Abstract
Thermophilic biohydrogen production by dark fermentation from a mixture (1:1) of C5 (arabinose) and C6 (glucose) sugars, present in lignocellulosic hydrolysates, and from Sargassum sp. biomass, is studied in this work in batch assays and also in a continuous reactor experiment. Pursuing the interest of studying interactions between inorganic materials (adsorbents, conductive and others) and anaerobic bacteria, the biological processes were amended with variable amounts of a zeolite type-13X in the range of zeolite/inoculum (in VS) ratios (Z/I) of 0.065–0.26 g g−1. In the batch assays, the presence of the zeolite was beneficial to increase the hydrogen titer by 15–21% with C5 and C6-sugars as compared to the control, and an increase of 27% was observed in the batch fermentation of Sargassum sp. Hydrogen yields also increased by 10–26% with sugars in the presence of the zeolite. The rate of hydrogen production increased linearly with the Z/I ratios in the experiments with C5 and C6-sugars. In the batch assay with Sargassum sp., there was an optimum value of Z/I of 0.13 g g−1 where the H2 production rate observed was the highest, although all values were in a narrow range between 3.21 and 4.19 mmol L−1 day−1. The positive effect of the zeolite was also observed in a continuous high-rate reactor fed with C5 and C6-sugars. The increase of the organic loading rate (OLR) from 8.8 to 17.6 kg m−3 day−1 of COD led to lower hydrogen production rates but, upon zeolite addition (0.26 g g−1 VS inoculum), the hydrogen production increased significantly from 143 to 413 mL L−1 day−1. Interestingly, the presence of zeolite in the continuous operation had a remarkable impact in the microbial community and in the profile of fermentation products. The effect of zeolite could be related to several properties, including the porous structure and the associated surface area available for bacterial adhesion, potential release of trace elements, ion-exchanger capacity or ability to adsorb different compounds (i.e. protons). The observations opens novel perspectives and will stimulate further research not only in biohydrogen production, but broadly in the field of interactions between bacteria and inorganic materials.
Similar content being viewed by others
Introduction
Hydrogen is a good energy carrier and an alternative to fossil fuels since it can be produced from renewable sources, it has a high efficiency of conversion to usable power, and non-polluting nature1. Dark fermentation is a biological process where hydrogen and carbon dioxide are produced by anaerobic microorganisms in one step2. This process is environmentally friendly and one of the most promising and sustainable processes for hydrogen production. Indeed, dark fermentation can be applied for the valorisation of organic wastes such as marine biomass3. Sargassum sp. is a genus of brown macroalgae that has a global occurrence and it is considered a marine waste. This macroalgae is composed by easily hydrolysable sugars and proteins and has low fractions of lignin and high fractions of hemicellulose as well as a good hydrolysis yield4. Due to its characteristics, Sargassum sp. can be a suitable candidate for biohydrogen production, turning dark fermentation more competitive and economically sustainable3.
Although dark fermentation is a promising process, the production of large quantities of soluble metabolic products, results in low hydrogen production efficiencies and low hydrogen yields (around 25%)5. Nevertheless, biological processes efficiencies under anaerobic conditions could be improved by the addition of different types of materials6. The mechanism of action of these materials depends on several properties such as electric conductivity or surface area. Zeolites is an aluminosilicate material that have been extensively studied in different biological processes due to its properties, i.e., ion exchange capacity, high surface area and porosity7. Several studies reported that the addition of zeolites can lead to a better performance of the anaerobic digestion process (AD) both under mesophilic and thermophilic conditions8,9. In these biological processes, zeolites were acting either as ion-exchangers for the removal of ammonia and/or as free ammonia adsorbing material, avoiding the ammonia inhibitory effect towards the microbial community, and therefore improving the overall process8,9. Compounds other than ammonia, i.e., cations such as Ca2+ and Mg2+ and long chain fatty acids, were also reported to be adsorbed to zeolites thus reducing their availability and potential toxicity, which resulted in significant improvements in methane production10,11. Moreover, zeolites induced changes in anaerobic microbial communities that apparently promoted the growth of a more efficient methanogenic community12.
Regarding the effect of zeolites on biohydrogen production, some studies reported enhancement of hydrogen yields during a two-step process of dark- and photo-fermentation after zeolites addition13,14. Again, the beneficial effect of zeolites was linked to its ion-exchanger property and the reduction of ammonium concentration. The hydrogen yield was enhanced in approximately 70% after ammonium removal by zeolites from dark fermentation residual solution13,14. Zeolites are also be used as adsorbents in industrial wastewaters for example to remove heavy metals15,16. In the majority of the studies, zeolites effect on the biological process of AD or photo-fermentation was assigned to the removal of ammonia or other compounds from the system. However, the high surface area of zeolites and its porosity are another important feature of zeolites for biological processes7. These characteristics allow this material to be used as a support for the immobilization of microorganisms in different types of bioreactor configurations improving its performance7,8. Recently, it was reported the enhancement of biohydrogen production in a hybrid bioreactor with integrated chlorinated polyethylene (CPE) fixed-bed and with natural and Fe-modified zeolite as a microorganism nutrition carrier (MNC)17. The authors observed the enhancement on biohydrogen production during AD process with the Fe-modified zeolite17. The authors suggested that Fe-modified zeolite coupled with the CPE fixed-bed could function not only for microbial immobilization but also could induced favourable pathways and enzymes, micronutrient supplementation and electrical conductivity when compared with natural zeolite17. Since zeolites successfully improved the efficiency of several biological processes, it was hypothesized that a commercially available zeolite could also improve biohydrogen production by dark fermentation. Therefore, in this study, the effect of zeolite (type-13X) in biohydrogen production by dark fermentation of a mixture (1:1) of C5 (arabinose) and C6 (glucose) sugars present in lignocellulosic hydrolysates and of Sargassum sp. biomass, was investigated under thermophilic conditions (70 ± 2 °C).
Results
Effect of zeolite on biohydrogen production by dark fermentation
The effect of zeolite on biohydrogen production from a mixture of C5 and C6-sugars was evaluated in batch experiments. The presence of zeolite type-13X influenced positively the maximum hydrogen production, the hydrogen yield, and the initial rate of hydrogen production (Fig. 1, Table 1). There was no correlation between the mass of zeolite/mass of inoculum (in VS) ratio (Z/I) and the maximum production or the hydrogen yield. The highest hydrogen production (24.54 mmol L−1), was achieved for the Z/I of 0.26 g g−1 followed by 22.88 mmol L−1 obtained for the Z/I of 0.065 g g−1, which were respectively 21% and 15% higher than the obtained for the control assay without zeolite (19.36 mmol L−1) (p < 0.05) (Fig. 1 A). Yet, no significant differences for hydrogen production between Z/I of 0.26 and 0.065 g g−1 were observed (p > 0.05). For the Z/I of 0.13 g g−1 of inoculum (in VS) hydrogen production reached 20.88 mmol L−1, which corresponded to an increment of only 7% compared to the control without zeolite (p > 0.05).
Cumulative hydrogen production and fermentative products produced in the batch assay with different zeolite to inoculum (in VS) ratios (0.26, 0.13, 0.065 g g−1) and for the control (without zeolite). (A) Glucose consumption:  0.26 g g−1;
0.26 g g−1;  0.13 g g−1;
0.13 g g−1;  0.065 g g−1;
0.065 g g−1;  control. Arabinose consumption:
control. Arabinose consumption:  0.26 g g−1;
0.26 g g−1;  0.13 g g−1;
0.13 g g−1;  0.065 g g−1;
0.065 g g−1;  control. Hydrogen production:
control. Hydrogen production:  0.26 g g−1;
0.26 g g−1;  0.13 g g−1;
0.13 g g−1;  0.065 g g−1;
0.065 g g−1;  control. (B), (C) and (D) Lactic acid, Acetic acid and formic acid production, respectively:
control. (B), (C) and (D) Lactic acid, Acetic acid and formic acid production, respectively:  0.26 g g−1;
0.26 g g−1;  0.13 g g−1;
0.13 g g−1;  0.065 g g−1;
0.065 g g−1;  control. The results are the average and standard deviations for duplicate assays.
control. The results are the average and standard deviations for duplicate assays.
Concerning the hydrogen yields, higher values were also obtained for the Z/I of 0.26 and 0.065 g g−1 and the lowest hydrogen yield (0.49 mmol mmol−1 as H2 per substrate consumed−1) was observed for the control (without zeolite) (Table 1). The pH at the end of the batch experiment was similar for all the Z/I ratios and for the control, approximately 5.7.
The rate of hydrogen production increased and was linearly correlated with the Z/I (Fig. 2). For the collected data it is also possible to estimate the rates of lactic, acetic and formic acid production. Apparently, there was an effect of the presence of zeolite in the decrease of lactate and formate production rates, but no significant effect was observed in the rate of acetate production (Table 2). It was also observed that for formic and lactic acid the presence of zeolite decreased the lag phase that preceded the onset of the production of these acids (about 2 days without zeolite and 1 day with zeolite for all Z/I and for both acids-not shown) (Fig. 1B,D).
Glucose and arabinose were totally consumed during the experiment for all Z/I ratios tested and for the control (Fig. 1A). The main end-product formed was acetic acid (reaching a maximum of 54.69 mmol L−1 for Z/I of 0.26 g g−1 (Fig. 1C). Lower amounts of lactic and formic acid were produced at similar concentrations for all the Z/I tested, and for the control (without zeolite) (Fig. 1B,D). Formic acid was produced in all the experimental conditions during the first days of operation, reaching a maximum concentration of 13 mmol L−1 (day 2). Afterwards, its concentration decreased to approximately 0.95 mmol L−1 (control Z/I of 0.26 g g−1) and 2.83 mmol L−1 Z/I of 0.13 and 0.065 g g−1) (day 8). This decrease was accompanied by an increase of hydrogen production and a decrease of the pH from 7 to approximately 5.7 (Table 1). The COD balance was closed in all the operational conditions with zeolite, showing that all metabolic products could be identified, except for the control (without zeolite) (Table 1).
Detailed information regarding the COD balance and the respectively soluble fermentation products formed can be found in the supplementary information (Table S1).
The zeolite effect on biohydrogen production by dark fermentation was further evaluated in a continuous bioreactor (Expanded Granular Sudge Bioreactor (EGSB)) fed with C5 and C6-sugars (Fig. 3). The bioreactor operation started at a hydraulic retention time (HRT) of 12 h and at an organic loading rate (OLR) of 8.8 kg m−3 day−1 of COD. During this operational period, the maximum hydrogen production rate achieved was 1016 mL L−1 day−1 after 15 days of operation, stabilizing afterwards in approximately 800 mL L−1 day−1 around day 21 (Fig. 3A). Moreover, the maximum hydrogen yield obtained was 2.33 mmol mmol−1 as H2 per substrate consumed (day 15) (Table 3). Under these operational conditions, acetate and lactic acid were the main metabolic products and their concentrations remained stable during this operation period (approximately 6.51 and 5.34 mmol L−1, respectively) (Fig. 3B).
After 25 days of operation, the HRT was decreased to 6 h and an OLR of 17.6 kg m−3 day−1 was applied. The hydrogen production rate decreased continuously and linearly over the time, reaching a minimum value of 143 mL L−1 day−1 after 46 days of operation (Fig. 3A). Hydrogen yield decreased as well to 0.19 mmol mmol−1 as H2 per substrate consumed) (Table 3). At this point, an increase in the lactic acid concentration was observed, reaching a maximum of 17.8 mmol L−1 (Fig. 3B).
No methane was detected during the experiment, which is in accordance with the low percentage of methanogenic archaea observed in the microbial community (0.02% of the total microbial community, Fig. 4), meaning that inoculum pre-treatment at high temperature and low pH was efficient at inhibiting methanogens.
After the drop of the hydrogen production rate to the minimum of 143 mL L−1 day−1, zeolite type-13X (Z/I of 0.26 g g−1) was added to the bioreactor. This Z/I ratio of 0.26 g g−1 was chosen since it achieved in absolute value the higher hydrogen production in batch experiments with C5 and C6-sugars. The system performance was immediately recovered after zeolite addition (Fig. 3A) and the hydrogen production increased linearly over time during the next 8 days, reaching 413 mL L−1 day−1, which was approximately 3 times higher than the previous condition (143 mL L−1 day−1). The addition of zeolites to the continuous operation with an OLR of 17.6 kg m−3 day−1 of COD allowed to improve the hydrogen yield from 0.19 to 0.53 mmol mmol−1 (Table 3). It was also observed that upon zeolite addition, formic acid concentration increased from 2.64 mmol L−1 to approximately 7.36 mmol L−1 (Fig. 3B). Propionate and n-butyrate acids were also detected during the reactor’s operation, however at concentrations lower than 1 mmol L−1 (Fig. 3B).
The pH remained constant during the entire operation, corresponding to approximately 5.9 ± 0.7, which is in the pH range (5.0–6.0) for optimum hydrogen production by dark fermentation18.
Microbial community analysis showed that Klebsiella sp. was the most abundant microorganism along all of the operation time (Fig. 4). However, after zeolite addition its relative abundance decreased from 77.5 to 51.8%, while Bacillus coagulans increased its relative abundance from 1.7 to 14.1%. (Fig. 4). Moreover, organisms belonging to the clostridia class namely Clostridium beijerinckii, Thermoanaerobacterium thermosaccharolyticums and Thermoanaerobacterium thermohydrosulfuricus also increased its relative abundance after zeolite addition to the bioreactor from 0.33 to 6.6%, 0.44 to 5.4% and 7.2 to 9.9%, respectively. Other microorganisms were also detected in the microbial community but in very low relative abundance (< 1%).
Glucose was detected in residual amounts while arabinose concentrations were maintained stable (around 10 mmol L−1) all along the operation time (Fig. 3B). The COD balance was closed during the bioreactor operation with an HRT of 6 h before and after zeolite addition, showing that all metabolic products could be identified, but not during the continuous operation with an HRT of 12 h (Table 3). Detailed information regarding the COD balance and the respectively soluble fermentation products formed can be found in the supplementary information (Table S2).
Zeolite effect in biohydrogen production from Sargassum sp
Hydrogen production by dark fermentation of Sargassum sp. biomass was also improved in the presence of zeolite type-13X (Fig. 5). The highest hydrogen production was obtained for the Z/I of 0.13 g g−1 (8.28 mmol L−1), which was approximately 27% higher than the control (6.10 mmol L−1) (Fig. 5). The Z/I of 0.26 g g−1 achieved a hydrogen production of 7.03 mmol L−1. Concerning the rates of hydrogen and lactic acid production (Table 4, Fig. 6) they were inversely related in the presence of zeolites, but no pattern was observed for the influence of the Z/I in the H2 production rate, with the maximum rate occurring for the intermediate Z/I of 0.13 g g−1.
Cumulative hydrogen production for the Z/I ratio of  0.26 g g−1,
0.26 g g−1,  0.13 g g−1,
0.13 g g−1,  0.065 g g−1, and for the
0.065 g g−1, and for the  control, and respectively lactic acid production for the Z/I ratio of
control, and respectively lactic acid production for the Z/I ratio of  0.26 g g−1;
0.26 g g−1;  0.13 g g−1,
0.13 g g−1,  0.065 g g−1 and for the
0.065 g g−1 and for the  control.
control.  Blank assay without zeolite and Sargassum sp. The results are the average and standard deviations for duplicate assays.
Blank assay without zeolite and Sargassum sp. The results are the average and standard deviations for duplicate assays.
No methane was detected during the assays in all of the conditions tested. In the blank assay, containing only anaerobic sludge, hydrogen was produced (2.79 mmol L−1), which means that part of the hydrogen produced, in the assays containing Sargassum sp., may be derived from the residual substrate associated with the inoculum sludge (Fig. 5).
Up to 13 mmol L−1 of acetic, iso-butyric, n-butyric, formic and propionic acid were formed (Figure S1). The COD balance was closed for the control and the Z/I of 0.26 g g−1 showing that all metabolic products could be identified, but not for the Z/I of 0.13 g g−1 (Table 5).
Detailed information regarding the COD balance and the soluble fermentation products can be found in the supplementary information (Table S3). The pH during the experiment decreased from 7 to approximately 6.6 in all operational conditions tested (Table 5).
Discussion
The addition of a zeolite Type 13X improved the yields and the rates of biohydrogen production by dark fermentation from simple carbohydrates (C5 and C6-sugars) and from a complex waste Sargassum sp. biomass.
For the experiments with sugars a linear correlation was observed between the H2 production rate and the Z/I ratio (Fig. 2). The production of other fermentative end-products rather than acetic acid by mixed-cultures can lead to low hydrogen yields comparing with the theoretical ones19. This was the case of the batch experiments performed with C5 and C6-sugars, were lactic and formic acid were produced besides acetic acid.
During high-rate dark fermentation systems operation, the decrease of the HRT, and thus increase of applied OLR, can often cause instability on the bioreactor performance, leading to lower hydrogen yields20. In the present study, the decrease of the HRT from 12 h (OLR of 8.8 kg m−3 day−1 of COD) to 6 h (ORL of 17.6 kg m−3 day−1 of COD) turned the performance of continuous C5 and C6-sugars fermentation unstable and led to a decrease on hydrogen production rate from approximately 800–143 mL L−1 day−1. Glucose was preferentially consumed compared with arabinose. The presence of a rapidly biodegradable source of carbon, as glucose, can inhibit the synthesis of enzymes involved in the metabolism of other carbon sources, leading to the repression of arabinose catabolism and, thus, to a preferential uptake of glucose instead of arabinose20,21.
The hydrogen production rates observed were lower than the ones reported in similar studies (Table 6). For instance, Abreu et al.20 reported a hydrogen production rate of 2700 mL L−1 day−1 at an HRT of 8 h in an extreme thermophilic EGSB reactor fed with C5 and C6-sugars (at 5 g COD L−1). In the same study, the hydrogen yield obtained at steady state was 0.77 mmol mmol−1 as H2 per substrate consumed−1 (HRT 8 h and fed concentration of 5 g COD L−1), which was 4 times higher than the obtained in this work (0.19 mmol mmol−1) (HRT 6 h and fed concentration of 4.4 g COD L−1)20 (Table 6). Nevertheless, in this study zeolite addition to the bioreactor has shown to be an interesting strategy to counteract the decrease on hydrogen yields caused by the increase of the applied OLR. A remarkable recovery of the system performance operating at high ORL (17.6 kg COD m−3 day−1), with a threefold improvement in hydrogen production, from 143 to 413 mL L−1 day−1 (Fig. 3A), as well as an increment in the hydrogen yield from 0.19 to 0.53 mmol mmol−1 (Table 3) was observed.
The metabolic profile obtained in the batch and continuous bioreactors, as well as the microbial community composition results, shows that several metabolic pathways were taking place simultaneously and were influenced by the presence of zeolites. In dark fermentation, hydrogen can be produced by two different pathways, i.e., mixed acid fermentation (or formic acid fermentation) and the clostridial-type fermentation (or butyric acid fermentation)22. Mixed acid fermentation is characteristic of bacteria that belong to the Enterobacteriaceae family, such as Klebsiella sp., while clostridial-type fermentation is typical in bacteria belonging to the Clostridium and Bacillus genera22. For both fermentation types, glycolysis is the first step in which glucose is converted into pyruvate with the formation of nicotinamide adenine dinucleotide (NADH)22. Depending on the fermentation type, different metabolic profiles can be obtained from pyruvate. In mixed acid fermentation the end products include lactate, formate, acetate, succinate, ethanol and the gases H2 and CO222. In butyric acid fermentation, butyrate can be produced along with acetate, lactate, ethanol and H2 and CO223. In both types of fermentation, acetate can be produced from acetyl-CoA in order to maximize ATP production, and to re-oxidize the NADH in order to provide NAD+ for the glycolytic pathway1. The accumulation of formic acid in batch experiments, during the first days of incubation, suggest that bacteria belonging to the Enterobacteriaceae family (known to perform the mixed-acid fermentation22) were the most active in this period (Fig. 1D). In this pathway, the pyruvate from glycolysis is cleaved by the enzyme pyruvate:formate lyase (Pfl) to form formic acid and acetyl-CoA22. Then, acetyl-CoA can be converted in acetate while formic acid can be degraded into hydrogen and carbon dioxide by formate:hydrogen lyase (FHL) that is activated at low pH1,22. Indeed, in the batch experiments, formic acid reached residual concentrations (below 2.8 mmol L−1), which was accompanied by the production of hydrogen and the decrease of the pH to 5.7.
A similar outcome was observed in the continuous experiment (Fig. 3). The production of acetic and formic acid during the process suggests that mixed-acid fermentation was the main metabolic pathway occurring. Analysis of the bioreactor microbial community showed the presence of potential hydrogen producing bacteria belonging to Klebsiella, Bacillus, Clostridium and Thermoanaerobacterium genera. Indeed, Klebsiella sp., that belongs to the Enterobacteriaceae family, was the most abundant microorganism in the continuous bioreactor operation before and after zeolite addition, although its relative abundance decreased from 77.5 to 51.8% (Fig. 4). Several studies reported the potential use of Klebsiella sp. to produce hydrogen under anaerobic conditions from a variety of carbohydrates and from more complex residues such as corn stalk hydrolysate20,24,25.
Moreover, Klebsiella sp. such as Klebsiella pneumoniae have been extensively studied due to its ability to produce several value-added products namely, lactic acid and ethanol under anoxic conditions25.
The presence of butyrate in the batch and continuous experiments can also be indicative of the performance of the clostridial-type fermentation, where the end products are hydrogen and acetate or butyrate, although relatively low butyrate concentrations could be detected (lower than 1 mmol L−1)23. Indeed, Clostridium, Thermoanaerobacterium and Bacillus genera, known to perform the clostridial-type fermentation were identified in the continuous bioreactor microbial community and account for 0.33%, 0.44% and 1.7% of the total microbial community before zeolite addition and 6.6%, 5.4% and 14.1% after zeolite addition (Fig. 4). Several species belonging to Thermoanaerobacterium genera namely T. thermosaccharolyticum were reported in literature to be promising candidates in lignocellulosic biomass conversion to biohydrogen with the simultaneous production of acetic and butyric acid as the principal fermentative end-products26,27. The analysis of the bioreactor microbial community showed the presence of microorganisms close related to B. coagulans and C. beijerinckii. These bacteria are capable of hydrogen production from a variety of carbon sources (e.g. l-arabinose, d-glucose, d-fructose, sucrose, maltose, starch and others)28. Masset et al.29 showed C. beijerinckii ability to produce hydrogen from formic acid. The authors pointed out that the ability of this bacteria to re-consume formate suggests the existence of a new metabolic pathway in clostridia29. Although, Bacillus genus is known to be hydrogen producer, some Bacillus species namely B. coagulans are also capable of producing lactic acid. Bischoff et al.30 isolated from a composted dairy manure a B. coagulans strain capable of converting mixed sugars of glucose, xylose and arabinose to l-lactic acid with 85% yield at a temperature of 50 °C. Indeed, lactic acid was detected in both batch and continuous experiments at considerable concentrations. The deviation of the metabolism for lactic acid production is associated with a decrease in hydrogen yields, since it is produced by a zero-hydrogen balance pathway16,20. In fact, the sharp increase in lactate concentration observed after lowering the HRT from 12 to 6 h in the continuous bioreactor was accompanied by a severe decrease in hydrogen production. The increase of lactic acid concentration with the decrease of the HRT and decrease on hydrogen production was already reported in EGSB reactors treating arabinose and glucose20. With the organic load increase there was, likely, an excess of reducing power overloading the H2 producing pathways and, thus, lactate was produced as a way to cope with the excess of protons generated. The addition of zeolite triggered a fluctuation in the production of lactate and formic acid leading to an increase in hydrogen production (Fig. 3A).
The link between hydrogen and lactate production was evident also in the batch assay with Sargassum sp. biomass. The rates of hydrogen and lactic acid production were inversely related in the presence of zeolites (Fig. 6), but no pattern was observed for the influence of the Z/I ratio in the H2 production rate, as observed for the sugars experiment. A maximum rate was observed for the intermediate Z/I of 0.13 g g−1 (Table 4). The H2 production rates were within a narrow range, but there is a clear indication that zeolite influenced in a higher extent the rate of lactate production.
Batch experiments performed with Sargassum sp. biomass showed that this residue can be efficiently used by mixed-cultures for hydrogen production via dark fermentation. The presence of zeolite improved hydrogen production from 6.10 mmol L−1 (control without zeolite) to approximately 8.3 mmol L−1 for Z/I of 0.13 g g−1 demonstrating the zeolite potential to enhance hydrogen production from lignocellulosic biomass. It was previously reported that Sargassum sp. biomass could be a suitable substrate for the generation of hydrogen enriched biogas (10–25% of H2) in a two-step system, combining dark fermentation from pure culture and AD process (Table 6)3. Therefore, zeolite addition to this two-step system can be a suitable strategy to enhance hydrogen production, increasing thereby the energy potential of the biogas produced from Sargassum sp. biomass.
Zeolites were used before in biological processes, such as AD and in a two-step process that coupled dark- and photo-fermentation in order to improve methane and hydrogen production, respectively8,13,14 (Table 6). In most of the studies, zeolite effect was attributed to the removal of NH4+ ions from the fermentation effluent due to its ion-exchanger capacity8,13,14. In this study, ammonium was not present in the fermentation medium and biohydrogen production was improved by adding zeolite in both batch and continuous experiments. Therefore, in this case, zeolites effect can be related to its ion-exchanger capacity or to its ability to adsorb molecules other than ammonium. In fact, besides NH4+, zeolites can adsorb different types of molecules. Specifically, zeolite type-13X was described as capable to adsorb CO2, CH4 and/or CO for hydrogen purification from gaseous mixtures31. In addition, other functions may be attributed to zeolites which can contribute to the observed improvement on hydrogen production, such as the presence of different catalytic sites (Brönsted and Lewis sites) in zeolites structure and their ability to function as a sink of trace elements which are important to provide optimal growth conditions to hydrogen producing microorganisms. Indeed, a previous study concluded that zeolites improved the biogas process efficiency, not only by reducing the ammonia concentration in the media, but also because its porous structure could function as a supplement of trace elements (i.e., Ca, Na, K and Ba), thus improving the activity of methanogens8,32. Another important feature of zeolites is the high-capacity to function as a matrix for the immobilization of microorganisms7,33. This was not likely the case in the present study, since the inoculum consisted of granular sludge where microorganisms are already self-immobilized.
The adsorption capacity of zeolite can be extended to protons. Since the dark fermentation process occurs at pH range between 5.0 and 6.0, protons in solution are most likely adsorbed through zeolite by ion-exchange. In this study it was hypothesized that these protons sink may reduce the NADH/NAD+ redox imbalance that is usually associated to formation of more reduced products such as lactate. In fact, it is worth to notice that, during the continuous operation, zeolite addition triggered a dynamic fluctuation between formate (a precursor of H2 production) and lactate, conducting ultimately to an increase in H2 production (Fig. 3A). The multitask potential of zeolites in several biotechnological processes suggests that its effect on dark fermentation may be the result of not one but several properties.
The improvement on the biohydrogen production can be linked to metabolic changes induced by zeolites as they affected the microbial community structure. The metabolic profile obtained in the batch and continuous bioreactors show that several metabolic pathways were taking place simultaneously and were influenced by the presence of zeolite, favoring hydrogen production.
Dark fermentation is a complex process that still has a long way to become a competitive technology for hydrogen production. The most suitable operational conditions still need to be optimized, since this fermentative process is influenced by several operational parameters such as inoculum source, temperature, pH, HRT, H2 partial pressure and fermentative end-products34. The obtained results demonstrate zeolite potential to improve thermophilic biohydrogen production from C5 and C6-sugars and from lignocellulosic wastes such as Sargassum sp. biomass, showing the versatility and suitability of this material, and opening a wide range of possibilities to be incorporated in biological processes for hydrogen production from renewable feedstocks.
Material and Methods
Batch experiments
Batch experiments were conducted in 120 mL serum bottles with a working volume of 50 mL, containing anaerobic medium with 0.6 mL g−1 of Chemical Oxygen Demand (COD) of macronutrients20, 10 mmol L−1 of sodium bicarbonate, resazurin at a final concentration of 0.5 mg L−1 and 15 mmol L−1 of sodium 2-bromoethanesulfonate (BES) to inhibit the methanogenic activity. Anaerobic granular sludge (with a final concentration of 7.72 g L−1 of volatile solids (VS)), collected from a brewery industry (Lisbon, Portugal), was used as inoculum and a mixture of l-arabinose and glucose monohydrated (25 mmol L−1, 1:1) as substrate. Three different amounts of zeolite type-13X to inoculum (in VS) ratios were tested: 0.26, 0.13 and 0.065 g g−1. The bottles were sealed and the headspace flushed with N2/CO2 (80/20%) to guarantee the anaerobic conditions and the buffer effect. Sodium sulfide (0.8 mmol L−1) was used as a reducing agent to eliminate the residual O2 concentrations in the medium. The assays were performed at 70 °C, 150 rpm and at an initial pH of 7.0. A schematic representation of the batch experiment is described in figure S2.
The zeolite used consisted in the commercial type-13X, known as molecular sieves and was obtained from Sigma Aldrich. It is composed by 1 Na2O:1 Al2O3:2.8 ± 0.2 SiO2:xH2O, the spherical bleads of the zeolite have a size of 4–8 mesh with an average pore diameter of 10 Å and are composed essentially by the faujasite structure35. This type of structure is composed by 3-dimensional channel system with supercages and 12-ring pore openings, with high surface area, low Si/Al ratio which confer higher cation exchange capacity and high thermal and chemical stability. In addition, the low Si/Al ratio, saturated in aluminium, offers excellent properties in terms of adsorption capacity36.
Consumption of arabinose and glucose and production of hydrogen and other soluble fermentation products (SFP) were monitored during the experiments.
A different set of batch assays was performed using Sargassum sp. as substrate, in the same conditions described for the batch assays with glucose and arabinose, with exception of the working volume (serum bottles and working volume of 160 and 60 mL, respectively).
Sargassum sp. was collected during spring from a location in the north coastline of Portugal (Póvoa de Varzim). The macroalgae was dried at room temperature and then milled into pieces with less than 0.5 cm3. The Sargassum sp. characterization can be found in3. To increase the soluble COD, the macroalgae was autoclaved at 121 °C and 1 bar for 10 min3.
In this experiment, a ratio of 1.54 g of inoculum (in VS) per g of Sargassum sp. (in VS) was used and the ratios 0.26 and 0.13 g zeolite per g of inoculum (in VS) were tested.
For both experiments a blank assay without inoculum and a control without zeolite were included. In the assay with Sargassum sp. an additional blank assay with only anaerobic granular sludge without any substrate was also performed for quantification of the hydrogen produced from the residual substrate present in the inoculum. For each condition, four replicates were performed. To avoid hydrogen leaks, gas composition was determined in two of the bottles and liquid samples were collected from the remaining two bottles for VFA, lactic acid and sugars quantification. pH was monitored in both experiments.
A schematic representation of the batch experiments for biohydrogen production by dark fermentation is shown in Fig. 7.
Continuous bioreactors operation
Anaerobic granular sludge from a brewery industry (Lisbon, Portugal) was previously acclimated for 2 months on an expanded granular sludge bed bioreactor (EGSB) at extreme thermophilic conditions (70 °C) and pH 5.5, to select for hydrogen producers and to inhibit methanogens. This sludge (0.08 ± 0.01 g VS g−1) was used as inoculum (300 mL) in a stainless steel EGSB reactor (internal diameter of 5.65 cm and total volume of 2.68 L) with a working volume of 2.14 L. The reactor was fed with l-arabinose and glucose monohydrated [1:1 (w/w)] at a concentration of 4.4 g L−1 (in COD) and operated in continuous mode at 70 ± 2 °C during 71 days. The feed was supplemented with 0.6 mL g−1 COD of macronutrients and sodium bicarbonate (NaHCO3) at a concentration of 2 g L−1 for pH control; and it was maintained at 4 °C to minimize acidification, as described elsewhere20. The pH was controlled by the addition of sodium bicarbonate (NaHCO3) to the bioreactor feed. Afterwards, the pH was monitored throughout the continuous operation (approximately 5.9 ± 0.7). The bioreactor was operated in the first 25 days with a hydraulic retention time (HRT) of 12 h and with an organic loading rate (OLR) of 8.8 kg m−3 day−1 of COD. Thereafter the HRT was decreased to 6 h during 45 days. At this operation period an OLR of 17.6 kg m−3 day−1 of COD was applied to the bioreactor. Zeolite type-13X was added to the bioreactor (in a ratio of 0.26 g of zeolite per g of inoculum (in VS)) when the hydrogen production dropped after 46 days of operation. A schematic representation of the continuous experiment is described in Fig. 7.
Gas samples were collected for hydrogen and methane measurements. Volatile fatty acids (VFA), lactic acid, glucose and arabinose concentrations were determined in liquid samples collected from the effluent.
Sludge samples (approximately 0.5 mL) were collected for microbial community taxonomic characterization at day 46 (immediately before zeolite addition) and at day 66 (after zeolite addition). Samples were centrifuged, washed with PBS and stored at − 20 °C. Total genomic DNA was extracted by using the FastDNA SPIN kit for soil (MP Biomedicals LLC, Santa Ana, CA). 16S rRNA genes were sequenced by Illumina MiSeq, using the universal prokaryotic primer pair 515-f/806-r37. Sequencing and data analysis were performed by RTL (Research testing Laboratory, Texas, US) as described elsewhere38. Sequencing reads were submitted to the European Nucleotide Archive (ENA) under the study accession number PRJEB23747 (sample SAMEA104420766, before zeolite addition; sample SAMEA104420767, after zeolite addition).
Analytical methods
Hydrogen and methane concentration in batch and continuous experiments were analyzed by gas chromatography using a BRUKER SCION 456 with a column molsieve (MS-13 × 80/100 mesh) and connected to a thermal conductivity detector. Argon was used as carrier gas at a rate of 30 mL min−1 and the injector, detector and column temperatures were set at 100, 130, and 35 °C, respectively. VFA, lactic acid and sugars (i.e., arabinose, glucose) were determined by high performance liquid chromatography using a chromatograph (Jasco, Japan) with an Aminex column (HPX-87H Ion 300 mm × 7.8 mm2); Sulfuric acid (0.01 N) at a flow rate of 0.7 mL min−1 was used as mobile phase. Column temperature was set at 60 °C and was used a retention time of 50 min. Detection of VFA’s and lactic acid was made through a UV detector at 210 nm, and the detection of glucose and arabinose was made by using an RI detector. pH was measured by using a benchtop metre InoLabVR pH 7110 (WTW, Weilheim, Germany). Determination of COD was performed using the commercial kits LCK 014 (colorimetric method) from Hach Lange, Düsseldorf, Germany, following the manufacturer’s instructions.
Statistical analysis
Significant differences between biological samples, collected from batch incubations with and without zeolite, were determined by applying a t test using Microsoft Excel (unequal variance t test was applied to batch experiments with Sargassum sp. and equal variance t test to the batch experiments with sugars, as determined by the results of the variance analysis obtained with the F test). The significant threshold was set at P = 0.05.
Abbreviations
- ATP:
-
Adenosine triphosphate
- AD:
-
Anaerobic digestion
- BES:
-
Sodium 2-bromoethanesulfonate
- COD:
-
Chemical oxygen demand
- DNA:
-
Deoxyribonucleic acid
- EGSB:
-
Expanded granular sludge bed bioreactor
- HRT:
-
Hydraulic retention time
- OLR:
-
Organic loading rate
- NADH:
-
Nicotinamide adenine dinucleotide
- SFP:
-
Soluble fermentation products
- VFA:
-
Volatile fatty acids
- VS:
-
Volatile solids
- Z/I:
-
Zeolite/inoculum ratio
References
Hallenbeck, P. C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrog. Energy 34, 7379–7389 (2009).
Rittmann, S. & Herwig, C. A comprehensive and quantitative review of dark fermentative biohydrogen production. Microb. Cell Fact. 11, 115 (2012).
Costa, J. C., Oliveira, J. V., Pereira, M. A., Alves, M. M. & Abreu, A. A. Biohythane production from marine macroalgae Sargassum sp. coupling dark fermentation and anaerobic digestion. Bioresour. Technol. 190, 251–256 (2015).
Nkemka, V. N. & Murto, M. Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J. Environ. Manag. 91, 1573–1579 (2010).
Hallenbeck, P. C., Abo-Hashesh, M. & Ghosh, D. Strategies for improving biological hydrogen production. Bioresour. Technol. 110, 1–9 (2012).
Martins, G., Salvador, A. F., Pereira, L. & Alves, M. M. Methane production and conductive materials: A critical review. Environ. Sci. Technol. 52, 10241–10253 (2018).
Montalvo, S., Huiliñir, C., Borja, R., Sánchez, E. & Herrmann, C. Application of zeolites for biological treatment processes of solid wastes and wastewaters—a review. Bioresour. Technol. 301, 122808 (2020).
Montalvo, S. et al. Application of natural zeolites in anaerobic digestion processes: A review. Appl. Clay Sci. 58, 125–133 (2012).
Kotsopoulos, T. A., Karamanlis, X., Dotas, D. & Martzopoulos, G. G. The impact of different natural zeolite concentrations on the methane production in thermophilic anaerobic digestion of pig waste. Biosyst. Eng. 99, 105–111 (2008).
Wang, X. et al. Biogas production improvement and C/N control by natural clinoptilolite addition into anaerobic co-digestion of Phragmites australis, feces and kitchen waste. Bioresour. Technol. 180, 192–199 (2015).
Nordell, E., Hansson, A. B. & Karlsson, M. Zeolites relieves inhibitory stress from high concentrations of long chain fatty acids. Waste Manag. 33, 2659–2663 (2013).
Poirier, S., Madigou, C., Bouchez, T. & Chapleur, O. Improving anaerobic digestion with support media: Mitigation of ammonia inhibition and effect on microbial communities. Bioresour. Technol. 235, 229–239 (2017).
Cheng, J. et al. Combination of dark- and photo-fermentation to improve hydrogen production from Arthrospira platensis wet biomass with ammonium removal by zeolite. Int. J. Hydrog. Energy 37, 13330–13337 (2012).
Androga, D. D., Özgür, E., Eroglu, I., Gündüz, U. & Yücel, M. Amelioration of photofermentative hydrogen production from molasses dark fermenter effluent by zeolite-based removal of ammonium ion. Int. J. Hydrog. Energy 37, 16421–16429 (2012).
Niaz, S., Manzoor, T. & Pandith, A. H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 50, 457–469 (2015).
Karakurt, C., Kurama, H. & Topçu, İB. Utilization of natural zeolite in aerated concrete production. Cem. Concr. Compos. 32, 1–8 (2010).
Zhao, C. et al. A developed hybrid fixed-bed bioreactor with Fe-modified zeolite to enhance and sustain biohydrogen production. Sci. Total Environ. 758, 143658 (2021).
Ginkel, S. V., Sung, S. & Lay, J.-J. Biohydrogen production as a function of pH and substrate concentration. Environ. Sci. Technol. 35, 4726–4730 (2001).
Hawkes, F., Hussy, I., Kyazze, G., Dinsdale, R. & Hawkes, D. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrog. Energy 32, 172–184 (2007).
Abreu, A. A. et al. Engineered heat treated methanogenic granules: A promising biotechnological approach for extreme thermophilic biohydrogen production. Bioresour. Technol. 101, 9577–9586 (2010).
Strobel, H. J. Evidence for catabolite inhibition in regulation of pentose utilization and transport in the ruminal bacterium Selenomonas ruminantium. Appl. Environ. Microbiol. 59, 40–46 (1993).
Sikora, A., Baszczyk, M., Jurkowski, M. & Zielenkiewicz, U. Lactic acid bacteria in hydrogen-producing consortia: On purpose or by coincidence? In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes (ed. Kongo, J. M.) (InTech, 2013). https://doi.org/10.5772/50364.
Saint-Amans, S., Girbal, L., Andrade, J., Ahrens, K. & Soucaille, P. Regulation of carbon and electron flow in Clostridium butyricum VPI 3266 grown on glucose-glycerol mixtures. J. Bacteriol. 183, 1748–1754 (2001).
Niu, K., Zhang, X., Tan, W.-S. & Zhu, M.-L. Characteristics of fermentative hydrogen production with Klebsiella pneumoniae ECU-15 isolated from anaerobic sewage sludge. Int. J. Hydrog. Energy 35, 71–80 (2010).
Liu, F. & Fang, B. Optimization of bio-hydrogen production from biodiesel wastes by Klebsiella pneumoniae. Biotechnol. J. 2, 374–380 (2007).
Zhao, L. et al. Unraveling hydrogen production potential by glucose and xylose co-fermentation of Thermoanaerobacterium thermosaccharolyticum w16 and its metabolisms through transcriptomic sequencing. Int. J. Energy Res. 44, 9617–9628 (2020).
Sheng, T., Gao, L., Zhao, L., Liu, W. & Wang, A. Direct hydrogen production from lignocellulose by the newly isolated Thermoanaerobacterium thermosaccharolyticum strain DD32. RSC Adv. 5, 99781–99788 (2015).
Lertsriwong, S. & Glinwong, C. Newly-isolated hydrogen-producing bacteria and biohydrogen production by Bacillus coagulans MO11 and Clostridium beijerinckii CN on molasses and agricultural wastewater. Int. J. Hydrog. Energy 45, 26812–26821 (2020).
Masset, J. et al. Fermentative hydrogen production from glucose and starch using pure strains and artificial co-cultures of Clostridium spp.. Biotechnol. Biofuels 5, 35 (2012).
Bischoff, K. M., Liu, S., Hughes, S. R. & Rich, J. O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 32, 823–828 (2010).
Brea, P., Delgado, J. A., Águeda, V. I., Gutiérrez, P. & Uguina, M. A. Multicomponent adsorption of H2, CH4, CO and CO2 in zeolites NaX, CaX and MgX. Evaluation of performance in PSA cycles for hydrogen purification. Microporous Mesoporous Mater. 286, 187–198 (2019).
Wang, H. et al. Anaerobic digestion technology for methane production using deer manure under different experimental conditions. Energies 12, 1819 (2019).
Emami Moghaddam, S. A., Harun, R., Mokhtar, M. N. & Zakaria, R. Potential of zeolite and algae in biomass immobilization. BioMed. Res. Int. 2018, 1–15 (2018).
Soares, J. F., Confortin, T. C., Todero, I., Mayer, F. D. & Mazutti, M. A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 117, 109484 (2020).
Molecular Sieves-Technical Information Bulletin, Sigma-Aldrich, 2019. http://www.sigmaaldrich.com/chemistry/chemical-synthesis/learning-center/technical-bulletins/al-1430/molecular-sieves.html.
Flaningen, E. M. Introduction to Zeolite Science and Practice. Studies in Surface Science and Catalysis (Elsevier, 1991).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. 108, 4516–4522 (2011).
Salvador, A. F. et al. Inhibition studies with 2-bromoethanesulfonate reveal a novel syntrophic relationship in anaerobic oleate degradation. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.01733-18 (2018).
Abreu, A. A., Karakashev, D., Angelidaki, I., Sousa, D. Z. & Alves, M. Biohydrogen production from arabinose and glucose using extreme thermophilic anaerobic mixed cultures. Biotechnol. Biofuels 5, 6 (2012).
Asada, Y. et al. Hydrogen production by co-cultures of Lactobacillus and a photosynthetic bacterium, Rhodobacter sphaeroides RV. Int. J. Hydrog. Energy 31, 1509–1513 (2006).
Acknowledgements
This study was supported by the Portuguese Foundation for Science and Technology (FCT, Portugal) under the scope of the strategic funding of UID/BIO/04469 unit and COMPETE 2020 (POCI-01-0145-FEDER-006684), Project SAICTPAC/0040/2015 (POCI-01-0145-FEDER-016403) and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020-Programa Operacional Regional do Norte.
Author information
Authors and Affiliations
Contributions
All authors contributed intellectually via scientific discussions during the work and have read and approved the final manuscript. M.A.P. and R.M.S. designed the study. R.M.S. executed the experimental work, interpreted the data and wrote the manuscript. A.A.A. and A.F.S. participated in the experimental work and in data interpretation. M.M.A., I.C.N. and M.A.P. contributed to data interpretation and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, R.M., Abreu, A.A., Salvador, A.F. et al. Zeolite addition to improve biohydrogen production from dark fermentation of C5/C6-sugars and Sargassum sp. biomass. Sci Rep 11, 16350 (2021). https://doi.org/10.1038/s41598-021-95615-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95615-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.

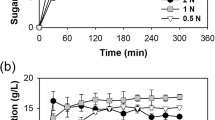




 Hydrogen production rate and
Hydrogen production rate and  HRT. (B)
HRT. (B)  Arabinose and
Arabinose and  glucose consumption and soluble fermentation products (SFP):
glucose consumption and soluble fermentation products (SFP):  Formic acid;
Formic acid;  Acetic acid;
Acetic acid;  Lactic acid;
Lactic acid;  n-butyrate acid;
n-butyrate acid;  Propionate acid.
Propionate acid.


