Abstract
While epigamic traits likely evolve via sexual selection, the mechanism whereby internal sexual dimorphism arises remains less well understood. Seeking clues as to how the internal sexual dimorphism evolved, we compared the abdominal musculature of 41 Drosophila montium group species, to determine whether any of these species carry a male-specific muscle of Lawrence (MOL). Our quantitative analysis revealed that the size of a sexually dimorphic MOL analog found in 19 montium group species varied widely from species to species, suggesting the gradual evolution of this sexually dimorphic neuromuscular trait. We attempted the ancestral state reconstitution for the presence or absence of the neuromuscular sexual dimorphism in the A5 segment; the neuromuscular sexual dimorphism existed in an old ancestor of the montium group, which was lost in some of the most recent common ancestors of derived lineages, and subsequently some species regained it. This loss-and-gain history was not shared by evolutionary changes in the courtship song pattern, even though both traits were commonly regulated by the master regulator male-determinant protein FruM. It is envisaged that different sets of FruM target genes may serve for shaping the song and MOL characteristics, respectively, and, as a consequence, each phenotypic trait underwent a distinct evolutionary path.
Similar content being viewed by others
Introduction
Sexually dimorphic characteristics have been a focus of evolutionary studies because they represent an important signature of the history of sexual selection1. Although male-specific external structures have attracted substantial interest among biologists1,2, sex differences in the structure and function of internal organs have not been thoroughly studied from an evolutionary point of view3. As an attempt to understand how internal sexual dimorphisms evolve, we studied a male-specific muscle called the muscle of Lawrence or MOL4 in the Drosophila montium group5,6, in which the male courtship display varies widely across species7,8, suggesting a unique evolutionary history of sexually dimorphic traits.
The MOL represents a single pair of longitudinal muscles running along the dorsal tergite of the fifth abdominal segment (A5) of adult males in some Drosophila species9,10. In D. melanogaster, where MOL formation has been studied in some depth, the MOL is readily recognizable as it is longer and wider than other longitudinal muscles. Gynandromorphic flies may or may not carry the MOL, depending on the sex chromosomal composition of the innervating motoneuron11,12, now known as the MOL-inducing (Mind) neuron13; the MOL forms when the composition is XO (the male) but not XX (the female), whereas the chromosomal sex of myocytes composing the muscle has nothing to do with the MOL formation14,15,16.Thus, the presence of MOL signifies that the innervating neuron is a male cell, whereas its absence indicates that the innervating neuron is a female cell. The MOL is larger than conventional longitudinal muscles as a result of a larger number of recruited myocytes, which fuse to form a fiber of the MOL.
Sex determination in Drosophila involves a cascade of genes that produce RNA splicing factors, the terminal effectors of which are two transcription factor genes, doublesex (dsx) and fruitless (fru). Expression of fru in the Mind neuron is essential for the MOL formation17,18,19,20, whereas dsx seems to play no role21,22. Indeed, female flies acquire the male-specific MOL if the male-specific fru gene product (i.e., the FruM protein) is transgenically produced in innervating motoneurons20.
FruM functions during the pupal stage as a master regulator of the formation of adult neural circuits underlying male mating behavior in D. melanogaster. In this species, ~ 2000 FruM-expressed neurons distributed through the entire nervous system from the sensory to the central and motor systems are interconnected, forming a circuit (i.e., the fru circuit) that operates to generate courtship behavior23,24. Although the MOL-innervating Mind neuron expresses FruM as aforementioned and thus likely contributes to the fru circuit, no role in courtship behavior has been assigned to the Mind-MOL neuromuscular system. Although the functional significance of the MOL remains obscure, this muscle offers an experimentally tractable system for the analysis of the fru-dependent developmental mechanism even in non-model species where the possibility of genetic manipulation of the fru circuit is limited. We therefore decided to use the MOL metrics as a convenient and reliable proxy of the FruM activity in the nervous system and compared them across species of the montium group to infer the evolutionary history of the fru circuit.
Although the fru circuit in non-melanogaster drosophilid flies is ill-defined due to technical difficulties in visualizing and manipulating specific neurons in these species, a few successful studies support the idea that the fru circuit plays a central role in executing courtship behavior also in these species25,26. Courtship song is a hallmark of Drosophila mating displays that are fru-dependent27,28 and montium group members exhibit spectacular variations in singing behavior29,30. Remarkably, males of many species of the montium group generate song not only before mounting (pre-mounting songs) but also after mounting (post-mounting songs), in contrast to most other Drosophila groups, in which males sing only pre-mounting songs29,30. Thoracic muscles on their own cannot produce any courtship song. They need to be driven by the FruM-dependent central neural circuit, a system distributed across the entire body, including the abdomen, in which the MOL and the FruM-positive innervating motoneurons exist. The interplay between the abdominal and thoracic circuitries is critical when a courting male makes an attempt at copulating, because the thoracic leg motor coordination is pivotal in bringing all his body parts into an appropriate position. Additionally, it is likely that males of some montium group species commence singing a post-mounting song when the thoracic song pattern generator becomes active upon receiving inputs from the abdominal ganglion that signals successful genital contact with a mating partner. These considerations suggest that the FruM-expressing neurons in the thorax and abdomen (and indeed the head) operate in coordination to generate courtship actions including singing, and therefore, it is logically possible that the MOL (as an abdominal muscle) and the song (powered by the thoracic muscles) evolved under common selective pressures.This might suggest that the montium group underwent substantial diversification in the neural fru activity across clades, prompting us to explore such evolutionary fru activity changes by comparing MOLs among the species of this group. This was our rationale for comparing the evolutionary history of the MOL formation with that of the song pattern.
A cross-species comparison of the MOL in the genus Drosophila (sensu lato) has been made by Gailey et al.9, who examined male flies of 95 species and classified them into two categories, i.e., species with the MOL (MOL+: 28 species) and those without the MOL (MOL−: 67 species), based on visual inspection of the abdominal musculature without quantification of the muscle size. That study included 12 species from the montium group, all of which were judged to be MOL−. In view of the fact that fru-dependent song traits are highly variable from species to species in the montium group, one may anticipate that the MOL formation, another fru-dependent trait, would also have been diversified, contrary to the observation by Gailey et al.9.
Through quantitative comparisons of the muscle size by standardized image analysis and statistical tests, here we show that some of the montium group species carry a single pair of sexually dimorphic muscles in the A5 abdominal segment, reminiscent of the MOL in D. melanogaster. These sexually dimorphic muscles in the montium group species are, however, much smaller in size than the MOL in D. melanogaster and exhibit substantial variations in size among species. We postulate that these sexually dimorphic muscles in the montium groups species represent the MOL analogs and suggest that the formation of MOL and its analogs evolved gradually, rather than emerging in an all-or-none manner across lineages. However, no apparent correlation was found between the putative MOL analogs and song characteristics in the montium group species, implying that these two traits evolved under distinct selective pressures.
Results
MOL quantification in the outgroup species
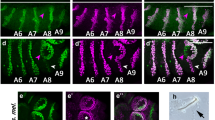
We first characterized the MOL and candidate MOL analogs in the following seven outgroup species: D. melanogaster, D. erecta, D. yakuba, D. subobscura, D. affinis, D. virilis and D. mercatorum (Table S1). In D. melanogaster males, the MOL is readily recognizable because of its large size and unique positioning in the A5 segment (Fig. 1a1; cf. Fig. 1a2). The length of the MOL also exceeds that of conventional muscles in D. subobscura, whose MOL has been extensively characterized because of its unusual feature, i.e., the presence of an additional pair of MOLs in A49,26. We therefore decided to use the length difference in distinguishing the MOL from conventional muscles. As a rigorous means to define the length, we adopted Feret’s diameter as calculated by the Fiji package, an ImageJ standard option (https://imagej.net/Welcome: see Materials and Methods; Fig. S1). Feret’s diameter was measured for the putative MOL (FA) and for the medial-most muscle running along the midline, which served as a control (FB) within the same hemi-segment for each fly. The frequency histogram of FA/FB constructed for the longest muscles in male A5 in D. melanogaster was fitted with a Gaussian distribution function, yielding the mean ± SEM of 1.71 ± 0.03 (Fig. 1a). Similar plots were constructed for the muscles in the male A4 and female A5 segments of the wild type and also the fru mutant male A5 in D. melanogaster, giving FA/FB values of 1.00 ± 0.01, 0.99 ± 0.01 and 1.00 ± 0.02, respectively (Fig. 1a; Table S1). The longest muscles in male A5 were significantly longer than conventional muscles in male A4 or female A5 segments (Fig. 1a), supporting the notion that the former muscles represent the analogs of MOL, which is male-specific and A5-specific in D. melanogaster.
Detection of sexual dimorphism in the abdominal musculature of D. melanogaster and some species of the montium group. Representative images (a1–a3,b1,b2,c1,c2,d1,d2) of abdominal muscles and the Gaussian fit to the FA/FB distribution of the largest muscle (a4,b3,c3,d3) in the reference species D. melanogaster (a) and 3 species of the montium groups, i.e., leontia (b), lactericornis (c) and cauverii (d). Images are typical examples of male abdominal musculatures with the exception of those for D. melanogaster, which are images of a wild-type male (a1), wild-type female (a2) and fru mutant male (a3). The MOL and its analogs are indicated with arrowheads, and the regions shown in Fig. 4 to visualize nuclei are boxed. D. cauverii is a species that did not show the neuromuscular sexual dimorphism in the A5 segment whereas 3 other species displayed the sexual dimorphism. Scale bar: 100 µm. The curves in a4, b3, c3 and d3 compare FA/FB distributions for the male A5 (red lines), the female (black lines) and male A4 (blue lines), and, only in a4, the male fru mutant A5 (magenta lines). The statistical differences were evaluated by the Brown-Forsythe and Welch ANOVA or Kruskal–Wallis test; ***P < 0.001.
Similar analysis in D. subobscura (Fig. S2) revealed that the longest muscles in male A5 (FA/FB = 1.93 ± 0.04) were significantly longer than conventional muscles in female A5 (1.12 ± 0.02) but not longer than the counterparts in male A4 (2.13 ± 0.04), an observation consistent with previous studies showing that D. subobscura males carry an additional pair of the MOL in A49,10,26. We also constructed a frequency histogram of FA/FB for the longest muscles in male A5, male A4 and female A5 in D. erecta, D. yakuba, D. affinis, D. virilis and D. mercatorum (Fig. S2). The histogram showed that the mean length of the longest muscles in male A5 (FA/FB = 1.34 ± 0.02) and also male A4 (1.32 ± 0.02) was longer than that in female A5 (1.13 ± 0.01) in D. affinis (Fig. S2), despite a previous work reporting that MOL was absent in this species9. The MOL-like muscles were not found in the remaining four outgroup species (Table S1).
Therefore, the quantification of the MOL and its potential analogs in the seven outgroup species described above suggests that the estimates of the muscle length with Feret’s diameter are in good agreement with their relative sizes judged by visual inspection. We thus used Feret’s diameter FA/FB to quantify the MOL-analog formation in the subsequent analysis with the montium group (Table 1).
Sex differences in MOL analogs of montium group species
To determine sexual dimorphisms in the neuromuscular system in the montium group flies, we first examined the A5 muscles in D. kikkawai (Figs. 2a–f and S3h1–h6). Although the histogram for the male A5 muscles extensively overlapped with that for the female A5 muscles, the male curve (the mean ± SEM of FA/FB: 1.51 ± 0.03) shifted to the right with a larger standard error compared with the female curve (1.07 ± 0.01; Figure S3h6 and Table 1). This sex difference in the FA/FB distribution was statistically significant (P < 0.001; Table 1). To further compare the distribution between the sexes, we plotted all data points for the male and female A5 muscles (Fig. 2g). Strikingly, all values for females were confined within a narrow range (the 99% confidence intervals are defined by colors in Fig. 2g), whereas the values for males were widely distributed, with some overlap with the female data distribution (Fig. 2g). We conclude that D. kikkawai exhibits a sexual difference in the formation of a pair of A5 muscles, but this sexual dimorphism forms to a lesser extent when compared with the male-specific MOL development in D. melanogaster.
Sexually dimorphic size variations of the largest A5 muscles in D. kikkawai. (a–f) MOL-analogs are visually recognizable bilaterally (a–c) or unilaterally (d) in most males but not females (f) and some males (e) from A5. (g) The scatter plot shows the distribution of mean FA/FB values as compared between the male and female. Each symbol represents the values estimated for a hemi-segment. The red and blue long lines represent the mean values for female and male values, respectively. The short bar represents the SEM. The blue and pink areas in the graph indicate the 99% confidence intervals (mean ± 3 × SD values) of the muscle size for males and females, respectively.
Next, we extended the above analysis to other members of the montium group species. We found that 19 out of 41 species examined exhibited significant sex differences in the FA/FB distribution of A5 muscles: these included D. baimaii, D. barbarae, D. birchii, D. bocki, D. diplacantha, D. fengkainensis, D. greeni, D. kikkawai, D. lacteicornis, D. leontia, D. mayri, D. ogumai, D. ohnishii, D. pectinifera, D. serrata, D. trapezifrons, D. truncata, D. tsacasi and D. watanabei (Fig. 1b1–d3 and S3 and Table 1). Among these 19 species, the male A5 muscles with large FA/FB values equivalent to FA/FB for the MOL in D. melanogaster (1.71 ± 0.03) were found in 2 species, D. barbarae (1.60 ± 0.03) and D. mayri (1.53 ± 0.02), both of which belong to the serrata subgroup (Fig. S3 and Table 1). The male A5 muscles in 5 other species exhibited slightly larger FA/FB values than that in D. affinis (1.34 ± 0.02); the 5 species included D. baimaii (1.38 ± 0.02) and D. lacteicornis (1.38 ± 0.02) in the montium subgroup and D. bocki (1.41 ± 0.02), D. kikkawai (1.51 ± 0.03) and D. leontia (1.40 ± 0.02) in the kikkawai subgroup (Figs. 1 and S3). In remaining 12 species, the sex differences in mean FA/FB values for the largest A5 muscles were less striking yet statistically significant (Table 1 and Figs. 1 and S3).
The FA/FB values for 41 species from the montium group are shown as a scatter plot in Fig. 3, which is conjoined with the phylogenetic tree of this fly group. We conclude that some montium group species have a MOL analog, the size of which varies markedly within an individual, within a species and/or across species. We note that most of the fly stocks of the montium species group used in this study were isofemale lines, and therefore, genetic factors should have contributed only minimally to the observed variations in muscle size within a species. However, we cannot exclude the possibility that the present study underestimates the muscle size variations in wild populations that are genetically heterogeneous.
Divergent neuromuscular sexual dimorphisms in A5 among species of the montium group. FA/FB values in the 41 species examined are shown on the phylogenetic tree. The scatter plot shows the distribution of mean FA/FB values as compared between the male and female. Each symbol represents the values estimated for a hemi-segment. The red and blue long lines represent the mean values for female and male values, respectively. The short bar represents the SEM. The blue and pink areas in the graph indicate the 99% confidence intervals (mean ± 3 × SD values) of the muscle size for males and females, respectively. The neuromuscular system in the A5 segment is sexually dimorphic (+) or sexually monomorphic (−). Courtship song-types reported in our previous paper (Chen et al.8) are indicated in the right-hand side of the plots. The song types for pre‐ and post‐mounting songs are shown separated by a hyphen (pre/post): P1–P3, pulse songs (P) distinct in certain song parameters; HPR, high pulse repetition song; S1 and S2, sine songs (S) distinct in certain song parameters; –, no song.
Variations in the number of fibers that constitute the MOL analog
As is the case with other muscles, the MOL in D. melanogaster is composed of several multinucleate muscle fibers. During development, a single founder myocyte recruits additional myocytes, forming a single fiber31. Therefore, the number of nuclei in a fiber corresponds to the total number of myocytes contributed, and the number of fibers coincides with the number of founder myocytes involved in the formation of an MOL. In the D. melanogaster species subgroup, the number of fibers contained in an MOL varies within a species and across the species, whereas the total number of myocytes in a fiber is invariant31. We therefore asked whether intra-species or inter-species variations in the fiber number exist in the montium group and if so, how such variations are correlated with the muscle size or fly phylogeny.
Staining for DNA with TO-PRO-3 iodide revealed an array of nuclei along the length of the MOL, and thus visualized the composite muscle fibers, allowing us to determine unequivocally the number of fibers composing an MOL analog (Fig. 4). We performed this analysis in 19 MOL analog-harboring species and 2 outgroup species, D. melanogaster and D. subobscura. It turned out that all examined species exhibited intra-species variations in the number of fibers composing a MOL analog (Table 2), ranging from 1 to 8 fibers. We also found significant inter-species variations: the largest number was found in D. barbarae (mean ± SEM: 5.3 ± 0.5) and the smallest in D. serrata (2.1 ± 0.1). However, the number of composite fibers did not appear to be correlated with the entire length of the MOL analog or phylogeny. For example, D. barbarae and D. mayri belong to the same serrata subgroup and have large MOL analogs of similar mean lengths, 1.60 ± 0.03 and 1.53 ± 0.02, respectively (Table 1), yet the MOL analogs of these 2 species are each composed of significantly different numbers of fibers, i.e., 5.3 ± 1.6 and 3.6 ± 0.5 (Table 2). We therefore suggest that the difference in the MOL-analog length across species may not result from a difference in the number of founder cells/composite fibers, which varies from species to species even within the same clade.
Inter-species variations in the number of fibers composing an MOL or MOL-analog. White dots represent the position of nuclei recorded on a transparency (see Materials and Methods), which aligns along a longitudinal axis, visualizing a single fiber composing the MOL or MOL-analog. Shown are representative examples of MOL nuclear alignments in D. melanogaster (a), D. subobscura (b), D. baimaii (c), D. barbarae (d), D. birchii (e), D. bocki (f), D. diplacantha (g), D. fengkainensis(h), D. greeni (i), D. kikkawai (j), D. lacteicornis (k), D. leontia (l), D. mayri (m), D. ogumai (n), D. ohnishii (o), D. pectinifera (p), D. serrata (q), D. trapezifrons (r), D. truncata (s), D. tsacasi (t) and D. watanabei (u). Scale bars: 50 μm.
Is Act79B expression a correlate of the MOL?
Among the 6 actin genes on the D. melanogaster genome, Act79B is known to be enriched in the MOL32,33. We therefore reasoned that Act79B expression may be sexually dimorphic. In keeping with this idea, RT-PCR of RNAs from lysates prepared from tergites showed strongly male-biased expression of Act79B in D. kikkawai and D. leontia, 2 montium group members that carry well-developed MOL analogs. Similar male-biased expression was detected in D. melanogaster and D. subobscura, 2 outgroup species that carry fully developed MOLs (Fig. 5a). Notably, Act79B transcript levels were too low to detect in females of D. kikkawai and D. leontia under our experimental conditions (Fig. 5a). We also measured the Act79B mRNA levels in 2 members of the montium group that carry smaller MOL analogs, i.e., D. ogumai and D. ohnishii (both from the kikkawai subgroup), as well as 2 members that do not display a neuromuscular sexual dimorphism in the A5 segment, i.e., D. lini (from the kikkawai subgroup) and D. seguyi (from the seguyi subgroup). These species exhibited distinct Act79B expression patterns: no apparent sex difference in D. lini, strongly female-biased expression in D. ogumai, moderately female-biased expression in D. ohnishii and strongly male-biased expression in D. seguyi (Fig. 5a).
Species differences in sex-biased expression of Act79B, an MOL-enriched actin transcript. (a) Quantitative RT-PCR analysis. Genomic DNA (lanes 1 and 2) and first-strand cDNA (lanes 3–6) were prepared from tergites of abdominal segments A3–A6 of males (lanes 1, 3 and 5) and females (lanes 2, 4 and 6) of D. melanogaster, D. subobscura, and 6 species of the montium group (indicated below each panel) for Act79B (lanes 1–4) and 2 control protein genes, α-Tubulin (lane 5) and Act5C (lane 6). M: DL2000 DNA marker. Primers used are as shown in Table S3. (b–d,f–l) in situ hybridization analysis with probes for coding (b–d) or non-coding (f–l) sequences of the Act79B transcript. Act79B expression in abdominal muscles in the wild-type (b,f) and Act79B mutant (d,h) males and wild-type females (c,g) of D. melanogaster and in wild-type males (i,k) and females (j,l) of D. ogumai (i,j) and D. ohnishii (k,l). (e) Phalloidin staining reveals the MOL in a Act79B mutant male of D. melanogaster even though the mutant lacks Act79B expression (d). The true MOLs with Act79B hybridization signals are indicated with arrowheads. The tergite regions typically occupied by the MOL are circled with dotted lines. Oenocytes emit autofluorescence, resulting in a segmentally repeated labelling pattern marked with *. Scale bars: 200 μm (b,e).
To determine the tissue distribution of Act79B, in situ hybridization analysis was conducted in tergites of D. melanogaster, D. ogumai and D. ohnishii using the RNA sequence coding for a part of D. melanogaster Act79B as a probe (Dm-Act79B probe). We also tested another probe (Dm-Act79B-O1-C1 probe) composed of a 3’UTR sequence of D. melanogaster Act79B that is conserved across species for Act79B orthologs but not among different actin forms. In D. melanogaster tergites, the Dm-Act79B probe yielded signals almost exclusively in the MOL in males and no signals in females under our experimental conditions (Fig. 5b–e). This result was rather unexpected because the sequences of all Actin forms are highly conserved, and the probe used here may cross-react with other Actin proteins. Importantly, Act79B mutant males exhibited no discernible hybridization signal in the MOL and other muscles under the same experimental conditions, supporting the notion that the Dm-Act79B probe detected Act79B and did not detect other actin mRNAs in the MOL of wild-type D. melanogaster males (Fig. 5b–e). A similar result was obtained with the Dm-Act79B-O1-C1 probe which selects for Act79B (Fig. 5f–h), suggesting that Act79B is primarily expressed in the MOL in D. melanogaster. When the Dm-Act79B-O1-C1 probe was used to detect Act79B in D. ogumai and D. ohnishii, hybridization signals were detected in many longitudinal muscles not only in males but also females, including the presumed MOL analogs in males (Fig. 5i–l).
We conclude that, although expression in the MOL is the primary causal factor for male-biased Act79B expression in D. melanogaster and possibly some other Drosophila species with well-developed MOL analogs, male-biased expression of Act79B may not be necessarily predictive of the presence of the MOL analogs in the montium species group members in which Act79B seems to be widely expressed in abdominal muscles irrespective of whether they are MOL analogs or not.
Ancestral reconstruction of the neuromuscular sexual dimorphism
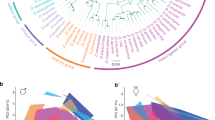
The maximum likelihood (ML) and Bayesian analyses generated almost the same tree topology (Figs. S4 and S5), which was largely congruent with those of Chen et al.8 and other studies34,35. The ancestral state for the presence or absence of a neuromuscular sexual dimorphism in the A5 segment in the subgenus Sophophora was reconstructed on the topology of the Bayesian tree (Fig. 6, left-hand side panel). The 19 montium group species with the sexually dimorphic neuromuscular system in A5 were scattered across 5 species subgroups, i.e., the montium subgroup, the kikkawai subgroup, the punjabiensis subgroup, the serrata subgroup and the seguyi subgroup, while in the remaining 2 subgroups, we examined only 1 or 2 species, which did not display the sexual dimorphism. To make the Bayesian tree more comprehensive, we included an additional 7 species outside of the montium group, i.e., those from the melanogaster, obscura, virilis and repleta species groups (Fig. 6). The reconstruction thus inferred that the most recent common ancestor (MRCA) of Sophophora likely carried the MOL. Then, the loss of the neuromuscular sexual dimorphism in the montium group would have independently occurred at the MRCAs of the parvula, seguyi, and punjabiensis-orosa subgroups and the auraria-rufa species complex (shaded in gray in Fig. 6), although the regain of the neuromuscular sexual dimorphism would have also occurred with the evolution of some species (D. diplacantha, D. tsacasi, D. greeni, D. watanabei, and D. lacteicornis).
Ancestral reconstruction of the neuromuscular sexual dimorphism in A5 for a Bayesian tree by BBM analysis. The presence or absence of the neuromuscular sexual dimorphism in the A5 segment was determined based on the results shown in Figs. 1 and S3 and Table 1. Pie charts along nodes indicate the probability of ancestral distribution of the neuromuscular sexual dimorphism estimated by BBM analysis (left-hand side panel). It is inferred that the neuromuscular sexual dimorphism was lost at the MRCAs of several lineages indicated by shading. The ancestral distribution of pre-mounting song states (right-hand side panel) is similarly constructed based on our previous observations (summarized in Fig. S5 according to Fig. 3 in Chen et al.8) and our unpublished result. The pre-mounting song was probably lost in the two lineages indicated by shading.
We wanted to determine whether the evolutionary history of the neuromuscular sexual dimorphism may have similarity to that of courtship songs in these species, because both traits are known to be controlled by the same master regulator gene fruitless in D. melanogaster 18,19,23 and D. subobscura26. The presence or absence of pre- and post-mounting songs in the montium group reported by Chen et al.8 is summarized in Fig. S5. In Fig. 6 (Fig. 6, right-hand side panel), we included the ancestral reconstruction for the presence or absence of pre-mounting song based on available data8, which revealed that pre-mounting song was probably lost in the MRCA of D. lacteicornis and D. neoasahinai and the D. kikkawai species subgroup (shaded in grey; Fig. 6, left-hand side panel). This result suggests that evolutionary changes in the neuromuscular sexual dimorphism in the A5 segment took place independently of those in pre-mounting song production.
Discussion
In this study, we identified the male-enlarged MOL analogs in 19 out of 41 species of the montium species group by rigorous quantification of the muscle size and subsequent statistical tests (Table 1). The MOL analogs found in this phylogenetic branch were much smaller in size than the MOL ortholog characterized in D. melanogaster (Tables 1 and S1), and this is probably the reason why Gailey et al.9 were unable to detect the MOL analogs in any montium group species they examined. 10 out of 12 species examined by Gailey et al.9 were evaluated in this study: 4 of them were judged to display the neuromuscular sexual dimorphism in the A5 segment, while the rest were not according to our quantitative criteria. To what extent the MOL analogs are sexually dimorphic varies from species to species (Table 1). Act79B mRNA preferentially enriched in the male-specific MOL in D. melanogaster appears to be expressed broadly in the abdominal muscles and in both sexes at lower levels in the montium group species, judging from our observations in a few select species of this group (Fig. 5). The majority of clades in the montium group contain both the species with and those without the neuromuscular sexual dimorphism in the A5 segment, as found in other Drosophila groups by Gailey et al.9, who explained this mixed configuration by random losses of the MOL in some species within a clade. Our ancestral state reconstitution of the neuromuscular sexual dimorphism favors an alternative view that the neuromuscular sexual dimorphism once present in an old ancestral species was subsequently lost in a few of the most recent common ancestors (MRCAs) of the lineage, and some species regained it thereafter (Fig. 6).
Because the MOL formation is a fru-dependent developmental process20, we compared the deduced history of the neuromuscular sexual dimorphism in the A5 segment with that of the courtship song patterns, which is another fru-dependent process36. The ancestral reconstitution suggested that pre-mounting song was lost from two lineages, i.e., the MRCA that delivered both D. lacteicornis and D. neoasahinai and the MRCA that delivered all kikkawai subgroup members (Fig. 6, shaded branches in the pie chart at right). Notably, the former MRCA was likely devoid of the neuromuscular sexual dimorphism in the A5 segment, whereas the latter MRCA probably retained it (Fig. 6, the pie chart at left). In contrast to pre-mounting song, which is limited to only certain species, post-mating song is shared by all montium group species thus far examined, precluding the possibility that the presence or absence of post-mating song is correlated with the MOL formation patterns. As Fig. 3 shows, song types were highly divergent from species to species irrespective of whether the species were MOL-sexually dimorphic or MOL-sexually monomorphic. Collectively, the results indicated that no concerted evolutionary changes existed between the neuromuscular sexual dimorphism in the A5 segment and the song pattern, even though both traits were strictly dependent on neural FruM expression. These findings seem to suggest that the neuromuscular sexual dimorphism in the A5 segment and song pattern were shaped under distinct selective pressures, even though both are developmentally controlled by the common master regulator FruM. FruM has been suggested to bind to approximately 13037–28038 genomic sites, resulting in repression or activation of the transcription of nearby genes39,40. It is plausible that FruM turns ON or OFF different sets of target genes in the two developmental contexts, i.e., the neuromuscular sexual dimorphism in the A5 segment and song circuit formation, and that selective pressures acted through distinct downstream genes in each of these cases, leading to the evolutionary paths unique to each trait.
Materials and methods
Drosophila stocks
The sources of fly stocks are described in Table S2. Flies were maintained on cornmeal-malt medium at 23 °C under a 12:12 h light:dark cycle and 50–60% humidity. Virgin flies were collected and separated into sexes within 12 h of emergence without anaesthesia. Male flies were kept individually in vials (9.5 cm height × 1.5 cm diameter) containing culture medium, whereas females were maintained in vials in groups of five. Reproductively mature virgin flies were used for all experiments: 8-day-old flies for the montium group41 and 4–6 day-old flies for the outgroup species30. No age-dependent change in the MOL size has been reported.
Tissue dissection, immunohistochemistry and imaging
The experimental procedures for dissection and staining of tissues have been described previously4. For observation of the MOL and its analogs, fly abdomens were cut along the dorsal midline and the intestines and other internal tissues were removed to expose the musculature on the internal surface of tergites. The exposed muscles on the tergites were fixed with 4% paraformaldehyde (DF0135; Leagene Biotechnology, China) for 20 min at room temperature (RT), stained with fluorescein isothiocyanate-labeled phalloidin (P5282; Sigma, St. Louis, MO, USA; 1 µg/ml) overnight at 4 °C and examined with a Nikon Eclipse Ti Inverted microscope. For the observation of muscular nuclei, adult dorsal abdomens dissected as above in PBT (0.5% Triton X-100 in PBS) were fixed in 4% paraformaldehyde, washed thoroughly, blocked with PBT + 5% normal goat serum (NGS) for 1 h and incubated with TO-PRO-3 iodide (T3605; Invitrogen, Carlsbad, CA, USA; 1:1000) and fluorescein isothiocyanate labeled phalloidin (P5282; Sigma; 1 µg/ml) overnight at 4 °C. Samples were imaged at 20 × magnification on a Nikon A1 confocal laser microscope. Adobe Photoshop CC software was used to show DNA staining alone in acquired images from the phalloidin-positive regions, so as to clearly visualize the rows of muscular nuclei. The nucleus position was marked on a transparency that overlay a computer screen, on which microscopic images of the MOL or its analogs stained for DNA were displayed.
Muscle size measurement and statistical analyses
Approximately 40 individuals obtained from several different vials were examined to estimate the size of the abdominal muscles for each genotype or species. The exact numbers of individuals and of hemisegments used are shown in Tables 1 and S1. Both the left and right sib muscles were subjected to size measurements and the two values obtained were treated as independent data, because the bilateral counterparts of a muscle pair form independently from each other13,14,16. This treatment unraveled quantitative differences in size between the bilateral counterparts of the MOL analog in single individuals of some montium group species.
The size of the MOL analogs and other abdominal muscles was measured by their Feret’s diameter, which is defined as the longest distance between any 2 points along the selection boundary and is also known as the maximum caliper. The Feret’s diameter of muscles was estimated on fluorescent microscopic images of muscles with the aid of the Fiji package of the ImageJ software (https://imagej.net/Welcome). Briefly, the threshold for the fluorescent intensity was set at an appropriate level (typically ~ 14% of the maximal value) to distinguish foreground pixels from background pixels (Fig. S1a) upon choosing an object area to measure with the ROI manager (Fig. S1b). The Fiji program then gave the Feret’s diameter for the object chosen for the measurement (Fig. S1c,d). In every abdominal hemi-segment, Feret’s diameters of the longest muscle as a candidate MOL analog (FA) and the most medial longitudinal muscle as a control (FB) were recorded. The standardized Feret's diameter, FA/FB, was used for the muscle size comparisons between the left and right hemi-segments within the same fly, among conspecific individuals or among the species. FA/FB can be a value smaller than 1.0 when the most medial longitudinal muscle is the longest muscle in the hemisegment. We chose the most medial muscle as the control because no known MOL and MOL analogs occupy the medial-most position, and no sexual dimorphism is detected in this muscle. Frequency histograms were constructed for the measured FA/FB for each fly group and fitted by a Gaussian distribution with or without log-transformations. The distributions were further analyzed with GraphPad Prism 8.0 for Mac by one-way ANOVA (the Brown-Forsythe and Welch ANOVA test) followed by the Games-Howell’s comparisons test. In cases where the Gaussian function failed to fit the data, the nonparametric Kruskal–Wallis test was used for statistical comparisons. The confidence interval was set at 99.9% unless otherwise stated. When FA/FB in males is larger than that in females at the statistically significant level P < 0.001, we judge that the males have the sexually dimorphic MOL analog.
Amplification of 79B actin mRNAs
Total RNA was isolated from the tergite of A3-A5 of a single fly using the TRIzol Reagent and Phasemaker Tubes Complete System (A33251, Invitrogen, USA). cDNA was synthesized using a PrimeScript RT reagent Kit with gDNA Eraser (RR047A, Takara, Japan). All PCR reactions were performed in a 25 μl mixture using Golden Star T6 Super PCR Mix (TSE101, TsingKe, China).
To facilitate the design of the 79B actin specific primers, we downloaded act79B, act5C and α-Tub84B cDNA sequences (Accession Numbers: NT_037436, NC_004354 and NT_033777, respectively) of D. melanogaster, and then used the BLAST tool of NCBI to download act79B and act5C cDNA sequences (Accession Numbers: XM_017167530 and XM_017180678) of D. kikkawai. The D. subobscura genome assembly42 was used for local BLAST via GENETYX-MAC software (version 18.0) to determine the act79B and α-Tub84B genomic DNA sequences. In particular, it should be noted that since the 5’ ends of act79B and act5C mRNAs are highly conserved43, we need to design primers at the 3’ end to ensure primer specificity. After PCR testing of multiple primer combinations, we selected the primer pairs in Table S3 to amplify the region of Act79B and reference gene cDNAs.
In situ hybridization
RNA scope-based FISH (Advanced Cell diagnostics (ACD), 320850) was performed according to the manufacturer’s protocol with some modifications, using the Dm-Act79B probe (ACD, 451771) or Dm-Act79B-O1-C1 probe to detect act79B mRNA. The dorsal muscles, including the MOL, were fixed in 4% PFA for 1 h at 4 °C. A series of MeOH concentrations, i.e., 25%, 50% and 75% in 0.01% PBT (0.01% Tween-20 in PBS), followed by 2 × 100% was used to dehydrate the muscles. After rehydration in 0.01% PBT, the muscles were digested by Protease III for 20 min at RT and post-fixed in 4% PFA for 30 min at 4 °C. The probe hybridization was performed at 40 °C overnight, followed by a second post-fixation in 4% PFA for 10 min at RT. The RNA signal was amplified by Amp 1–4 at 40 °C. After each hybridization step, embryos were washed with 0.02% SSCT (0.02% Tween-20 in 1xSSC). Amp 4 Alt A-FL was used for the fluorescent labeling.
Phylogenetic analysis
A total of 48 species in the subgenus Sophophora of the genus Drosophila were used for the phylogenetic analysis, including the 41 species of the montium group8. Sequences of 2 mitochondrial (COI and COII) and 3 nuclear (Adh, Amy1, and Amyrel) genes were obtained from GenBank (Table S4). Intron sequences of the nuclear genes were removed before the analysis due to a high degree of alignment ambiguity. Nucleotide sequences of individual gene regions were aligned using MUSCLE44 implemented in SeaView 4.745 or MEGA X46 with default settings. Individual alignments were concatenated by using FASconCAT 1.047. Phylogenetic trees were constructed based on concatenated sequences, using the maximum likelihood (ML) and Bayesian methods, in which sequences were partitioned according to the best partitioning scheme obtained with PartitionFinder 2.1.148 under the options AICc, “greedy” algorithm, and “models = all”. ML analyses were conducted by using RAxML 8.1.2149 with raxmlGUI 1.5beta50, in which the “GTRGAMMA” model was applied for all data partitions. A bootstrap analysis of 1000 replicates was performed by using an “ML + rapid bootstrap” search. Bayesian analyses were conducted by using MrBayes 3.2.7a51. The best-fit substitution model for each data partition was obtained with PartitionFinder 2.1.1. A Markov-Chain Monte-Carlo (MCMC) search was performed with 4 chains, each of which was run for 10 million generations. Trees were sampled every 100 generations, and the first 25% of the samples were discarded as burn-in. The trace file generated by the Bayesian MCMC runs was inspected in TRACER 1.7.152 to check whether the number of sampling generations and effective sample sizes (ESS) were large enough for reliable parameter estimates.
Ancestral state reconstruction
Ancestral state reconstruction was carried out for the presence or absence of two characteristics: first, the neuromuscular sexual dimorphism in the A5 segment identified in the present study (Tables 1 and S1), and second, pre-mounting songs based on our previous paper8. The reconstruction was performed by using Bayesian binary MCMC (BBM) analysis53 implemented in RASP 4.254. Taking phylogenetic uncertainty into account, 10,000 trees randomly selected from 150,000 post burn-in trees generated by MrBayes were used as input trees. The BBM analysis was then run on a consensus Bayesian topology, with the maximum number of areas set to 1 and without allowing null root distribution. The MCMC chain was run for 50,000 generations using 10 chains and sampled every 100 generations. An estimated F81 model55 with default Dirichlet distribution (0.5 and 0.5) and equal rates for among-site rate variation was used for the analysis.
Data availability
We agree to deposit our data to a public repository.
References
Darwin, C. The Decent of Man, and Selection in Relation to Sex 2nd edn, Vol. 688 (D. Appleton and Company, 1889).
Carroll, S. B. Endless Forms Most Beautiful: The New Science of Evo Devo Vol. 331 (W.W. Norton & Company, 2005).
Sato, K., Tanaka, R., Ishikawa, Y. & Yamamoto, D. Behavioral evolution of Drosophila: unraveling the circuit basis. Genes (Basel) https://doi.org/10.3390/genes11020157 (2020).
Gailey, D. A., Taylor, B. J. & Hall, J. C. Elements of the fruitless locus regulate development of the muscle of Lawrence, a male-specific structure in the abdomen of Drosophila melanogaster adults. Development (Cambridge, England) 113, 879–890 (1991).
Toda, M. J. DrosWLD a Database on Taxonomy of Drosophilidae https://bioinfo.museum.hokudai.ac.jp/db/modules/stdb/index.php?start=0&perpage=20&action=data&tbl_id=21 (2021).
Yassin, A. Phylogenetic biogeography and classification of the Drosophila montium species group (Diptera: Drosophilidae). Ann. Soc. Entomol. Fr. 54, 167–175. https://doi.org/10.1080/00379271.2018.1447853 (2018).
Chen, C.-C. et al. Courtship patterns in the Drosophila montium species subgroup: Repeated loss of precopulatory courtship?. Zool. Sci. 30, 1056–1062. https://doi.org/10.2108/zsj.30.1056 (2013).
Chen, A.-L. et al. Evolution and diversity of the courtship repertoire in the Drosophila montium species group (Diptera: Drosophilidae). J. Evol. Biol. 32, 1124–1140. https://doi.org/10.1111/jeb.13515 (2019).
Gailey, D. A. et al. The muscle of lawrence in Drosophila: A case of repeated evolutionary loss. Proc. Natl. Acad. Sci. U. S. A. 94, 4543–4547. https://doi.org/10.1073/pnas.94.9.4543 (1997).
Takayanagi, S. et al. A fruitless upstream region that defines the species specificity in the male-specific muscle patterning in Drosophila. J. Neurogenet. 29, 23–29. https://doi.org/10.3109/01677063.2014.983101 (2015).
Currie, D. A. & Bate, M. Innervation is essential for the development and differentiation of a sex-specific adult muscle in Drosophila melanogaster. Development (Cambridge, England) 121, 2549–2557 (1995).
Taylor, B. J. & Knittel, L. M. Sex-specific differentiation of a male-specific abdominal muscle, the Muscle of Lawrence, is abnormal in hydroxyurea-treated and in fruitless male flies. Development (Cambridge, England) 121, 3079–3088 (1995).
Nojima, T., Kimura, K.-I., Koganezawa, M. & Yamamoto, D. Neuronal synaptic outputs determine the sexual fate of postsynaptic targets. Curr. Biol. 20, 836–840. https://doi.org/10.1016/j.cub.2010.02.064 (2010).
Kimura, K.-I., Usui, K. & Tanimura, T. Female myoblasts can participate in the formation of a male-specific muscle in Drosophila. Zool. Sci. 11, 247–251 (1994).
Lawrence, P. A. & Johnston, P. The genetic specification of pattern in a Drosophila muscle. Cell 36, 775–782. https://doi.org/10.1016/0092-8674(84)90357-x (1984).
Lawrence, P. A. & Johnston, P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell 45, 505–513. https://doi.org/10.1016/0092-8674(86)90282-5 (1986).
Billeter, J.-C. et al. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 16, 1063–1076. https://doi.org/10.1016/j.cub.2006.04.039 (2006).
Ito, H. et al. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. U. S. A. 93, 9687–9692. https://doi.org/10.1073/pnas.93.18.9687 (1996).
Ryner, L. C. et al. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089. https://doi.org/10.1016/s0092-8674(00)81802-4 (1996).
Usui-Aoki, K. et al. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat. Cell Biol. 2, 500–506. https://doi.org/10.1038/35019537 (2000).
Taylor, B. J. Differentiation of a male-specific muscle in Drosophila melanogaster does not require the sex-determining genes doublesex or intersex. Genetics 132, 179–191 (1992).
Shirangi, T. R., Wong, A. M., Truman, J. W. & Stern, D. L. Doublesex regulates the connectivity of a neural circuit controlling Drosophila male courtship song. Dev. Cell 37, 533–544. https://doi.org/10.1016/j.devcel.2016.05.012 (2016).
Demir, E. & Dickson, B. J. fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794. https://doi.org/10.1016/j.cell.2005.04.027 (2005).
Yamamoto, D. & Koganezawa, M. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 14, 681–692. https://doi.org/10.1038/nrn3567 (2013).
Seeholzer, L. F., Seppo, M., Stern, D. L. & Ruta, V. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559, 564–569. https://doi.org/10.1038/s41586-018-0322-9 (2018).
Tanaka, R., Higuchi, T., Kohatsu, S., Sato, K. & Yamamoto, D. Optogenetic activation of the fruitless-labeled circuitry in Drosophila subobscura males induces mating motor acts. J. Neurosci. 37, 11662–11674. https://doi.org/10.1523/jneurosci.1943-17.2017 (2017).
Hall, J. C. The mating of a fly. Science 264, 1702–1714. https://doi.org/10.1126/science.8209251 (1994).
Philipsborn, A. C. et al. Cellular and behavioral functions of fruitless isoforms in Drosophila courtship. Curr. Biol. 24, 242–251. https://doi.org/10.1016/j.cub.2013.12.015 (2014).
Li, Y.-F., Wen, S.-Y. & Ritchie, M. G. Copulatory song in three species of the Drosophila montium subgroup extends copulation and shows unusual genetic control. Anim. Behav. 83, 233–238. https://doi.org/10.1016/j.anbehav.2011.10.032 (2012).
Spieth, H. T. Mating behavior within the genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist. 99, 399–474 (1952).
Orgogozo, V., Muro, N. M. & Stern, D. L. Variation in fiber number of a male-specific muscle between Drosophila species: A genetic and developmental analysis. Evol. Dev. 9, 368–377. https://doi.org/10.1111/j.1525-142X.2007.00174.x (2007).
Courchesne-Smith, C. L. & Tobin, S. L. Tissue-specific expression of the 79B actin gene during Drosophila development. Dev. Biol. 133, 313–321. https://doi.org/10.1016/0012-1606(89)90036-5 (1989).
Ohshima, S., Villarimo, C. & Gailey, D. A. Reassessment of 79B actin gene expression in the abdomen of adult Drosophila melanogaster. Insect Mol. Biol. 6, 227–231. https://doi.org/10.1046/j.1365-2583.1997.00176.x (1997).
Schawaroch, V. Phylogeny of a paradigm lineage: The Drosophila melanogaster species group (Diptera: Drosophilidae). Biol. J. Linn. Soc. 76, 21–37. https://doi.org/10.1111/j.1095-8312.2002.tb01711.x (2002).
Yassin, A. et al. The pdm3 locus is a hotspot for recurrent evolution of female-limited color dimorphism in Drosophila. Curr. Biol. 26, 2412–2422. https://doi.org/10.1016/j.cub.2016.07.016 (2016).
Villella, A. et al. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics 147, 1107–1130 (1997).
Ito, H. et al. Fruitless recruits two antagonistic chromatin factors to establish single-neuron sexual dimorphism. Cell 149, 1327–1338. https://doi.org/10.1016/j.cell.2012.04.025 (2012).
Neville, M. C. et al. Male-specific fruitless isoforms target neurodevelopmental genes to specify a sexually dimorphic nervous system. Curr. Biol. 24, 229–241. https://doi.org/10.1016/j.cub.2013.11.035 (2014).
Ito, H., Sato, K., Kondo, S., Ueda, R. & Yamamoto, D. Fruitless represses robo1 transcription to shape male-specific neural morphology and behavior in Drosophila. Curr. Biol. 26, 1532–1542. https://doi.org/10.1016/j.cub.2016.04.067 (2016).
Sato, K., Ito, H., Yokoyama, A., Toba, G. & Yamamoto, D. Partial proteasomal degradation of Lola triggers the male-to-female switch of a dimorphic courtship circuit. Nat. Commun. 10, 166. https://doi.org/10.1038/s41467-018-08146-1 (2019).
Wen, S.-Y. et al. Copulatory courtship behavior and sine song as a mate recognition cue in Drosophila lini and its sibling species. Zool. Sci. 28, 469–475. https://doi.org/10.2108/zsj.28.469 (2011).
Seabra, S. G. et al. Different genomic changes underlie adaptive evolution in populations of contrasting history. Mol. Biol. Evol. 35, 549–563. https://doi.org/10.1093/molbev/msx247 (2018).
Fyrberg, E. A., Mahaffey, J. W., Bond, B. J. & Davidson, N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell 33, 115–123. https://doi.org/10.1016/0092-8674(83)90340-9 (1983).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. https://doi.org/10.1093/nar/gkh340 (2004).
Gouy, M., Guindon, S. & Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224. https://doi.org/10.1093/molbev/msp259 (2010).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Kück, P. & Meusemann, K. FASconCAT: Convenient handling of data matrices. Mol. Phylogenet. Evol. 56, 1115–1118. https://doi.org/10.1016/j.ympev.2010.04.024 (2010).
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T. & Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773. https://doi.org/10.1093/molbev/msw260 (2017).
Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033 (2014).
Silvestro, D. & Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 12, 335–337. https://doi.org/10.1007/s13127-011-0056-0 (2012).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. https://doi.org/10.1093/sysbio/sys029 (2012).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904. https://doi.org/10.1093/sysbio/syy032 (2018).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics (Oxford, England) 19, 1572–1574. https://doi.org/10.1093/bioinformatics/btg180 (2003).
Yu, Y., Harris, A. J., Blair, C. & He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 87, 46–49. https://doi.org/10.1016/j.ympev.2015.03.008 (2015).
Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 17, 368–376. https://doi.org/10.1007/bf01734359 (1981).
Acknowledgements
We thank Dr. Jun-zheng Zhang, Prof. Kai Yuan, the Drosophila Stock Center of Ehime University (Ehime, Japan), Prof. Toshiro Aigaki, Prof. Richard Cripps and the Drosophila Species Stock Center (San Diego, CA, USA) for fly stocks. We appreciate the assistance of Dr. Jian-jun Gao in identifying outgroup species and the secretarial assistance of Ms. Y. Takamura. Our appreciation also to Prof. Yi-kang Rong and Dr. Lin Cheng for sharing their experimental protocols. This work was supported in part by the National Scientific Foundation of China (31372187 to SYW), the Doctoral Joint Training Scholarship from South China Agricultural University (2017LHPY023 to HQL) and Grants-in-Aid for Scientific Research from the Japan Ministry of Education, Culture, Sports, Science and Technology (16H06371, 19H04923 and 21H04790 to DY; 17K07040, 19H04766 to KS). We thank Prof. Masanori J. Toda for his long-term support of our studies on the montium group.
Author information
Authors and Affiliations
Contributions
All authors of this article have directly participated in the planning, execution, or analysis of this study. Conceptualization: S.Y.W.; Experiments: H.Q.L., K.S.; Analysis: H.Q.L., T.K., Writing-original draft: H.Q.L., D.Y., S.Y.W.; Writing-review and editing: H.Q.L., T.K., D.Y., S.Y.W.; Visualization: H.Q.L., T.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, Hq., Katoh, T., Sato, K. et al. Evolution of a neuromuscular sexual dimorphism in the Drosophila montium species group. Sci Rep 11, 15272 (2021). https://doi.org/10.1038/s41598-021-94722-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94722-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.