Abstract
Vascular endothelial growth factor (VEGF) has been implicated in the pathophysiology of stress-related mental disorders. However, VEGF levels have seldom been compared across mental disorders and never by isoforms. Pathophysiological processes involving leakage of astrocyte-derived extracellular vesicles (EVs) across the blood–brain barrier could be associated with VEGF levels in patients with stress-related mental disorders. This cross-sectional study compared plasma levels of VEGF121, VEGF165, and VEGF121 + VEGF165 (VEGFtotal) in patients with stress-induced exhaustion disorder (SED) (n = 31), patients with major depressive disorder (MDD) (n = 31), and healthy controls (n = 61). It also analyzed the correlation between VEGF and astrocyte-derived EVs in plasma. An enzyme-linked immunosorbent assay (ELISA) was used to measure VEGF121 and VEGF165 in citrate plasma, and flow cytometry was used to measure astrocyte-derived EVs in plasma. The mean concentration of soluble VEGF121 (sVEGF121) was significantly higher in patients with SED than healthy controls (P = 0.043). Mean sVEGF165 was significantly lower in patients with MDD than patients with SED (P = 0.004) or healthy controls (P = 0.037). Mean sVEGFtotal was significantly higher in patients with SED than in patients with MDD (P = 0.021) and also higher in patients with SED than healthy controls (P = 0.040). Levels of sVEGF121 were positively correlated with levels of astrocyte-derived EVs only in patients with SED (P = 0.0128). The same was true of levels of sVEGFtotal and astrocyte-derived EVs (P = 0.0046). Differing levels of VEGF isoforms may reflect different pathophysiological mechanisms in SED and MDD. Further research is needed to better understand the potential roles of VEGF isoforms and astrocyte-derived EVs in mental disorders.
Similar content being viewed by others
Introduction
Acute and chronic stress are important in the development of many mental disorders, such as posttraumatic stress disorder, schizophrenia, and major depressive disorder1. The pathophysiological pathways between stress and these disorders remain unclear2,3, but research suggests that cerebrovascular and endothelial dysfunction may play a role4. Vascular endothelial growth factor (VEGF) is one potential component of the pathways. VEGF is important in angiogenesis and blood vessel formation, has neurotrophic and neuroprotective effects5, and promotes blood–brain barrier permeability6,7. Research shows that it is involved in the pathophysiology of major depressive disorder (MDD)8 and in the effects of antidepressant treatment9,10,11.
Previous studies have examined VEGF concentrations in the peripheral blood of patients with MDD8,12,13,14 and stress-induced exhaustion disorder (SED)15,16,17,18. SED is a clinical condition defined by at least 6 months of chronic stress without sufficient recovery19. It has been classified as a disorder in the Swedish version of the ICD-10 since 2004. In other countries, SED is sometimes classified as a kind of depression (i.e. job stress-induced depression)20 or may be referred to as clinical burnout21 or chronic burnout syndrome22.
Previous studies of VEGF in patients with SED15,16,17,18 and MDD12,13,14 have produced conflicting results, which might be caused, at least in part, by differing study designs. For instance, studies may have measured different isoforms of the VEGF family. VEGF121 and VEGF165 are the two major isoforms in mammals23. VEGF121, the main isoform in circulating blood, probably plays a minor role in angiogenesis but a major role in vascular permeability24. The heavier isoform, VEGF165, has higher mitogenic potential and appears to induce angiogenesis25,26,27,28. Previous research has investigated plasma levels of VEGF in people with different mental disorders14, but to the best of our knowledge, no previous research has compared plasma concentrations of different isoforms of VEGF in patients with different mental disorders.
Additionally, research indicates that VEGF mediates increased permeability of the blood–brain barrier (BBB)29,30,31,32. Particles that may indicate increased BBB permeability have been identified in the peripheral blood of patients with SED, and to a lesser extent, MDD33. These particles, astrocyte-derived extracellular vesicles (EVs), are important in intercellular communication and are released during cellular activation or death34,35. They include both smaller exosomes and larger microvesicles (sometimes called microparticles)35. Because the presence of elevated levels of EVs in peripheral blood may be related to stress and BBB permeability33, we hypothesized that levels of VEGF in plasma would be correlated with levels of EVs in plasma.
To better understand the role of VEGF in stress-related mental disorders, the present study aimed to compare plasma levels of different isoforms of VEGF, including VEGF121, VEGF165, and VEGF121 + VEGF165 (VEGFtotal) in patients with SED, patients with MDD, and healthy controls. We also analyzed the correlation between levels of VEGF and astrocyte-derived EVs in plasma.
Materials and methods
Study population
Between 2014 and 2018, patients with common mental disorders treated at the psychiatric outpatient clinic at Ersta Hospital, Stockholm, were consecutively recruited to the study. During 2018, patients with MDD attending an outpatient clinic in Stockholm, the Capio Anxiety and Depression Clinic, were also consecutively recruited. Recruitment continued until the SED and MDD groups reached predefined sizes calculated on the basis of a pilot study.
Patients who fulfilled the diagnostic criteria for SED19 (Table 1) or MDD (DSM-5) were asked to participate in the study by their physician, occupational therapist, or nurse. Inclusion criteria were ongoing SED or MDD diagnosed less than 3 months prior to inclusion, age 18–65 years, the ability to understand Swedish, and the capacity to undergo 30–40 min of clinical examination. Patients with SED could also fulfill criteria for depression if the physician considered the depressive symptoms secondary to SED. Exclusion criteria were anemia, vitamin B12 deficiency, subclinical thyroid disease, alcohol overconsumption, and a somatic (e.g. anemia) or psychiatric (e.g. post-traumatic stress disorder) diagnosis that better explained the patient’s symptoms.
Physician examination and blood analyses were used to check for inclusion and exclusion criteria. Patients were diagnosed with SED or MDD by their physician. To exclude other psychiatric diagnoses, patients underwent the Swedish version of the Mini International Neuropsychiatic Interview (M.I.N.I.) 6.0.036,37. M.I.N.I. was administered by a member of the research staff, a clinic psychologist, or a physician, all of whom were familiar with the instrument. Clinical characteristics such as height and weight were also gathered, as was information on use of antidepressant medication. 24 patients with MDD, 25 patients with SED, and no healthy controls had antidepressant medication.
Controls, matched as closely as possible for age and sex, were chosen from a group of 165 healthy subjects described in detail in a previous publication17. In brief, the healthy subjects were recruited by Statistics Sweden, Sweden’s national statistics agency, in 2009. Inclusion criteria were being between the ages of 28 and 55 and being a permanent resident of the Stockholm area. The exclusion criteria were current or previous physical and/or mental disorders. Physician examination, including with the Structured Clinical Interview for Mental Disorders (SCID)38, and blood samples was used to check for inclusion and exclusion criteria. The healthy controls provided blood samples.
Symptom rating scales
Two self-rated scales were used to measure depressive and cognitive symptoms. Severity of depressive symptoms was assessed with the 9-item self-reported version of the Montgomery-Asberg Depression Rating Scale (MADRS-S). Higher scores on the scale reflect more severe symptoms39,40. The 25-item Cognitive Failures Questionnaire (CFQ) was used to quantify cognitive problems. The CFQ measures self-reported cognitive failures in daily life. High scores indicate a high degree of subjective cognitive problems41,42. Patients completed the CFQ; people in the control group did not.
Sample collection
Blood samples were obtained from healthy controls in 2009 in accordance with a standardized protocol presented elsewhere33. In summary, all participants were asked to abstain from consuming alcohol prior to their blood test. They were also asked to fast from midnight on the previous night, to avoid physical activity prior to blood sampling, and to put off sampling if they had any symptoms of infection. Blood samples were drawn in the morning after the participant had rested for at least 15 min. The samples were drawn into citrated tubes through direct venepuncture from an antecubital vein using a 21G sampling needle. They were centrifuged within 1 h at 2000g for 20 min at room temperature and subsequently stored at − 80 °C as platelet poor plasma. Blood samples were obtained from patients at inclusion using the same procedure as for controls. Samples from patients and controls were analyzed in the same batch at Danderyd University Hospital, Sweden.
Measurement of sVEGF121 and sVEGF165
Soluble(s) VEGF121 and sVEGF165 were measured with an enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s recommendations (LSBio, Seattle, WA, USA). Citrate plasma was thawed in a water bath for approximately 5 min at 37 °C. Standards and samples were added in pre-coated wells with either sVEGF121 or sVEGF165. After incubation (1 h at 37 °C) and wash, the detection antibody was added. After a second incubation (1 h at 37 °C) and a second wash, conjugate was added, and the plates were incubated for 30 min at 37 °C. The plates were washed, Tetramethylbenzidine substrate was added, and after 20 min of incubation (37 °C), 50 µl of stop solution was added to each well. Optical density was read using a microplate reader set to 450 nm with the correction set to 570 nm. Intra-assay and inter-assay variation were less than 15% for both assays. Data are presented as picograms (pg)/ml sVEGF121, sVEGF165, and sVEGFtotal (VEGF121 plus VEGF165).
Analysis of astrocyte-derived extracellular vesicles
A detailed description of the analysis of EVs has been presented elsewhere33. In summary, EVs were measured in platelet poor plasma collected as described above. After thawing, 20 µl of samples were incubated with markers specific to the astrocytes aquaporin-4 (AQP4) and glial fibrillary acidic protein (GFAP). These markers were anti-Aquaporin-4 Dylight 488 (corresponding to Human Aquaporin 4 aa 50–150, Abcam, Cambridge, UK), and/or anti-GFAP Dylight 755 (Abcam, Cambridge, UK). The samples were measured on a Beckman Gallios instrument (Beckman coulter, Brea, CA, USA), a flow cytometer with the threshold set to forward scatter.
Astrocyte-derived EVs were defined by size (forward/side scatter characteristics, ≤ 0.9 µm) and expression of AQP4 and GFAP. EVs were grouped into three subgroups: single AQP4 expression, single GFAP expression, and double expression (AQP4 and GFAP) Results are presented as EVs/µl plasma. Both AQP-4 and GFAP are predominately expressed astrocytes43 but can also be found in detected in smaller quantities elsewhere44,45,46. Thus, we chose to include double-positive EVs (i.e. AQP4-positive and GFAP-positive) in the analysis because we could be more confident that they derived from astrocytes. Results are presented as EVs/µl plasma.
Statistical methods
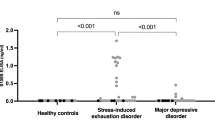
Clinical and demographic characteristics were compared between groups, as appropriate, with non-parametric or parametric tests. The Kruskal–Wallis test was used for non-parametric tests of three groups and the Mann–Whitney test for two groups. ANOVA was used for parametric tests of three groups and un-paired t-tests for parametric tests of two groups. Bonferroni was used as a post-hoc test of Kruskal–Wallis and ANOVA tests (Table 2). Plasma levels of sVEGF isoforms (sVEGF121, sVEGF165, and sVEGFtotal) were compared between groups with ANOVA (after log-transformation) together with Bonferroni post-hoc test (Fig. 1). Correlations between EV subtypes (AQP4-positive, GFAP-positive, and double-positive) and sVEGF isoforms (sVEGF121, sVEGF165, and sVEGFtotal) were investigated with linear regression after log-transformation (Table 3). Correlations between symptom rating scales and sVEGF and EVs were investigated by Pearson Correlation tests. P values of ≤ 0.05 were considered significant. Statistical analysis was performed with SPSS Statistics (IBM SPSS Statistics for Windows, v 26.0. Armonk, NY: IBM Corp.) and JMP software (SAS Institute, v12.0, Cary, North Carolina, USA).
Ethics
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden, http://www.epn.se/en/start/, d.nr. 2008/0061-31, 2014/585-31/1, 2016/1239-32, 2017/2088-32. It was carried out in accordance with the recommendations of the Local Ethics Committee, Karolinska Institutet, Stockholm, and the Declaration of Helsinki. All participants received verbal and written information about the study and provided written informed consent prior to participation. Data were pseudonymized before they were linked and analyzed.
Results
Clinical and demographic characteristics
The study included 31 patients with SED, 31 patients with MDD, and 61 healthy controls. The three groups were similar in age, sex, and body mass index (Table 2). Differing mean MADRS-S scores reflected the participants’ diagnoses or lack thereof. They were highest in patients with MDD, second highest in patients with SED, and lowest in healthy controls (P = 0.001). Higher mean CFQ scores were observed in patients with SED than in patients with MDD (P = 0.025).
In those with SED, there was no significant difference in plasma levels of sVEGF121, sVEGF165, or sVEGFtotal in patients who received and who did not receive antidepressants (P = 0.385 for sVEGF121, P = 0.957 for sVEGF165 and P = 0.746 for sVEGFtotal). In addition, there were no significant differences in concentration of leucocytes, erythrocytes, or platelets between patients with SED who received antidepressant medication and patients with SED who did not receive such medication.
Isoforms of VEGF
There were statistically significant differences between the groups (Fig. 1). The mean plasma concentration of sVEGF121 was significantly higher in patients with SED (15.4, SD ± 1.9 pg/ml) than in healthy controls (8.7, SD ± 1.4 pg/ml), P = 0.043. Mean sVEGF165 was significantly lower in patients with MDD (9.5, SD ± 1.8 pg/ml) than in patients with SED (15.9, SD ± 1.8 pg/ml, P = 0.004) or healthy controls (12.3, SD ± 1.2 pg/ml, P = 0.037). The largest differences between the groups were observed in plasma levels of sVEGFtotal. These levels were significantly higher in patients with SED (31.2, SD ± 3.3 pg/ml) than in patients with MDD (21.1, SD ± 3.4 pg/ml, P = 0.021) and also higher in patients with SED than in healthy controls (21.1, SD ± 2.4 pg/ml, P = 0.040). There was a significant positive correlation between MADRS-S scores and levels of AQP4 (r = 0.196 P = 0.042) and between MADRS-S scores and levels of GFAP (r = 0.210 P = 0.029). There was no significant correlation between MADRS-S scores and levels of VEGFtotal or between scores on the CFQ symptom rating scale and VEGF isoforms or EVs.
Correlation between sVEGF isoforms and astrocyte-derived extracellular vesicles
In patients with SED, there was a significant positive correlation between the plasma concentration of sVEGF121 and astrocyte-derived EVs concurrently expressing AQP4 and GFAP (P = 0.0128), as well as between sVEGFtotal and astrocyte-derived EVs concurrently expressing these markers (P = 0.0046) (Table 3).
Discussion
In the present study, we demonstrated that patients with SED had sVEGF121 levels that were significantly higher than the levels in healthy controls. Patients with MDD had significantly lower levels of sVEGF165 than either patients with SED or healthy controls. Additionally, we observed that levels of sVEGF121 and of sVEGFtotal were positively correlated with plasma levels of astrocyte-derived EVs in patients with SED but not in patients with MDD or healthy controls.
It is challenging to compare our findings with those of previous studies on the connection between VEGF and either SED or MDD because in prior work, results have not been reported by VEGF isoform. The results of previous studies of VEGF (isoforms not reported) in people with SED differ. Some researchers have found higher levels of VEGF in people with SED than healthy controls17,18, others have found similar levels15, and still others have found lower levels16. Findings of elevated levels of VEGF might be related to measuring VEGF121, and findings of no difference, to measuring VEGF165. Different isoforms are thus a potential cause of variation in plasma levels of VEGF across studies.
Differences in methodology probably contributed to the heterogeneity in study findings. Choice of protein assay15 can lead to significantly different results, as can the decision to use ELISA methods or multiplex assays47 and the decision to measure VEGF in plasma or serum48. Measuring circulating extracellular VEGF in plasma is often more accurate than measuring it in serum49 because peripheral VEGF can be stored in blood cells such as platelets and released during the clotting process. Centrifugation time and speed may also affect VEGF concentrations50.
Our finding of significantly lower levels of sVEGF165 in patients with MDD than in healthy controls contradicts the findings of two previous meta-analyses, which found that levels of sVEGF (isoforms not reported) were higher in the peripheral blood of patients with MDD than in the peripheral blood of healthy controls12,13. However, a 2020 meta-analysis14 that had a larger sample size (> 4000 participants; isoforms not reported) examined the levels of VEGF in people with different psychiatric disorders, including MDD, and healthy controls. The analysis showed significant elevation of blood levels of VEGF, but only in patients with MDD who were treated with antidepressants. Levels of VEGF may also vary across the course of a disorder17, which would mean that the timing of measurement is important, and inclusion and exclusion criteria could be crucial.
Different isoforms, disease severity, and antidepressant treatment could all play a role in the inconsistent findings regarding VEGF in people with mental disorders, and it is still unclear whether changes in VEGF levels are part of the causal pathway in depression, a result of depression13, or both.
If confirmed in other studies, our finding of elevated levels of VEGF121 in the peripheral blood of patients with SED may help illuminate physiological changes associated with the disorder. Previous research from our group suggests that patients with SED may have increased BBB permeability33. In that study, we found raised levels of astrocyte-derived EVs in the peripheral blood of patients with SED. Those findings were consistent with other researchers’ findings of leakage or release of astrocyte-derived EVs through the blood brain barrier in patients with traumatic brain injury43,51. The sVEGF121 isoform is the one most closely associated with increased vascular permeability52. Thus, raised levels of sVEGF121 in the peripheral blood of patients with SED could help explain increased BBB permeability in these patients.
In the current study, we also found that levels of sVEGF121 were correlated with levels of circulating astrocyte-derived EVs in patients with SED. This finding is consistent with the hypothesis that sVEGF121 is involved in the physiological changes that result in increased levels of astrocyte-derived EVs in the peripheral blood of patients with SED.
Limitations
This study has several limitations. First, blood samples were obtained from patients and healthy controls at different times, and differences in storage times could have affected the results. However, the blood sampling routines were the same in both groups, and the samples were analyzed in the same batches. Second, patients with SED and MDD may have been in different stages of their diseases. To minimize this diversity, all patients included in the study, regardless of diagnosis, had to be diagnosed less than three months before blood sampling. Third, the cross-sectional design makes it impossible to draw causal inferences on the basis of the observed associations.
Conclusions
Our study indicates that plasma levels of VEGF isoforms vary in patients with SED, patients with MDD, and healthy controls. These heterogeneous levels may reflect different pathophysiological mechanisms in SED and MDD. Further research is needed to better understand the potential roles of isoforms of VEGF in mental disorders, including whether stress can influence processes involving VEGF and BBB permeability in people with stress-related mental disorders such as SED and MDD.
Data availability
The dataset generated and analyzed during the study is available from the corresponding author on reasonable request.
Change history
23 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-37259-x
References
McEwen, B. S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. NY Acad. Sci. 1032, 1–7. https://doi.org/10.1196/annals.1314.001 (2004).
Theorell, T. et al. A systematic review including meta-analysis of work environment and depressive symptoms. BMC Public Health 15, 738. https://doi.org/10.1186/s12889-015-1954-4 (2015).
OConghaile, A. & DeLisi, L. E. Distinguishing schizophrenia from posttraumatic stress disorder with psychosis. Curr. Opin. Psychiatry 28, 249–255. https://doi.org/10.1097/yco.0000000000000158 (2015).
Burrage, E., Marshall, K. L., Santanam, N. & Chantler, P. D. Cerebrovascular dysfunction with stress and depression. Brain Circ. 4, 43–53. https://doi.org/10.4103/bc.bc_6_18 (2018).
Sondell, M., Sundler, F. & Kanje, M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 12, 4243–4254. https://doi.org/10.1046/j.0953-816x.2000.01326.x (2000).
Mayhan, W. G. VEGF increases permeability of the blood-brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am. J. Physiol. 276, C1148-1153. https://doi.org/10.1152/ajpcell.1999.276.5.C1148 (1999).
Zhang, Z. G. et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 106, 829–838. https://doi.org/10.1172/jci9369 (2000).
Clark-Raymond, A. & Halaris, A. VEGF and depression: A comprehensive assessment of clinical data. J. Psychiatr. Res. 47, 1080–1087. https://doi.org/10.1016/j.jpsychires.2013.04.008 (2013).
Deyama, S. & Duman, R. S. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol. Biochem. Behav. 188, 172837. https://doi.org/10.1016/j.pbb.2019.172837 (2020).
Nowacka, M. M. & Obuchowicz, E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: A new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 46, 1–10. https://doi.org/10.1016/j.npep.2011.05.005 (2012).
Fournier, N. M. & Duman, R. S. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: Implications for the pathophysiology and treatment of depression. Behav. Brain Res. 227, 440–449. https://doi.org/10.1016/j.bbr.2011.04.022 (2012).
Tseng, P. T., Cheng, Y. S., Chen, Y. W., Wu, C. K. & Lin, P. Y. Increased levels of vascular endothelial growth factor in patients with major depressive disorder: A meta-analysis. Eur. Neuropsychopharmacol. 25, 1622–1630. https://doi.org/10.1016/j.euroneuro.2015.06.001 (2015).
Carvalho, A. F. et al. Peripheral vascular endothelial growth factor as a novel depression biomarker: A meta-analysis. Psychoneuroendocrinology 62, 18–26. https://doi.org/10.1016/j.psyneuen.2015.07.002 (2015).
Pu, J. et al. Vascular endothelial growth factor in major depressive disorder, schizophrenia, and bipolar disorder: A network meta-analysis. Psychiatry Res. 292, 113319. https://doi.org/10.1016/j.psychres.2020.113319 (2020).
Jonsdottir, I. H., Hägg, D. A., Glise, K. & Ekman, R. Monocyte chemotactic protein-1 (MCP-1) and growth factors called into question as markers of prolonged psychosocial stress. PLoS ONE 4, e7659. https://doi.org/10.1371/journal.pone.0007659 (2009).
Sjörs Dahlman, A., Blennow, K., Zetterberg, H., Glise, K. & Jonsdottir, I. H. Growth factors and neurotrophins in patients with stress-related exhaustion disorder. Psychoneuroendocrinology 109, 104415. https://doi.org/10.1016/j.psyneuen.2019.104415 (2019).
Wallensten, J. et al. Possible biomarkers of chronic stress induced exhaustion—A longitudinal study. PLoS ONE 11, e0153924. https://doi.org/10.1371/journal.pone.0153924 (2016).
Åsberg, M. et al. Novel biochemical markers of psychosocial stress in women. PLoS ONE 4, e3590. https://doi.org/10.1371/journal.pone.0003590 (2009).
Åsberg, M., Glise, K., Herlofson, J., Jacobsson, L., Krakau, I. & Nygren, Å et al. Socialstyrelsen. Utmattningssyndrom Stressrelaterad psykisk ohälsa. ISBN 91-7201-786-4 (Artikel nr: 2003-123-18).
Rydmark, I. et al. Neuroendocrine, cognitive and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biol. Psychiat. 60, 867–873. https://doi.org/10.1016/j.biopsych.2006.04.029 (2006).
Grossi, G., Perski, A., Osika, W. & Savic, I. Stress-related exhaustion disorder–clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scand. J. Psychol. 56, 626–636. https://doi.org/10.1111/sjop.12251 (2015).
Sandstrom, A., Rhodin, I. N., Lundberg, M., Olsson, T. & Nyberg, L. Impaired cognitive performance in patients with chronic burnout syndrome. Biol. Psychol. 69, 271–279. https://doi.org/10.1016/j.biopsycho.2004.08.003 (2005).
Bates, D. O. et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 62, 4123–4131 (2002).
Zhang, Y., Furumura, M. & Morita, E. Distinct signaling pathways confer different vascular responses to VEGF 121 and VEGF 165. Growth Factors 26, 125–131. https://doi.org/10.1080/08977190802105909 (2008).
Finley, S. D. & Popel, A. S. Predicting the effects of anti-angiogenic agents targeting specific VEGF isoforms. Aaps J. 14, 500–509. https://doi.org/10.1208/s12248-012-9363-4 (2012).
Vempati, P., Popel, A. S. & Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 25, 1–19. https://doi.org/10.1016/j.cytogfr.2013.11.002 (2014).
Ferrara, N. Binding to the extracellular matrix and proteolytic processing: Two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 21, 687–690. https://doi.org/10.1091/mbc.e09-07-0590 (2010).
Ruhrberg, C. Growing and shaping the vascular tree: Multiple roles for VEGF. BioEssays 25, 1052–1060. https://doi.org/10.1002/bies.10351 (2003).
Davis, B. et al. Role of vasodilator stimulated phosphoprotein in VEGF induced blood-brain barrier permeability in endothelial cell monolayers. Int. J. Dev. Neurosci. 28, 423–428. https://doi.org/10.1016/j.ijdevneu.2010.06.010 (2010).
Valable, S. et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J. Cereb. Blood Flow Metab. 25, 1491–1504. https://doi.org/10.1038/sj.jcbfm.9600148 (2005).
Lange, C., Storkebaum, E., de Almodóvar, C. R., Dewerchin, M. & Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 12, 439–454. https://doi.org/10.1038/nrneurol.2016.88 (2016).
Van Dyken, P. & Lacoste, B. Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Front. Neurosci. 12, 930. https://doi.org/10.3389/fnins.2018.00930 (2018).
Wallensten, J. et al. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: A cross-sectional study. Sci. Rep. 11, 2009. https://doi.org/10.1038/s41598-021-81453-8 (2021).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. https://doi.org/10.1038/nrm.2017.125 (2018).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 7, 1535750. https://doi.org/10.1080/20013078.2018.1535750 (2018).
Allgulander, C. Humble, M. Andersch, S. Ågren H. Karolinska institutet—Stockholm, Sahlgrenska akademin—Göteborg. M.I.N.I. Mini Internationell Neuropsykiatrisk Intervju Svensk version 6.0.0.
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl 20), 22–33 (1998) (Quiz 34–57).
Ekselius, L., Lindström, E., von Knorring, L., Bodlund, O. & Kullgren, G. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr. Scand. 90, 120–123. https://doi.org/10.1111/j.1600-0447.1994.tb01566.x (1994).
Montgomery, S. A. & Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Mental Sci. 134, 382–389. https://doi.org/10.1192/bjp.134.4.382 (1979).
Svanborg, P. & Asberg, M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatr. Scand. 89, 21–28. https://doi.org/10.1111/j.1600-0447.1994.tb01480.x (1994).
Broadbent, D. E., Cooper, P. F., FitzGerald, P. & Parkes, K. R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 21(Pt 1), 1–16. https://doi.org/10.1111/j.2044-8260.1982.tb01421.x (1982).
Rast, P., Zimprich, D., Van Boxtel, M. & Jolles, J. Factor structure and measurement invariance of the cognitive failures questionnaire across the adult life span. Assessment 16, 145–158. https://doi.org/10.1177/1073191108324440 (2009).
Nekludov, M., Bellander, B. M., Gryth, D., Wallen, H. & Mobarrez, F. Brain-derived microparticles in patients with severe isolated TBI. Brain Inj. 31, 1856–1862. https://doi.org/10.1080/02699052.2017.1358395 (2017).
Hainfellner, J. A. et al. Fibroblasts can express glial fibrillary acidic protein (GFAP) in vivo. J. Neuropathol. Exp. Neurol. 60, 449–461. https://doi.org/10.1093/jnen/60.5.449 (2001).
Riol, H., Tardy, M., Rolland, B., Lévesque, G. & Murthy, M. R. Detection of the peripheral nervous system (PNS)-type glial fibrillary acidic protein (GFAP) and its mRNA in human lymphocytes. J. Neurosci. Res. 48, 53–62 (1997).
Takata, K., Matsuzaki, T. & Tajika, Y. Aquaporins: Water channel proteins of the cell membrane. Prog. Histochem. Cytochem. 39, 1–83. https://doi.org/10.1016/j.proghi.2004.03.001 (2004).
Dupuy, A. M. et al. Performance evaluation of human cytokines profiles obtained by various multiplexed-based technologies underlines a need for standardization. Clin. Chem. Lab Med. 51, 1385–1393. https://doi.org/10.1515/cclm-2012-0648 (2013).
Banks, R. E. et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br. J. Cancer 77, 956–964. https://doi.org/10.1038/bjc.1998.158 (1998).
Okamoto, Y. et al. Determination of age-related changes in human vascular endothelial growth factor in the serum and urine of healthy subjects. Clin. Lab. 54, 173–177 (2008).
Hormbrey, E. et al. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: Is the current literature meaningful?. Clin. Exp. Metastasis 19, 651–663. https://doi.org/10.1023/a:1021379811308 (2002).
Agoston, D. V. & Elsayed, M. Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front. Neurol. 3, 107. https://doi.org/10.3389/fneur.2012.00107 (2012).
Tepper, O. M. et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105, 1068–1077. https://doi.org/10.1182/blood-2004-03-1051 (2005).
Acknowledgements
Funding for this work came from Skandia; Söderström Königska Foundation; the Foundation for Rehabilitation and Medical Research, Karolinska Institutet (to Kristian Borg); and Region Stockholm, including the Department of Rehabilitation Medicine at Danderyd University Hospital and the Network Health Care (Nätverkssjukvård) project. The authors thank Kimberly Kane, medical writer certified, of Region Stockholm’s Academic Primary Health Care Centre for help with editing the language used in the manuscript.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
J.W., A.N., M.Å., K.B., F.M., and A.W. conceived and designed the study. J.W., A.B., and A.W. acquired the data. J.W., A.N., F.M., and A.B. analyzed and interpreted the data. J.W., A.N., and F.M. drafted the manuscript. All authors revised the manuscript for important intellectual content, approved the final version for publication, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Materials and methods as well as the Discussion sections. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wallensten, J., Mobarrez, F., Åsberg, M. et al. Isoforms of soluble vascular endothelial growth factor in stress-related mental disorders: a cross-sectional study. Sci Rep 11, 16693 (2021). https://doi.org/10.1038/s41598-021-96313-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96313-8
This article is cited by
-
Psychological Treatment of Exhaustion Due to Persistent Non-Traumatic Stress: A Scoping Review
International Journal of Behavioral Medicine (2024)
-
Extracellular vesicle approach to major psychiatric disorders
European Archives of Psychiatry and Clinical Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.