Abstract
Novel mutant camelina has become a crop of interest inspired by its short growing season, low harvesting costs and high oil composition. Despite those advantages, limited research has been done on novel mutant lines to determine applicability for biodiesel production. Jatropha is an extremely hardy, frugal and high oil yielding plant species. The major aim of the present study was not only to compare biodiesel production from jatropha and camelina but was also to test the efficacy of camelina mutant lines (M6 progenies) as superior feedstock. The biodiesel yield from camelina oil and jatropha oil was 96% and 92%, respectively. The gas chromatographic analysis using flame ionization detector (GC-FID) showed that mutant camelina oil biodiesel sample contain major amount of oleic acid (46.54 wt%) followed by linolenic acid (20.41 wt%) and linoleic acid (16.55 wt%). Jatropha biodiesel found to contain major amount of oleic acid (45.03 wt%) followed by linoleic acid (25.07 wt%) and palmitic acid (19.31 wt%). The fuel properties of produced biodiesel were found in good agreement with EN14214 and ASTM D6751 standards. The mutant camelina lines biodiesel have shown comparatively better fuel properties than jatropha. It has shown low saponification value (120.87–149.35), high iodine value (130.2–157.9) and better cetane number (48.53–59.35) compared to jatropha biodiesel which have high saponification value (177.39–198.9), low iodine value (109.7–123.1) and lesser cetane number (47.76–51.26). The results of the present student of utilizing novel mutant camelina lines for biodiesel production are quite promising and are helpful in turning out the outcomes of the previous studies suggesting that C. sativa biodiesel presents serious drawbacks for biodiesel applications.
Similar content being viewed by others
Introduction
Energy is an essential element for all types of economic and social development. Renewable energy technologies use the natural phenomena of converting feedstock into useful forms of energy1. The socioeconomic impacts of providing power through renewable resources on a local economy instead of conventional generation technologies are very important. The four most important reasons why the world needs biofuels are (i) Combating climate change (ii) Responding to higher energy consumption (iii) Securing energy supply (iv) Making the most of scarce resources. Combating climate change making to look for alternative energy and fuel sources having low carbon. By the 2050, the world population expected to increase to 8 or even 10.5 billion. In addition, there will be substantially increased energy consumption due to growth in emerging economies. Increasing energy demand in future will pose critical challenges to security of energy supply as fuel resources are scattered around the globe. Reducing the wasted energy amount and making the most of our valuable natural resources is another important crucial thing for our future survival. Biofuels offer a solution to all these problems2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17.
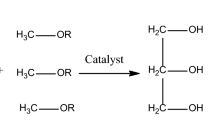
Biodiesel is the most valuable form of renewable energy that can be used directly in any existing, unmodified diesel engine. Biodiesel is best renewable substitute for diesel engines4,18,19. Chemically, biodiesel is made up of methyl esters of fatty acid obtained by the transesterification reaction of vegetable oil with alcohol18,20. Biodiesel burns much cleaner with low emissions of pollutants than petroleum diesel. On combustion, it produces fewer air pollutants such as carbon monoxide, particulates, hydrocarbons, sulfur dioxide, and air toxics21,22,23. Energy Scientists are exploring different cheap biodiesel feedstock to divert the world from high priced pollution producing fossil fuel to the alternate renewable energy resources. The potential of using biodiesel as a fuel for combustion engine has opened up a new horizon for using a wide range of feedstocks for extraction of oil grouped as edible oils, non-edible oils, algal oils (both micro and macroalgae) and genetically engineered plant oils4. Microalgae are also being utilized for biodiesel production and its utilization is dependent upon its biomass, fatty acid profile and lipid productivity24,25. The genetically engineered feedstocks are most recent feedstock for biodiesel production26. There is no doubt that Jatropha is a good feedstock for biodiesel production at mass scale. However, it is a poisonous plant and its crude oil (also called hell oil) is carcinogenic for human skin, the seed is poisonous for human as only 4–5 seeds ingestion is enough to cause death27. Its negative impact in case of mono-cropping in flora and fauna of terrestrial and aquatic life has been reported. It has been declared as the natural disaster by the environmentalists28. On critical comparison between camelina and jatropha following extremely important fact were found and all these observations supports the use of mutant camelina lines for biodiesel production. As for as the comparison of jatropha with camelina for biofuel production is concerned a detailed study in Thailand (one of the biggest growers of Jatropha) showed that camelina has the net energy ratio as high as 5.22 as compared to the Jatropha with 3.74. C. sativa have positive energy balance even for the production of biodiesel only (net energy ratio = 1.47) whereas Jatropha has negative energy balance based on such criterion (net energy ratio = 0.68)29,30. One of the myths about jatropha is that it can grow anywhere without care, but studies have shown that in the initial 3–4 years it needs more irrigation water than any other cultivated crops. The cultivation of Jatropha is not economical unless proper inputs are provided31,32. Camelina seeds contain average 37% of oil 25–45% protein, 35–49% lipid32,33. Camelina oil naturally contains over 50% fatty acids including oleic acid (14.5–19.7%), linolenic acid (32.6–38.2%), linoleic acid (16.9–19.6%) and gadoleic acid (12.4–16.2%). The fatty acid composition of camelina oil varies for different genotypes under a range of locations and environmental conditions31,32. These unsaturated fatty acids are good for cardiovascular health and heart because it reduces the low-density lipoprotein (LDL) and cholesterol level in the blood. The oil contains many natural antioxidants, such as tocopherol, which enhance the shelf life of oil34. Many previous studies critically noted that camelina is not a good feedstock as its biodiesel presents serious drawbacks for biodiesel applications. These draws backs were found due to exhibited the poorest oxidative stability, highest distillation temperature and has the highest potential to form coke during combustion, all which attributed to the high percentage of polyunsaturated fatty acid (n − 3) methyl esters in camelina oil35,36,37.
The present study reports the comparative use of novel mutant camelina and jatropha seeds oils as a low-cost feedstock for biodiesel-production. The findings of the present study will be helpful in commercializing novel mutant camelina for biodiesel production.
Material and methods
Materials
The seed of 3000 camelina mutant lines (M5 progenies) were obtained from the Department of Biological Sciences, University of California Davis, USA. The seeds of these lines were sown under randomized complete block design in three replications to get M6 progenies at the experimental farm of Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad-Pakistan. Fifty high yielding and drought tolerant lines were selected from 3000 mutant lines. The oil was extracted from the seed of harvested 50 mutant M6 drought tolerance lines. Jatropha oil was obtained from the local market of Faisalabad, Pakistan. Methanol, KOH, H2SO4, Na2S2O3.5H2O, and Wijs reagent (Iodine chloride) used in the present study were of analytical grade and purchased from Merck.
Extraction of oil and production of biodiesel
Healthy seeds of camelina from M6 population obtained by M5 mutant lines were washed, sun dried, and oil was extracted from cold press method. The jatropha oil was purchased from the local market of Faisalabad, Pakistan. Cold pressed seed oils of mutant camelina and jatropha were transesterified into biodiesel and glycerol (by-product). For acid catalyzed transesterification reaction, a mixture of 25 g of mutant camelina/jatropha seed oil, 6:1 methanol to oil molar ratio and required amount of H2SO4 (25%, 50% and 100% in weight as compared to oil) was heated at 60 °C for 4.5 h with constant stirring. For base catalyzed transesterification, a mixture of 25 g of mutant camelina/jatropha seed oil, 1:3 methanol to oil molar ratio and required amount of KOH (0.125%, 0.25% and 0.5% in weight compared to oil) was heated at 60 °C for 1.5 h with constant stirring. The produced biodiesel was separated from reaction mixture by separatory funnel after the settling of solution in two layers, upper biodiesel layer and the lower glycerol layer. Biodiesel was washed with hot water for the removal of soap and excess methanol.
Determination of fuel properties
Fatty acid composition of methyl esters was determined by GC-FID analysis. These analyses were performed at University of Agriculture Faisalabad. HI-8014 HANNA instruments pH meter was used for measuring pH of the produced biodiesel. For the determination of density (g/ml) of all biodiesel samples, mass of 1 ml of each produced biodiesel sample was weighed. Standard specific gravity bottle was used to determine specific gravity of biodiesel. The iodine value (IV) (Degree of unsaturation) was measured by dissolving the 0.5 g of biodiesel in 20 ml of CCl4 and 25 ml of Wijs solution which taken in glass stoppered iodine flask of 250 ml capacity. The flask was vigorously shaken and placed in the dark for half an hour. Then added 15% solution of KI and 100 ml of distilled water. The solution was titrated against 0.1 N Na2S2O3.5H2O using starch as indicator till the disappearance of yellow color of iodine. The procedure was repeated for the blank. The IV was calculated using following formula18:
where S and B represent the volume of titrant used for sample and blank.
For the determination of saponification value (SV) of biodiesel, 0.5 g biodiesel sample and 20 ml alcoholic KOH solution was taken in a 250 ml round bottom flask connected with a condenser. The mixture was heated gently until completion of saponification reaction (a clear solution was indication of completion of reaction). Two drops of phenolphthalein indicator were added into reaction mixture at room temperature. The solution was titrated against 0.5 N HCl until the pink color disappeared. Same procedure was used to measure the bank value. It was measured by using the following formula18:
where B and S are volume of titrant (ml) used for the blank and biodiesel sample while W is the mass of biodiesel sample used, N is the normality of HCl solution and 56.1 is the molecular weight of KOH (g/mol).
The acid value of biodiesel was measured by taking 0.5 g of biodiesel, 10 ml of ethanol and 1–2 drops of phenolphthalein in a 250 ml titration flask. It was titrated against 0.1 N NaOH solution until the appearance of pink color. Free fatty acids (FFA) were calculated from following formula38.
where V and N is volume and normality of NaOH titrant used, weight of biodiesel used and 282 is the molecular weight of oleic acid as equivalent molecular weight of oil. The percentage of FFA free fatty acid can be converted to acid value by the following formula18.
Cetane number (CN) of the produced biodiesel samples was calculated using iodine value (IV) and saponification value (SV) by the following equation39.
Results and discussions
Effect of catalysts on biodiesel yield (%)
Effect of catalyst concentration for maximizing the biodiesel yield from mutant camelina and jatropha seed oil was evaluated by using H2SO4 and KOH as catalysts at three concentrations shown levels (Figs. 1, 2). Maximum yield of biodiesel was recorded to be 96.28% and 92.8% for mutant camelina and jatropha seed oil at 0.125% KOH as alkaline catalyst with a reaction conditions of 3:1 oil to methanol molar ratio, 60 °C temperature, 1.5 h reaction time at constant stirring (120 rpm). In base catalyzed transesterification reaction higher catalyst concentrations resulted in reduced biodiesel production. Bases favor esterification reaction along with transesterification at higher concentrations which results in the production of soap. This not ultimately reduce biodiesel yield but also makes separation of byproducts from biodiesel difficult. It was reported in a previous study that camelina biodiesel conversion rate decrease by increase the concentration and the maximum biodiesel yield (92.6%) was obtained at conventional homogeneous alkaline-catalyst concentration (0.75 wt.%) at 40 °C for 40 min with 8:1 methanol to oil molar ratio20. In case of acid catalyzed transesterification reaction, biodiesel yield increased with increase in the H2SO4 concentration for mutant camelina oil. A similar trend of biodiesel production was reported in a previous studied biodiesel production from waste tallow40. A slight decrease in biodiesel yield was recorded for jatropha oil. The biodiesel produced from mutant camelina was not only better in respect of yield but was also have better stability and fuel properties when compared to camelina biodiesel reported in the previous studies35,36,37. Optimized concentration for biodiesel yield was obtained at 0.125% of KOH catalyst. Mutant camelina (96.28%) exhibited higher biodiesel yield than jatropha biodiesel yield (92.8%). In short, mutant camelina could be another new potential feedstock for biodiesel production along with jatropha.
Characterization of biodiesel profile
The fuel properties of biodiesel are related to the nature of fatty acids present in the feedstock oil. The degree of unsaturation and chain length determine the physical properties of biodiesel. Tranesterification does not alter fatty acid composition of raw feedstocks41. The fatty acid composition of studied biodiesel was shown in Table 1. Triglycerides present in vegetable oil have great potential for biodiesel production, but its quality depends upon the composition of oil. The good quality of biodiesel produced from the oil which have high amount of monounsaturated fatty acid and lower saturated and polyunsaturated fatty acid. The biodiesel produced from sunflower oil have poor oxidation stability due to high polyunsaturated fatty acid. Similarly biodiesel production from palm oil have poor flow properties or may be solid at room temperature and perform better in temperate regions42. The mutant camelina and jatropha biodiesel profile was identified by gas chromatographic analysis equipped with flame ionization detector (GC-FID).
The gas chromatographic analysis using flame ionization detector (GC-FID) (Table 1) showed that mutant camelina oil biodiesel sample contain major amount of oleic acid (46.54 wt%) followed by linolenic acid (20.41 wt%) and linoleic acid (16.55 wt%). Jatropha biodiesel found to contain major amount of oleic acid (45.03 wt%) followed by linoleic acid (25.07 wt%) and palmitic acid (19.31 wt%). The fatty acid composition of mutant camelina was quite variable from camelina oil reviewed previously43. Mutant camelina lines found to have higher amounts of oleic acid than non-mutant varieties and could serve as a potential feedstock in biofuel sector in upcoming years. However, jatropha fatty acid profile was similar to previously reported work44. One pervious study has reported that biodiesel containing fatty acids more than 15 carbon atoms is considered to be of superior quality45. Both mutant camelina and jatropha biodiesels contains high quantity of fatty acids having more than 15 carbon atoms and could serve as good sources for renewable energy.
Assessment of fuel properties
Physical properties
The physical properties of both mutant camelina and jatropha biodiesel were evaluated and summarized in Tables 2, 3, 4and 5. The pH of biodiesel produced from mutant camelina (M6) and jatropha are presented in Table 2. The densities of various jatropha and mutant camelina biodiesel samples were found ranging from 0.67–0.72 g/ml and 0.74–0.89 g/ml, respectively. Most of the values of densities for mutant camelina biodiesel are in the specified range by European standards (0.86–0.90 g/ml) while those of jatropha biodiesel values found below the recommended limits. The maximum density 0.89 g/ml was observed for the biodiesel produced from mutant camelina at 0.50% KOH (w/w% of oil). The minimum value of density (0.68 g/ml) was observed for jatropha biodiesel at catalyst concentration of 100% H2SO4 (w/w% of oil) (Table 3). The density of biodiesel has influence the spray properties, injection timing and injection system46. Greater density of biodiesel, more mass of fuel will be injected in the cylinder which increases the energy of the engine and vice versa. Generally the density of biodiesel decrease with molecular weight and increase with unsaturation level of oil42. Viscosity defined as “the resistance in flowing liquid”. The viscosity of mutant camelina and jatropha biodiesel produced by using various acid and alkali catalysts concentrations was 113–119 centipoise (CP) and 64–82 CP, respectively (Table 4). In the previous study 130 centipoise viscosity was recorded in camelina sativa biodiesel47. The viscosity of biodiesel decreases with increasing unsaturation level and temperature but increases with molecular weight48,49,50. “The point at which crystals of biodiesel starts to appear known as cloud point”. “The temperature at which fuel converted to gel like appearance which do not flow known as fuel's pour point”. Cloud point of mutant camelina was found varying from − 2 to − 2.5 °C and jatropha from 0.2 to − 2 °C. A lower cloud point makes biodiesel even suitable during winters or in cold areas47,51. There is no limit defined for cloud point and pour point in European and American standards and it changes with respect to climatic zone37. Higher cloud point causes poor flow problem in the combustion chamber, delayed startup and misfire. The cloud point of biodiesel decreases with increase in the degree of unsaturation and increases by increase in the fatty acid chain length47,52. Mutant camelina biodiesel have present good fuel properties for its utilization as renewable energy resource.
Chemical properties
Experimental results showed that mutant camelina and jatropha biodiesel produced by acid and base catalysts have free fatty acid (FFA) in the range of 0.35–0.43% and 10.56–12.98%, respectively (Table 5). FFA and moisture content has significant effect on transesterification of triglycerides with alcohol using catalyst53. The high FFA content (> 1% w/w) will produce the soap in large quantities and cause difficulty during the separation of biodiesel layer. This ultimately decrease biodiesel yield54. In the present study, the mutant camelina biodiesel was found to contain much lower amount of FFA which is one of requirements for the production of good quality biodiesel55. The acid value determined for mutant camelina and jatropha biodiesel were 0.59 to 0.67 and 21 to 23.55, respectively (Table 6). Acid value indicates the concentration of free fatty acids in the biodiesel sample. Experimental results showed that jatropha biodiesel have high free fatty acids as compared to mutant camelina. Jatropha biodiesel exhibited much higher acid values than European and American standards. The saponification values of mutant camelina and jatropha biodiesel produced by acid and base catalysts are summarized in Table 7. The observed saponification values mutant camelina and jatropha were 120.87–149.35 and 177.39–198.9, respectively. The saponification value of 242 was recorded for date palm biodiesel18 and 193.33 for jatropha biodiesel previously53. Mutant camelina has exhibited quite suitable saponification values for good quality biodiesel production. The iodine value is a measure of the degree of unsaturation of fats and oils. Higher iodine value indicates higher unsaturation in fats and oils56,57. The iodine value of mutant camelina and jatropha oil was found to be 130.2–157.9 and 109.7–123.1, respectively (Table 8). Iodine value relies on the unsaturated fatty acid and indicate double bound present in biodiesel sample58. According to European standards iodine values equal or less than 120 is preferable for biodiesel produced for consumption. However, in American standards no iodine value is specified. Unsaturation represented by iodine value reflects biofuel chance of solidification45. Two previous studies reported camelina biodiesel having iodine values of 15147 and 15352. Iodine value for mutant camelina biodiesel was observed to be 120 by using catalyst 0.125% KOH at 60 °C for 1.5 h in the present study. Higher iodine values indicate the presence of the higher amounts of unsaturated fatty acids in the biodiesel. Such higher amounts undergo polymerization reactions due to high availability of unsaturated bonds at high temperature in the internal combustion engine. The ultimate result is the deposition or deterioration of biodiesel in the engine41. Fuels that have such properties (e.g. safflower oil, sunflower oil and soybean oil) also considered to produce thick sludges in the sump of the engine during fuel seeping down the sides of the cylinder into crankcase42. Jatropha oil is placed in the semi-drying oil group according to its iodine values. High iodine values of mutant camelina and jatropha oils are due to the high contents of unsaturation fatty acids such as linoleic acid and oleic acid. Cetane number (CN) is very important quality parameter for evaluating the biodiesel. Generally, it is similar to the octane number of gasoline, and give us information about fuel ignition performance inside the engine56. It give idea that how quickly fuel burn during the combustion inside the cylinder of engine. The biodiesel which have higher cetane number ignite quickly and completely. On the contrary, low cetane number lead to incomplete combustion, emission of NOx and may cause knocking during combustion59. According to European and American standards, the minimum cetane number is 47 and 51, respectively. The cetane number of conventional petro-diesel is 40 which is lower than biodiesel56. It mainly depends on the fatty acid chain length and degree of unsaturation. Cetane number increases by increasing chain length and decreases by increasing number of double bonds60. In the present study, the cetane number of mutant camelina biodiesel ranged between 48.53–59.35 and that of jatropha biodiesel ranged between 47.76–51.26 (Table 9). The cetane number of mutant camelina is higher than jatropha and meet well the European and American standards. The difference in cetane number of the biodiesel from mutant camelina and previously reported non mutant camelina is due to slight change in fatty acid profile52.
Conclusions
The research in this paper depicted the feasibility of biodiesel production from mutant camelina seed oil. Mutant camelina seed oil is perhaps the best source of oil for biodiesel production when depletion of petroleum and environmental issues are the major threats in the current century. The fuel properties of both (mutant camelina and jatropha biodiesel) were evaluated and all parameters compared with American and European standards. The mutant camelina biodiesel have high cetane number and low viscosity as compared to jatropha biodiesel. The mutant camelina oil is easily and readily converted into biodiesel as compared to jatropha seed oil and gave 96.28% yield on alkaline transesterification under optimized condition.
References
Kalogirou, S. A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 30(3), 231–295 (2004).
Lin, L., Cunshan, Z., Vittayapadung, S., Xiangqian, S. & Mingdong, D. Opportunities and challenges for biodiesel fuel. Appl. Energy 88(4), 1020–1031 (2011).
Ragit, S., Mohapatra, S., Kundu, K. & Gill, P. Optimization of neem methyl ester from transesterification process and fuel characterization as a diesel substitute. Biomass Bioenerg. 35(3), 1138–1144 (2011).
Atabani, A. E. et al. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 16(4), 2070–2093 (2012).
Ikram, M. M., Hanif, M. A., Khan, G. S., Rashid, U. & Nadeem, F. Significant seed oil feedstocks for renewable production of biodiesel: a review. Curr. Org. Chem. 23(14), 1509–1516 (2019).
Inam, S., Khan, S. & Nadeem, F. Impacts of derivatization on physiochemical fuel quality parameters of fatty acid methyl esters (FAME)-a comprehensive review. Int. J. Chem. Biochem. Sci. 15, 42–49 (2019).
Kalsoom, M., El Zerey-Belaskri, A., Nadeem, F. & Shehzad, M. R. Fatty acid chain length optimization for biodiesel production using different chemical and biochemical approaches–a comprehensive. Int. J. Chem. Biochem. Sci. 11, 75–94 (2017).
Nadeem, F., Inam, S., Rashid, U., Kainat, R. & Iftikhar, A. A review of geographical distribution, phytochemistry, biological properties and potential applications of Pongamia pinatta. Int. J. Chem. Biochem. Sci. 10, 79–86 (2016).
Nadeem, F., Shahzadi, A., El Zerey-Belaskri, A. & Abbas, Z. Conventional and advanced purification techniques for crude biodiesel–a critical review. Int. J. Chem. Biochem. Sci. 12, 113–121 (2017).
Shahzadi, A., Grondahl, L. & Nadeem, F. Development of effective composite supports for production of biodiesel-a detailed review. Int. J. Chem. Biochem. Scie. 16, 76–86 (2019).
Waseem, H. H., El Zerey-Belaskri, A., Nadeem, F. & Yaqoob, I. The downside of biodiesel fuel–a review. Int. J. Chem. Biochem. Sci. 9, 97–106 (2016).
Yaqoob, I., Rashid, U. & Nadeem, F. Alumina supported catalytic materials for biodiesel production-a detailed review. Int. J. Chem. Biochem. Sci. 16, 41–53 (2019).
Hanif, M. A., Nisar, S., Akhtar, M. N., Nisar, N. & Rashid, N. Optimized production and advanced assessment of biodiesel: a review. Int. J. Energy Res. 42(6), 2070–2083 (2018).
Hanif, M. A., Nisar, S. & Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 59(2), 165–188 (2017).
Mehboob, A. et al. Reactor designs for the production of biodiesel. Int. J. Chem. Biochem. Sci. 10, 87–94 (2016).
Nisar, S. et al. Biodiesel by-product glycerol and its products. Int. J. Chem. Biochem. Sci. 9, 92–96 (2016).
Nisar, S., Ishaq, A., Asia Sultana, F. & Shehzad, M. R. Reactions other than transesterification for biodiesel production. Int. J. Chem. Biochem. Sci. 12, 141–146 (2017).
Azeem, M. W. et al. Production of biodiesel from low priced, renewable and abundant date seed oil. Renew. Energy 86, 124–132 (2016).
Schenk, P. M. et al. Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res. 1(1), 20–43 (2008).
Wu, X. & Leung, D. Y. Optimization of biodiesel production from camelina oil using orthogonal experiment. Appl. Energy 88(11), 3615–3624 (2011).
Sudhakar, M. P., Kumar, B. R., Mathimani, T. & Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 228, 1320–1333 (2019).
Mathimani, T., Senthil Kumar, T., Chandrasekar, M., Uma, L. & Prabaharan, D. Assessment of fuel properties, engine performance and emission characteristics of outdoor grown marine Chlorella vulgaris BDUG 91771 biodiesel. Renew. Energy V 105, 637–646 (2017).
Saravanan, A. P., Mathimani, T., Deviram, G., Rajendran, K. & Pugazhendhi, A. Biofuel policy in India: a review of policy barriers in sustainable marketing of biofuel. J. Clean. Prod. 193, 734–747 (2018).
Anto, S., Pugazhendhi, A. & Mathimani, T. Lipid enhancement through nutrient starvation in Chlorella sp. and its fatty acid profiling for appropriate bioenergy feedstock. Biocatal. Agric. Biotechnol. 20, 101179 (2019).
Deviram, G. et al. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J. Clean. Prod. 253, 119770 (2020).
Altın, R., Cetinkaya, S. & Yücesu, H. S. The potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers. Manag. 42(5), 529–538 (2001).
Bart, J. C. J., Palmeri, N. & Cavallaro, S. Feedstocks for biodiesel production. In Biodiesel Science and Technology (eds Bart, J. C. J. et al.) 130–225 (Woodhead Publishing, Sawston, 2010).
Williams, G. C. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought (Princeton University Press, Princeton, 2008).
Johnson, D. Introduction and Production of Camelina (Montana State University, Northwest Agricultural Research Center, Kalispell, MT, 2006).
Ehrensing, D. T. & Guy, S. O. Camelina (Extension Service, Oregon State University, Corvallis, Or., 2008).
Zubr, J. Qualitative variation of Camelina sativa seed from different locations. Ind. Crops Prod. 17(3), 161–169 (2003).
Jiang, Y., Caldwell, C. D. & Falk, K. C. Camelina seed quality in response to applied nitrogen, genotype and environment. Can. J. Plant Sci. 94(5), 971–980 (2014).
Gugel, R. & Falk, K. Agronomic and seed quality evaluation of Camelina sativa in western Canada. Can. J. Plant Sci. 86(4), 1047–1058 (2006).
Crowley, S. Composition in the university: Historical and polemical essays (University of Pittsburgh, Pittsburgh, 1998).
Soriano, N. U. & Narani, A. Evaluation of biodiesel derived from Camelina sativa oil. J. Am. Oil. Chem. Soc. 89(5), 917–923 (2012).
Yang, J., Caldwell, C., Corscadden, K., He, Q. S. & Li, J. An evaluation of biodiesel production from Camelina sativa grown in Nova Scotia. Ind. Crops Prod. 81, 162–168 (2016).
Ciubota-Rosie, C., Ruiz, J. R., Ramos, M. J. & Pérez, Á. Biodiesel from Camelina sativa: a comprehensive characterisation. Fuel 105, 572–577 (2013).
Nielsen, S. S., Examination of foods for extraneous materials. In Food Analysis Laboratory Manual. 113–118 (Springer, 2003).
Amani, M. A., Davoudi, M. S., Tahvildari, K., Nabavi, S. M. & Davoudi, M. S. Biodiesel production from< i> Phoenix dactylifera</i> as a new feedstock. Ind. Crops Prod. 43, 40–43 (2013).
Bhatti, H. N., Hanif, M. A. & Qasim, M. Biodiesel production from waste tallow. Fuel 87(13–14), 2961–2966 (2008).
Mittelbach, M. & Remschmidt, C. Biodiesel. The comprehensive handbook 27–35 (Boersedruck Ges. MBH, Vienna, 2004).
Gunstone, F. D. Rapeseed and canola oil: production, processing, properties and uses (CRC Press, Boca Raton, 2004).
Moser, B. R. Camelina (Camelina sativa L.) oil as a biofuels feedstock: golden opportunity or false hope?. Lipid Technol. 22(12), 270–273 (2010).
Kawakami, K., Oda, Y. & Takahashi, R. Application of a Burkholderia cepacialipase-immobilized silica monolith to batch and continuous biodiesel production with a stoichiometric mixture of methanol and crude Jatropha oil. Biotechnol. Biofuels 4(1), 42 (2011).
Canakci, M. & Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 35(5), 431–441 (2008).
Akbar, E., Yaakob, Z., Kamarudin, S. K., Ismail, M. & Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. Eur. J. Sci. Res. 29(3), 396–403 (2009).
Fröhlich, A. & Rice, B. Evaluation of Camelina sativa oil as a feedstock for biodiesel production. Ind. Crops Prod. 21(1), 25–31 (2005).
Noureddini, H., Teoh, B. & Clements, L. D. Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 69(12), 1189–1191 (1992).
Pramanik, K. Properties and use of Jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew. Energy 28(2), 239–248 (2003).
Banapurmath, N., Tewari, P. & Hosmath, R. Performance and emission characteristics of a DI compression ignition engine operated on Honge, Jatropha and sesame oil methyl esters. Renew. Energy 33(9), 1982–1988 (2008).
Saloua, F., Saber, C. & Hedi, Z. Methyl ester of [Maclura pomifera (Rafin.) Schneider] seed oil: Biodiesel production and characterization. Bioresource Technol. 101(9), 3091–3096 (2010).
Moser, B. R. & Vaughn, S. F. Evaluation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra low-sulfur diesel fuel. Biores. Technol. 101(2), 646–653 (2010).
Goodrum, J. Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass Bioenerg. 22(3), 205–211 (2002).
Veljković, V., Lakićević, S., Stamenković, O., Todorović, Z. & Lazić, M. Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel 85(17), 2671–2675 (2006).
Crabbe, E., Nolasco-Hipolito, C., Kobayashi, G., Sonomoto, K. & Ishizaki, A. Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties. Process Biochem. 37(1), 65–71 (2001).
Knothe, G. Analyzing biodiesel: standards and other methods. J. Am. Oil. Chem. Soc. 83(10), 823–833 (2006).
Kyriakidis, N. B. & Katsiloulis, T. Calculation of iodine value from measurements of fatty acid methyl esters of some oils: comparison with the relevant American oil chemists society method. J. Am. Oil. Chem. Soc. 77(12), 1235–1238 (2000).
Azam, M. M., Waris, A. & Nahar, N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenerg. 29(4), 293–302 (2005).
Monyem, A. & Van Gerpen, J. H. The effect of biodiesel oxidation on engine performance and emissions. Biomass Bioenerg. 20(4), 317–325 (2001).
Ramírez-Verduzco, L. F., Rodríguez-Rodríguez, J. E. & del Rayo Jaramillo-Jacob, A. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91(1), 102–111 (2012).
Author information
Authors and Affiliations
Contributions
M.M.A. He has conducted lab experiments for biofuel production A.A.K. Prof. K. is senior author and designed whole study. H.M.N.C. She has helped in sample collection and field experimentation M.A.H. He has provided lab facilities and provided help in giving article final shape M.W.A. He was responsible for fuel properties testing M.A.A. He carried out fatty acid profiling.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aslam, M.M., Khan, A.A., Cheema, H.M.N. et al. Novel mutant camelina and jatropha as valuable feedstocks for biodiesel production. Sci Rep 10, 21868 (2020). https://doi.org/10.1038/s41598-020-78680-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78680-w
This article is cited by
-
Automation of the control system for drying grain crops of the technological process for obtaining biodiesel fuels
Scientific Reports (2023)
-
Utilizing Carica papaya seeds as a promising source for bio-oil production: optimization and characterization
Biomass Conversion and Biorefinery (2023)
-
Production of biodiesel from non-edible feedstocks using environment friendly nano-magnetic Fe/SnO catalyst
Scientific Reports (2022)
-
Parameter optimization for enhanced biodiesel yield from Linum usitatissimum oil through solar energy assistance
Biomass Conversion and Biorefinery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.