%, and the rates of every 2 years were 67.5%, 57.7%, and 46.1%, consecutively. Among patients with LOAS (Fig. 1B), adalimumab continuously had the highest and etanercept had the lowest drug retention rates without any significant differences (adalimumab vs golimumab, Log-rank test p = 0.895; adalimumab vs infliximab, Log-rank test p = 0.621; adalimumab vs etanercept, Log-rank test p = 0.104). In the YOAS group (Fig. 1C), golimumab consistently had the highest drug retention rate during the 7 years (golimumab vs etanercept and infliximab, Log-rank test p < 0.001; golimumab vs adalimumab, Log-rank test p = 0.05). The lowest retention rates were at the 3-year follow-up for secukinumab, 4- to 7-year follow-up for infliximab, without significant differences with other bDMARDs. The most common reasons for discontinuation were inefficacy (LOAS, 38/105 [36.2%]; YOAS, 158/546 [28.9%]) followed by adverse events (LOAS, 31/105 [29.5%]; YOAS, 127/546 [23.3%]) in both groups. In the LOAS group, etanercept demonstrated the highest inefficacy (25.0%) and remission (8.3%) while infliximab had the highest rate of adverse events (16.9%). In the YOAS group, infliximab had the highest inefficiency (15.4%) and etanercept had the highest rate of adverse events (12.4%) (Supplementary Table S4).
Drug retention rates of biologic disease modifying antirheumatic drug (bDMARD). (A) Drug retention rate of overall late onset ankylosing spondylitis (LOAS) and young onset ankylosing spondylitis (YOAS). LOAS showed lower drug retention rate than YOAS. Drug retention rates of each bDMARDs in LOAS (B) and YOAS (C).
Predictors of bDMARD discontinuation
In the Cox regression analysis of drug discontinuation, LOAS was not significant in the PS-matched or the covariate-adjusted results (HR 1.229; 95% CI 0.931–1.622 and HR 1.184; 95% CI 0.934–1.501, respectively). The HR for bDMARD discontinuation was 1.291 for female sex (95% CI 1.030–1.617), 1.381 for current smokers (95% CI 1.122–1.700), 1.473 for HLA-B27 negativity (95% CI 1.155–1.878), 0.719 for adalimumab (95% CI 0.567–0.9110), and 0.570 for golimumab (95% CI 0.433–0.751) [in reference to etanercept] (Table 5).
Discussion
In this study, the efficacy and retention of bDMARDs were examined in patients with LOAS for the first time using a large nationwide registry. Patients with LOAS had a higher prevalence of peripheral arthritis and were less likely to be male or HLA-B27 positive. A lesser decline in BASDAI was observed, in comparison to a greater decline in ESR and CRP in patients with LOAS at the 1-year follow-up compared to those with YOAS. LOAS was negatively associated with achieving ASDAS-CII, ASAS 20, and ASAS 40. The overall drug retention time was shorter in LOAS, without any differences based on bDMARD type. There was no association between LOAS and bDMARD discontinuation.
The use of bDMARDs in older patients with AS is usually associated with poor drug response. In one meta-analysis, older age was negatively associated with achieving BASDAI 50 at the 12-week and 24-week follow-up (OR 0.91, 95% CI 0.84–0.99 and OR 0.98, 95% CI 0.97–0.99, respectively)21. The difference between previous studies and ours is that we demonstrated whether LOAS could impact clinical response and bDMARD retention, whereas previous study showed association between age of bDMARDs initiation and efficacy of bDMARDs. Most of the baseline characteristics of LOAS in our study were consistent with those of previous reports6,15. In our study, LOAS had higher a prevalence of female sex, peripheral arthritis, ESR, and CRP and a lower prevalence of HLA-B27 positivity compared to YOAS. Moreover, disease-associated parameters of PGA and ASDAS-ESR/CRP were higher in LOAS. Inflammatory back pain was not significantly different between YOAS and LOAS. Several of our findings were different from other reports. Skare et al. found that among those with LOAS, there was a lower prevalence of inflammatory low back pain and uveitis, higher prevalence of dactylitis and psoriasis, and comparable CRP levels compared to that of patients with YOAS6. Karaarslan et al. showed that peripheral arthritis was equivalent between LOAS and YOAS7. Endo et al. found that LOAS was less likely to be associated with inflammatory back pain and more likely to be associated with dactylitis. It is important to note that they chose the age of 57 years as the differentiating point between YOAS and LOAS5. These variations may be due to inclusion criteria differences as our study only enrolled bDMARDs users. Other factors such as ethnic background and symptom duration prior to diagnosis may have also contributed to these differences. Our study enrolled 236 patients with LOAS, which is much higher compared to other studies. Since LOAS has several different characteristics than YOAS, it should be considered as a unique subgroup of patients with AS.
Therapeutic options for LOAS are based on recommendations from the management of younger patients11,12,15. Considering the comorbidities are associated with older age, such as peptic ulcer and cardiovascular disease, physicians are often reluctant to prescribe effective drugs such as NSAIDs and TNF-α inhibitors to patients with LOAS. TNF-α inhibitor use in elderly patients with rheumatoid arthritis has shown lower efficacy and increased risk of tuberculosis reactivation, serious infections, and skin cancer22. Nonetheless, as far as treating AS, they have shown high efficacy and safety according to the ASAS/EULAR23. Moreover, secukinumab has been emerging as a highly effective remedy in these patients24,25. Since bDMARDs have been proven efficacious and safe, the demand for this treatment has been increasing. It is important to note that there are mixed results regarding the type of bDMARDs depending on the time of disease onset. Etanercept was found to be well-tolerated and safe in elderly patients with AS26,27, whereas the same demographic were more likely to discontinue infliximab and develop severe pyogenic infections28,29.
In our study, ASDAS-CII and ASDAS-MI for overall bDMARDs were comparable between LOAS and YOAS; however, ASAS 20 and ASAS 40 were significantly more likely to be achieved in YOAS. In the multivariate logistic regression analysis, we demonstrated that bDMARD use in patients with LOAS patients was negatively associated with achieving clinical response (ASDAS-CII, ASAS20, ASAS40). In addition, objective parameters such as ESR and CRP were associated with a greater decline in LOAS, whereas BASDAI, a subjective patient grading score, was associated with a lesser decline in LOAS. Arends et al. reported that the predictors of achieving clinical response to TNF-α inhibitors were increased acute-phase reactants, higher disease activity, higher functional status, younger age, and HLA-B27 positivity30. Rusman et al. reported that female sex was associated with a higher disease activity score, lower quality of life score, and lower response to TNF inhibitors31. Several previous studies have found that male sex is a predictor for clinical response and drug retention32,33,34 despite having more severe disease and radiologic damage31. It is possible that the clinical response of LOAS could be affected by higher prevalence of female sex or higher level of inflammatory reactant. Therefore we performed PS-matched and multivariate covariate adjusted regression analyses, and being LOAS was still associated with lower clinical response. The lower clinical response and shorter retention time may arise as a consequence of multiple co-morbidities, resulting in polypharmacy, that prompts changes in pharmacokinetics and pharmacodynamics of bDMARDs in the LOAS group35,36. However, in vivo measurement of pharmacokinetic and pharmacodynamic interactions between bDMARDs and other medications in patients with LOAS should be unmasked to reveal the mechanisms of lower clinical efficacy and shorter retention time of bDMARDs in the LOAS group.
Similar to previous studies26,27,28, the drug retention time was shorter in the LOAS group than in the YOAS group. However, in the co-variate adjusted and PS-matched results of the Cox regression analysis, LOAS was not significantly associated with drug discontinuation. Although LOAS was negatively associated with achieving clinical efficacy at the 1-year follow- up and had a shorter drug retention time, bDMARDs did diminish disease activity. Therefore, physicians should not hesitate in initiating bDMARDs in LOAS. One of the main strengths of our study is that it was based on real-world data of patients with AS, with relatively large sample size and long follow-up period5,6,7. Moreover, non-radiographic axSpA, which has been shown to be different from AS, was excluded37. The results of our study could be specific and helpful for patients with LOAS considering initiating bDMARDs. In addition, the National Health Insurance Service in Korea strictly monitors the diagnosis of AS (through the mNY criteria14) and the use of bDMARDs for every rheumatic disease. The initiation, continuation, and discontinuation of bDMARDs are carefully observed as physicians check the disease activity in the designated cycle according to the type of bDMARD. These help in drawing accurate conclusions about the efficacy and drug retention rate of bDMARDs in patients with AS.
Smoking is a well-known aggravating factor for the pathogenesis of AS. Current smokers have more observable progression of spinal structural damage38 and lower bDMARDs response39,40. However, none of the previous studies demonstrated significant association between smoking status and drug discontinuation of bDMARDs among patients with AS41,42. In this study, current smokers had higher risk for bDMARDs discontinuation (HR 1.381, 95% CI 1.233–1.700). This could be meaningful in the sense that smoking could be negatively impacting retention of bDMARDs among patients with AS.
There are some limitations to this study. Firstly, data from KOBIO did not represent the entirety of patients with AS, and only patients receiving bDMARDs were included. Therefore, there could be a difference in baseline characteristics between this group and patients with AS not receiving bDMARDs. Secondly, the pharmacokinetics of each bDMARD was not considered. Each drug has its own time of onset for showing effects in the human body. Disease activity scores and treatment response could not demonstrate the potency of each bDMARD at the first follow-up. Thirdly, the choice of bDMARDs was solely dependent on each rheumatologist’s preference, without a specific protocol. Fourthly, there was no data on secukinumab in LOAS, therefore, its efficacy and retention rate could not be evaluated. Finally, although the cut-off values governing the age range for YOAS and LOAS were reckoned from prior studies4,10,15, the cut-off age range for LOAS is still debated. This cut-off age for LOAS (over 50 years old) may consequently influence the results of this study. Despite these limitations, data from the KOBIO registry could reflect the real-world and give useful evidence for starting bDMARDs for patients with LOAS.
In conclusion, patients with LOAS had lower bDMARDs efficacy and drug retention rate when compared to those with YOAS, however being LOAS was not significant factor for predicting drug discontinuation. There were no differences in the efficacy and drug retention rate of each bDMARD in LOAS. These results could be helpful for patients with LOAS who require bDMARD initiation.
References
Sieper, J. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: A guide to assess spondyloarthritis. Ann. Rheum. Dis. 68 Suppl 2, ii1-44. https://doi.org/10.1136/ard.2008.104018 (2009).
Feldtkeller, E., Khan, M. A., van der Heijde, D., van der Linden, S. & Braun, J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol. Int. 23, 61–66. https://doi.org/10.1007/s00296-002-0237-4 (2003).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783. https://doi.org/10.1136/ard.2009.108233 (2009).
Toussirot, E. & Wendling, D. Late-onset ankylosing spondylitis and related spondylarthropathies: clinical and radiological characteristics and pharmacological treatment options. Drugs Aging 22, 451–469. https://doi.org/10.2165/00002512-200522060-00001 (2005).
Endo, Y. et al. Characteristics of late-onset spondyloarthritis in Japan: A retrospective cohort study. Medicine (Baltimore) 98, e14431. https://doi.org/10.1097/md.0000000000014431 (2019).
Skare, T. L. et al. Effect of age at disease onset in the clinical profile of spondyloarthritis: a study of 1424 Brazilian patients. Clin. Exp. Rheumatol. 30, 351–357 (2012).
Karaarslan, A., Yilmaz, H., Aycan, H., Orman, M. & Kobak, S. Demographic, clinical, and laboratory features of Turkish patients with late onset ankylosing spondylitis. Bosn J. Basic Med. Sci. 15, 64–67. https://doi.org/10.17305/bjbms.2015.511 (2015).
Chen, H. A. et al. Clinical, functional, and radiographic differences among juvenile-onset, adult-onset, and late-onset ankylosing spondylitis. J. Rheumatol. 39, 1013–1018. https://doi.org/10.3899/jrheum.111031 (2012).
Montilla, C. et al. Clinical features of late-onset ankylosing spondylitis: comparison with early-onset disease. J. Rheumatol. 39, 1008–1012. https://doi.org/10.3899/jrheum.111082 (2012).
Toussirot, É. Diagnosis and management of late-onset spondyloarthritis: implications of treat-to-target recommendations. Drugs Aging 32, 515–524. https://doi.org/10.1007/s40266-015-0280-y (2015).
van der Heijde, D. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991. https://doi.org/10.1136/annrheumdis-2016-210770 (2017).
Ward, M. M. et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 71, 1599–1613. https://doi.org/10.1002/art.41042 (2019).
Kim, S. K., Choe, J. Y., Lee, S. S. & Shin, K. Body mass index is related with the presence of syndesmophyte in axial spondyloarthritis: Data from the Korean College of Rheumatology BIOlogics (KOBIO) registry. Mod. Rheumatol. 27, 855–861. https://doi.org/10.1080/14397595.2016.1265637 (2017).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368. https://doi.org/10.1002/art.1780270401 (1984).
Toussirot, E. Late-onset ankylosing spondylitis and spondylarthritis: an update on clinical manifestations, differential diagnosis and pharmacological therapies. Drugs Aging 27, 523–531. https://doi.org/10.2165/11315970-000000000-00000 (2010).
Mok, C. C., van der Kleij, D. & Wolbink, G. J. Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases. Clin. Rheumatol. 32, 1429–1435. https://doi.org/10.1007/s10067-013-2336-x (2013).
Strand, V. et al. Immunogenicity of biologics in chronic inflammatory diseases: A systematic review. BioDrugs 31, 299–316. https://doi.org/10.1007/s40259-017-0231-8 (2017).
Machado, P. et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann. Rheum. Dis. 70, 47–53. https://doi.org/10.1136/ard.2010.138594 (2011).
Anderson, J. J., Baron, G., van der Heijde, D., Felson, D. T. & Dougados, M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 44, 1876–1886. https://doi.org/10.1002/1529-0131(200108)44:8%3c1876::Aid-art326%3e3.0.Co;2-f (2001).
Micheroli, R. et al. Effectiveness of secukinumab versus an alternative TNF inhibitor in patients with axial spondyloarthritis previously exposed to TNF inhibitors in the Swiss Clinical Quality Management cohort. Ann. Rheum. Dis. 79, 1203–1209. https://doi.org/10.1136/annrheumdis-2019-215934 (2020).
Maneiro, J. R., Souto, A., Salgado, E., Mera, A. & Gomez-Reino, J. J. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 1, e000017. https://doi.org/10.1136/rmdopen-2014-000017 (2015).
Lahaye, C., Tatar, Z., Dubost, J. J. & Soubrier, M. Overview of biologic treatments in the elderly. Joint Bone Spine 82, 154–160. https://doi.org/10.1016/j.jbspin.2014.10.012 (2015).
Baraliakos, X., van den Berg, R., Braun, J. & van der Heijde, D. Update of the literature review on treatment with biologics as a basis for the first update of the ASAS/EULAR management recommendations of ankylosing spondylitis. Rheumatology (Oxford) 51, 1378–1387. https://doi.org/10.1093/rheumatology/kes026 (2012).
Baeten, D. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713. https://doi.org/10.1016/s0140-6736(13)61134-4 (2013).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548. https://doi.org/10.1056/NEJMoa1505066 (2015).
Fleischmann, R. et al. Long term safety of etanercept in elderly subjects with rheumatic diseases. Ann. Rheum. Dis. 65, 379–384. https://doi.org/10.1136/ard.2005.035287 (2006).
Fleischmann, R. & Iqbal, I. Risk: benefit profile of etanercept in elderly patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis. Drugs Aging 24, 239–254. https://doi.org/10.2165/00002512-200724030-00005 (2007).
Chevillotte-Maillard, H. et al. Survival and safety of treatment with infliximab in the elderly population. Rheumatology (Oxford) 44, 695–696. https://doi.org/10.1093/rheumatology/keh562 (2005).
Maillard, H. et al. Severe pyogenic infections in patients taking infliximab: a regional cohort study. Joint Bone Spine 72, 330–334. https://doi.org/10.1016/j.jbspin.2004.09.003 (2005).
Arends, S., van der Veer, E., Kallenberg, C. G., Brouwer, E. & Spoorenberg, A. Baseline predictors of response to TNF-α blocking therapy in ankylosing spondylitis. Curr. Opin. Rheumatol. 24, 290–298. https://doi.org/10.1097/BOR.0b013e32835257c5 (2012).
Rusman, T., van Vollenhoven, R. F. & van der Horst-Bruinsma, I. E. Gender differences in axial spondyloarthritis: Women are not so lucky. Curr. Rheumatol. Rep. 20, 35. https://doi.org/10.1007/s11926-018-0744-2 (2018).
Arends, S. et al. Baseline predictors of response and discontinuation of tumor necrosis factor-alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res. Ther. 13, R94. https://doi.org/10.1186/ar3369 (2011).
Glintborg, B. et al. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years’ surveillance in the Danish nationwide DANBIO registry. Ann. Rheum. Dis. 69, 2002–2008. https://doi.org/10.1136/ard.2009.124446 (2010).
Kristensen, L. E. et al. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: An observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res. (Hoboken) 62, 1362–1369. https://doi.org/10.1002/acr.20258 (2010).
Mangoni, A. A. & Jackson, S. H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 57, 6–14. https://doi.org/10.1046/j.1365-2125.2003.02007.x (2004).
Jansen, P. A. & Brouwers, J. R. Clinical pharmacology in old persons. Scientifica (Cairo) 2012, 723678. https://doi.org/10.6064/2012/723678 (2012).
Kiltz, U. et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis?. Arthritis Care Res. (Hoboken) 64, 1415–1422. https://doi.org/10.1002/acr.21688 (2012).
Poddubnyy, D. et al. Cigarette smoking has a dose-dependent impact on progression of structural damage in the spine in patients with axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort (GESPIC). Ann. Rheum. Dis. 72, 1430–1432. https://doi.org/10.1136/annrheumdis-2012-203148 (2013).
Glintborg, B. et al. Impact of tobacco smoking on response to tumour necrosis factor-alpha inhibitor treatment in patients with ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Rheumatology (Oxford) 55, 659–668. https://doi.org/10.1093/rheumatology/kev392 (2016).
Shimabuco, A. Y. et al. Factors associated with ASDAS remission in a long-term study of ankylosing spondylitis patients under tumor necrosis factor inhibitors. Adv. Rheumatol. 58, 40. https://doi.org/10.1186/s42358-018-0040-x (2018).
Jeong, H. et al. Drug survival of tumor necrosis factor α inhibitors in patients with ankylosing spondylitis in Korea. Korean J. Intern. Med. 33, 407–416. https://doi.org/10.3904/kjim.2016.042 (2018).
Ki Min, H. et al. Retention rate and effectiveness of secukinumab vs TNF inhibitor in ankylosing spondylitis patients with prior TNF inhibitor exposure. Rheumatology (Oxford) https://doi.org/10.1093/rheumatology/keab245 (2021).
Acknowledgements
Thanks for the KOBIO registry which made data affordable to prepare for journal.
Author information
Authors and Affiliations
Contributions
S.H.K. participated in concept/design, data analysis and interpretation, and writing the original manuscript. H.R.K. participated in concept/design and interpretation. S.H.L. participated in data collection and interpretation. K.S. participated in data collection and interpretation. H.A.K. participated in data collection and interpretation. H.K.M. participated in concept/design, data analysis and interpretation, supervision, and revising the manuscript. All authors participated in drafting, critical revision and final approval of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.H., Kim, HR., Lee, SH. et al. Effectiveness and drug retention of biologic disease modifying antirheumatic drugs in Korean patients with late onset ankylosing spondylitis. Sci Rep 11, 21555 (2021). https://doi.org/10.1038/s41598-021-01132-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01132-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.