Abstract
A long-term change of a subtidal macroalgal assemblage has been investigated in Maxwell Bay, King George Island (KGI) of the Antarctic coast by a revisit survey after 30 years. Field surveys were done by SCUBA diving at six sites in 2016–2018 to directly compare with the previous survey conducted in 1988–1993 at the same sites. The total number of macroalgal species was similar between the previous and the present survey, 25 and 27 species respectively. However, the macroalgal assemblage changed substantially with the average similarity of 48.2% between the two surveys. Also, the species-level abundance showed a high variability between surveys. On the other hand, over the 30 years interval there was little overall change at the between-site level hierarchical structure in the subtidal communities of Maxwell Bay. The sites near the penguin rookery consistently showed the highest biodiversity, indicating the importance of land-based nutrients input in Antarctic coastal habitats. A noticeable pattern change over 30 years was the increase of Desmarestia complex and Plocamium cartilagineum and the decrease of Himantothallus grandifolius. Both groups are still dominant, but the shift from Himantothallus to Desmarestia-Plocamium may reflects temperature rise on the Maxwell Bay coast compared to the past.
Similar content being viewed by others
Introduction
Man-induced global climate change is causing gradual changes in biological systems1,2,3,4. As warming continues, the area where life can be inhabited extends to the polar regions, high mountains, and deep seas, but at the same time ecological succession occur due to changes in biological niche, that is, the extinction and invasion of organisms5,6. It has been predicted that coastal benthic ecosystems7, marine benthic communities8, marine fauna9 and kelp forests10 will change gradually caused by global warming. Many studies on various responses of macroalgae to climate changes have been done in the temperate and tropical regions11,12,13,14,15, these studies focused on population fluctuations and assemblage changes of macroalgae related to changes in seawater temperature and acidification.
The polar region has warmed more than twice as much as the global average since 185016, and the Antarctic Peninsula has the highest surface temperature rise in the Southern Hemisphere17. Despite this climatic significance in Antarctica, a few experimental studies have been recently reported which predict the fate of future populations from the algal demographic data such as mortality, growth and reproduction in relation to water temperature rise and pH drop18,19. Such studies, however, predicted assemblage changes on the base of population level fluctuations, thus direct observation of macroalgal assemblage level changes were still lacking. Recently, investigations on long-term assemblage change through the comparison with previously collected data, so called historical research, have been done in the Arctic region. For example, Kortsch, et al.20 reported changes, after 30 years, of macroalgal coverage and marine benthic assemblage and Bartsch, et al.21 compared seaweed biomass data with that of 16 years ago. Also, Filbee-Dextor et al.10 summarize the positive and negative effects of warming on kelp forests through kelp biomass information from 1973 to 2016. In the Antarctic, Mystikou, et al.22 compared seaweed diversity of the islands in the Antarctic Peninsula with the data collected 35 years ago. Due to the mismatch of the study sites between past and present, it is difficult to explain explicitly diversity change over time. However, our present study provides valuable information on seaweed species checklist that can be an useful reference for comparative study in the future.
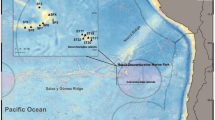
South Shetlands Island (SSI) archipelago is located 120 km away from the north end of the Antarctic Peninsula and consists of many islands including King George Island (KGI). SSI is known for the highest biodiversity area in the Antarctic coast23,24, also related to the relatively low latitude compared to the rest of Antarctica. KGI is a region where warming is very visible. Since 1956, more than 1.7 km and 1.0 km of glaciers have retreated from Marian Cove and Potter Cove, respectively8,25 (Fig. 1). Also, since 1947, the warming trend of the annual mean atmospheric temperature showed 0.022 °C a×1, corresponding to an increase of about 1.1 °C over 49 years. The warming trend of air temperature during winter was 0.038 °C a−1, which was about two times stronger than in the other season26. King Sejong Station (KSS), located in KGI, was established in 1988. Macroalgal research was initiated at the beginning of KSS and focused on taxonomy and assemblage27,28. Chung and his colleagues investigated seaweed distribution and biomass at 17 intertidal and subtidal sites in Maxwell Bay, KGI, in 1988–1993, and compared assemblage between the sites. This pioneer research contains important reference data for a long-term monitoring of this macroalgal assemblage, but follow-up monitoring has not been carried out for the last decades.
The purpose of our study is to conduct a comparative study of the long-term changes at the same sites used in the past survey of about 30 years ago as well as to provide more detailed ecological data on current vertical and spatial distribution as baseline information for possible future monitoring. The present monitoring can also be another historical study in the Antarctic region, if such successive research continues, and will contribute to our understanding on community dynamics and ecosystem changes with relation to global warming and other various causes.
Results
Long-term change of algal assemblage
The species with notable quantitative increase were Desmarestia complex (mixed with D. anceps and D. menziesii: mainly composed of D. menziesii) and Plocamium cartilagineum in the past 30 years (Tables 1, 2). In the Chung’s 1988–93 survey (here-after Chung’s survey), an islet in the entrance of Potter Cove site (site PC) showed the highest diversity (Shannon-Wiener diversity index; H’, the parameters presented here are based on the abundance derived from the IV value; see Materials and Methods) at 2.59 (Table 1). In the present survey in 2016–18 (here-after present survey), an islet off Ardley Island site (site AD) showed the highest diversity of 2.08 and site PC showed relatively low at 1.38. It was noteworthy that the diversity of site PC was low due to dominance of Desmarestia complex (Table 2, Fig. 2). Despite a time difference of about 30 years, the macroalgal assemblage was little overall change in the hierarchical structure based on the similarity of assemblage between sites (Chung’s survey vs. present survey; Fig. 3). A closer look at the results of cluster analysis through similarity analysis, based on the similarity (Bray-Curtis similarity) of 40%, the Chung’s survey was divided into two groups of less disturbed site PR (an islet off penguin rookery site), PC, AD and disturbed site KS (pier of King Sejong Station site), WP (a southern shore of Weaver Peninsula site). Similarity of assemblage within sampling time was 40.5% in the Chung’s survey and 41.7% in the present survey (Fig. 3; Supplementary Table S1).
The pattern of change of the species causing the long-term change of the assemblage, the species that contributed to the dissimilarity of the Chung’s survey and the present survey communities derived from SIMPER analysis were 20 species; these species included Desmarestia complex and Plocamium cartilagineum, which were the most prominent species base on abundance during the past 30 years (Fig. 2, Supplementary Table S2). These two species were quantitatively (mean IV value) increased 2.8 and 1.6 times compared to the Chung’s survey. The abundance of Himantothallus grandifolius and Palmaria decipiens was very high at 10.4 in the Chung’s survey but decreased to 9.1 and 6.2 in the present survey, respectively (Fig. 2, Supplementary Table S2). Although the abundance of the two species present in high abundance in the assemblage decreased, the contribution to the assemblage change between survey times was less than 5.0%.
We tried to see how the species changed in the assemblage and how much the change contributed to the assemblage (Table 2). The changes in macroalgal assemblage in the survey site, i.e., within-site variation, were 38.2%, 49.1%, 70.1% and 43% in sites KS, PR, PC and AD, respectively (Table 2). Regardless of the survey time, there were 14 abundant species with an average abundance of more than 5%. The sum of the contribution of these species to the within-site variation was 60.8% − 77.8%, and explained the dissimilarity of the macroalgal assemblage between the survey times by at least 60%. Desmarestia complex, which made the largest contribution to between-survey difference (Supplementary Table S2), increased in sites PR and PC, and the contribution to changes in each site was 10.2% and 16.9%, respectively. Interestingly, the abundance of Himantothallus grandifolius was complementary to Desmarestia complex in site AD, where abundance of Desmarestia complex decreased, abundance of H. grandifolius increased, whereas site PR and PC showed the opposite pattern (Table 2, Fig. 2).
Species composition and vertical distribution
In the present survey, Daewang Rock site (site DW) was added to five sites analyzed in comparative analysis, thus the average coverage of each species, total algal cover of each site, and diversity were analyzed for six sites. Desmarestia complex, Himantothallus grandifolius, Melobesioideae (crustose coralline algae; CCA), Palmaria decipiens, Gigartina skottsbergii, Plocamium cartilagineum, and Picconiella plumosa appeared at least five of six sites, considered as common species in the study area (Fig. 4, Supplementary Table S3). Site DW, PR, PC and AD were high in macroalgal abundance with an average coverage of >50%. Particularly, site AD and PC showed 93.3% and 85.5% in macroalgal coverage, respectively, which were highest among the sites.
The distribution of seaweed species in each site and by depth derived from SIMPER analysis was as follows. Desmarestia complex, the most dominant species of the present survey, showed the highest coverage at 5 m depth and appeared most frequently at 5–15 m depth (Figs. 4 and 5). This appeared mainly in sites PR, PC, and AD outside Marian Cove (less disturbed site) and showed high site variability. Especially in site PC, the coverage of Desmarestia complex was about 90% at the depth of 5 m (Fig. 4), which resulted in low species diversity (Supplementary Table S3). Plocamium cartilagineum exhibited the highest coverage at 25 m depth, most frequently at 15–25 m depth (Fig. 5). Himantothallus grandifolius was mainly present at 15 m on all sites except site WP and was the most dominant species in site DW (Fig. 5, Supplementary Table S3). The major species of site WP was only one species, P. decipiens and it was found mainly in the shallow zone (Fig. 4).
In the sites PR and AD located near the penguin rookery, the macroalgal coverage was over 80%, which was much higher than the inner bay sites (site KS, DW, WP) and the number of species was as high as 17 and 18 species. In this area, the coverage was 65% or more even excluding the CCA, Melobesioidea. The diversity derived from the Shannon index was 2.05 and 2.23 at sites PR and AD, respectively, which were highest among the survey sites (Supplementary Table S3). These results were observed in both the present survey and the Chung’s survey (Table 1).
Discussion
In most of the sites, notable pattern in the macroalgal assemblage over the last 30 years was the complementary increase or decrease of the Desmarestia complex and Himantothallus grandifolius populations which have the highest biomass in the Antarctic region and dominate in the sublittoral zone18,29,30 (Table 2). The complementary change and abundance shift can be interpreted in terms of temperature and inter-specific competition. First, Schoenrock, et al.18 reported that Desmarestia menziesii population would increase if the temperature increase due to global warming persisted. Glacier retreat has intensified due to warming over the past 50 years and the average temperature of the atmosphere has increased by over 1 °C in Marian Cove and Potter Cove, KGI8,25,26 (Fig. 1). Therefore, the increase of Desmarestia populations in KGI can not rule out the effects of climate change warming. Second, Klöser, et al.31 suggests that D. anceps and D. menziesii have a competitive advantage over H. grandifolius under favorable conditions (e.g. less ice impact). The inter-specific competition between H. grandifolius and Desmarestia complex was determined by disturbance caused by iceberg and sea ice fraction. Assuming an increase in temperature due to climate change and an increase in iceberg (from glacier retreat), an increase in H. grandifolius can be expected in site KS, an ice-abraded zone located inside the Marian Cove. Desmarestia complexes are expected to increase in site PR, PC and AD, where icebergs stay relatively short before they leave the open ocean. Also, Desmarestia spp. and H. grandifolius functionally act as kelps in Antarctic region18, and variation in their abundance can be understood as an increase or decrease of canopy. That is, changes in canopy can affect the fitness of understory species by inter-specific interactions10. Excessive canopy in site PC may have a negative effect on understory macroalgal assemblage. At the same time, canopies provide clues to understand the increase of the filamentous and foliose red algae Plocamium cartilagineum and Gigartina skottsbergii in sites PR and AD (Table 2)32,33.
The sites near the penguin rookery (site PR and AD) consistently showed the highest biodiversity, indicating an Antarctic coastal biodiversity hot spot. The presence of penguin breeding colonies is a major source of nutrients in the terrestrial ecosystem of Antarctica, affecting the formation of land vegetation and zonation34. The long-term change of macroalgal vegetation carried out on the Stora Bornö Island in the inner Gullmar Fjord on the Swedish Skagerrak coast was associated with an increase in nutrient availability35. Seabird guano has been shown to have a positive effect on some species (fleshy crustose algae, Prasiola and Ulva) at lower guano concentration in the intertidal zone, but in most cases guano had no direct effects36. Although seabirds can fertilize costal benthic communities through pelagic-benthic coupling, it can not be directly influenced by benthic primary production37. Nevertheless, one interesting point is that in site PR, unlike other sites, a rich benthic invertebrate assemblage was observed (personal observation, Supplementary Fig. S1). In particular, sponge, bryozoan, and soft coral populations were developed at depths of 15 m or more, suggesting that the state of hyper nutrients originating from the penguin rookery positively affects the richness of benthic animals as well as seaweeds.
Antarctica is generally known to have relatively low seaweed diversity29,38. For example, temperate southern Australia recorded approximately 1,500 species of macroalgal species32, while 130 macroalgal species have been recorded in Antarctica38,39,40. This low level of species richness is attributed to infrequent sampling, difficulty of access, and logistic difficulties41. Also, the use of quadrat photo is difficult to identify understory species or cryptic species, and because Antarctica is a relatively unexplored region, macroalgae is still less taxonomically resolved. About 30 species of algal species were found in this study and 25–66 species of seaweed were reported in the previous studies conducted in KGI. Studies of taxonomic reports have shown relatively high species richness41,42,43, but are also closely related to relatively low latitudes compared to other regions of Antarctica. In case of ecological studies or a small number of survey sites, the level of species richness was similar to our study44,45.
30 years snapshot survey revealed that there were significant changes in the species composition (Fig. 2, Table 1). This shows that the assemblage in this area was changing very dynamically, but the heterogeneity or homogeneity between sites determined by various environmental factors did not seem to change. We chose ecologically diverse sites that could represent Maxwell Bay among the sites of the Chung’s survey, so the similarity of the macroalgal assemblage among the sites was inevitably low. The past and present hierarchical structures based on similarity are clearly distinguished from sites KS and WP inside of Marian cove (frequently disturbed by ice) and sites PR, PC and AD exposed to open ocean (less disturbed by ice). The changes in the benthic community in Potter Cove, KGI showed that the community in the same site showed a statistically significant difference in four to six years46. Sahade, et al.46 reported that sudden shifts in the benthic community occurred due to sedimentation caused by glacier retreat. In the Antarctic area, the community is determined by various environmental factors such as iceberg scouring, sedimentation from glacier retreat, ice cover and light, but it is very difficult to interpret community dynamics due to the complexity between these environmental conditions. The macroalgal assemblage, we report here, which was analyzed over 30 years, maintained a hierarchical structure based on the similarity between the five sites despite the dynamic change of the assemblage. Unfortunately, our long-term snapshot study does not include the causes of macroalgal assemblage changes (eg. temperature, disturbance, sediments) and the patterns of change (eg. linear, sudden, threshold). Despite the apparent change in assemblage, maintaining the hierarchy could be considered an example of an alternative stable state47,48,49 in Antarctica. Therefore, it is necessary to confirm whether the variability of macroalgal assemblage and the stability of hierarchical structure are maintained regardless of the time interval, through short term monitoring in the future.
Finally, our study has great significance in trying to analyze the long-term change of algal assemblage at the same sites over an interval of about 30 years in the Antarctic region. In Gullmar Fjord on the Swedish Skagerrak coast, macroalgal vegetation was discovered at the same site as it was 57 years ago. Its continuing presence was interpreted as related to the nutrient dynamics of Baltic Sea and Kattegat water as an exceptional example of analyzing historical assemblage change through comparison with existing research results35. In Arctic fjords along the coast of Svalbard, where sea water temperature increases and sea ice area decreases, the macroalgal coverage increased 5 to 8 times over 30 years20. Warming in the Arctic region increases turbidity under the influence of sediments in sea ice and decreases salinity under the influence of melting ice. Those increase of turbidity and decrease of salinity have a negative effect on kelp recruitment, growth, and survival. As a result, the positive effects of warming and increased light can be offset10. In the sub-Antarctic Prince Edward Islands, a comparison of an epibenthic assemblage over a 25-year time difference confirmed the increase in polychaete Lanice marionensis (a suspension feeder); the cause was interpreted as a change in the local primary productivity due to climate change, that was, the change in the flux of food50. In the Arctic region, the biomass of Laminaria digitata was reported to have increased about 8.2 times compared to 16 years ago21. Thus, recent studies on the long-term change of cold water communities have been reported with regard to climate change or warming but there are not many cases in the Antarctic region. Mystikou, et al.22 compared the similarity and shared species among sites several kilometers to several hundred km away from the islands near the south-western Antarctic Peninsula. Unfortunately, this study was not a site based comparative survey because the sites, surveyed over a 35-year time period, were more than 350 km apart. Therefore, our study is very meaningful in that it is a site based comparative survey in Antarctica.
In conclusion, we compared the macroalgal assemblages of about 30 years ago and later in Maxwell Bay, KGI to initiate a long-term research in the Antarctic region. We observed that large brown algae populations dominant in the Antarctic regions shifted from the Himantothallus grandifolius to the Desmarestia complex. Previous studies have shown that this population shift is strongly suspected to have resulted from a competitive replacement of H. grandifolius by Desmarestia complex. If global warming intensifies in the future, the Desmarestia complex population will expand directly due to the increase in water temperature. As seen from the site nearest the glacier (site WP), a change to a simple assemblage dominated by r-type algae such as Palmaria decipiens or Acanthococcus antarcticus can be expected. In addition, considerable changes were observed in the species composition of past and present macroalgal communities, that is, within-site variation was very high. We expected that the spatial variation would also be very large if this dynamic transition of macroalgal communities was common in the KGI coast, but between site heterogeneity did not appear to be significant. This suggests that climate change such as global warming leads to drastic change of macroalgal assemblage in local micro scale (- up to 1 km2; within sites) but it shows a gradual change in regional meso scale (1–10,000 km2; among sites) at Maxwell Bay in KGI. Also we hope that this study will serve as another important basis for the interpretation of future macroalgal assemblage changes in the Antarctic region due to climate change.
Materials and methods
Study area
This study was conducted in Maxwell Bay, King George Island (KGI), the largest island of the South Shetland Islands, about 120 km far from the Antarctic Peninsula. KGI is about 95 km long and 25 km wide with a land area of 1,150 km2. More than 90% of the land area is covered with permanent glaciers, and ice-free land areas are limited to the coast. Coastal areas connected to exposed land areas are regularly frozen from late July to mid-September of the austral winter, and after late October, the sea ice begins to crack and floating ice starts to cover the coastal zone. This study was carried out at four sites (KS, DW, PR, PC) around Barton Peninsula, one site (WP) around Weaver Peninsula and one site (AD) near Ardely Island (Fig. 1, Table 3). Site KS was located in front of the pier of King Sejong Station, so an anthropogenic effect is expected. Sites PR and AD were located in the vicinity of the penguin rookery, where a large amount of organic nutrients are introduced from the land. Sites KS, DW and WP (located in Marian Cove) were influenced by the sediments that flowed along with melted snow water and turbid plume from glacial retreat51. Predominant winds direction (NW-W) increase the residence time of the ice within the cove, and strong wave generated by strong wind intensity forms the ice-abraded zone. These hydrodynamics of ice in Marian Cove are expected to be similar to the hydrodynamic pattern shown in Potter Cove52. On the other hand, sites PR, PC and AD are relatively less affected by disturbance caused by ice. Especially, the terrain near site WP formed a steep slope, and large and small pebbles rolled down from the slope and piled up on the shore and underwater. Here, mud was piled up on the bottom and the development of seaweed assemblage was poor.
Sampling for the present survey and the Chung’s data
The field survey was conducted at the six sites in Maxwell Bay, KGI, during the austral summer 2016–18. The sampling areas were all located outside of the Antarctic Specially Protected Area (ASPA no. 171, site PR; ASPA no. 150, site AD), and all surveys were performed with non-destructive sampling to minimize disturbance to the ecosystem. Algal assemblage of subtidal habitats was investigated by SCUBA diving with underwater camera and video. Diving survey was performed at four depths (1 m, 5 m, 15 m, and 25 m) and were conducted along a virtual vertical line. Five 50 × 50 cm (0.25 m2) quadrats were randomly located at each depth and photographed. Quadrats, within the same depth, were placed >2 m apart from each other were horizontally placed. The underwater video device was used to record the substrate configuration and the surrounding environment while diving down to the depth of 25 m. We counted the coverage (percentage) of each macroalgal species using virtual grids on the photo. Algal identification was done to the lowest possible taxonomic level. Exceptionally, crustose coralline algae were classified at subfamily level.
Unfortunately, there is no raw data available in the Chung’s survey, except for the published data. Those data was coverage and/or biomass and then they converted them to IV (Supplementary Table S4). Along the transect line, macroalgal assemblage data in quadrat (1 m × 1 m) were collected at different depth intervals (2 m interval between 0–10 m and 5 m interval between 10–30 m). Also, the data were presented as the average value of each site and there was no depth profile (more details in Chung’s literatures27,28,53).
Data analysis for comparing surveys
All the data collected in the present survey were converted to the same format as the data published in the Chung’s survey, to achieve direct comparison between the Chung’s survey and the present survey. Major species were selected to adequately express the differences in communities in both surveys. Major species were defined by the following method. The similarity of percentage (SIMPER) analysis54 was conducted between the surveys at the average abundance data of each site. Eight species with the contribution of more than 5% to the difference between the two surveys were defined as the major species (for Fig. 3 and Supplementary Table S2). The composition of assemblage is presented by calculating the cumulative contribution or by sorting the contribution of all species in ascending order55,56,57,58,59. To understand the variation of the assemblage (for Table 2), we defined the species with an average abundance of 5% or more in the Chung’s survey and the present survey as abundant species. Despite the 30-years time lag of the two surveys, such species that showed more than 5% abundance in the Chung's survey and the present survey each were expressed as maintained species because the macroalgal population remained stable. Moreover, we observed the patterns of variation of the communities by examining how much the increase or decrease of the population contributed to the within-site variation. The biomass and coverage of species in site WP were very low compared to other sites, so site WP was excluded from the comparison, preventing a distortion in major species calculation and their contribution. Cluster analysis was performed to investigate the spatial variation between sites for each survey. For this analysis, Bray Curtis similarity between sites was calculated, then a resemblance matrix was created and a hierarchical cluster analysis was performed to create a dendrogram (for Fig. 3 and Supplementary Table S1). The algorithm for calculating the distance between clusters was group average. Prior to SIMPER and cluster analysis, all data were subjected to a pre-treatment procedure through standardized and square root transformation54. Shannon-Wiener diversity index was calculated to compare the diversity of the algal assemblage between sites60.
Macroalgal assemblage and vertical distribution in the present survey
We attempted to identify the distribution of major species by depth at each site using the extracted coverage-based data in the present survey. Major species were selected for each site to describe the vertical distribution of macroalgal assemblage (for Figs. 4 and 5). Major species were selected based on the contribution of each species (90% cut-off) to similarity between the site through SIMPER analysis. Cluster analysis, diversity index and the pre-treatment process of data for assemblage analysis were done as described above. The SIMPER, cluster analysis, and diversity index analysis applied to this study were performed using PRIMER 6 software (PRIMER-E, Ltd., UK).
References
Pecl, G. T. et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355, eaai9214 (2017).
Smale, D. A. et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 9, 306–312 (2019).
Hoegh-Guldberg, O. & Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528 (2010).
Wernberg, T. et al. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Biol. Ecol. 400, 7–16 (2011).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37 (2003).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Burrows, M. T. et al. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (2011).
Moon, H.-W., Hussin, W. M. R. W., Kim, H.-C. & Ahn, I.-Y. The impacts of climate change on Antarctic nearshore mega-epifaunal benthic assemblages in a glacial fjord on King George Island: responses and implications. Ecol. Indic. 57, 280–292 (2015).
Fields, P., Graham, J., Rosenblatt, R. & Somero, G. Effects of expected global climate change on marine faunas. Trends Ecol. Evol. 8, 361–367 (1993).
Filbee-Dexter, K., Wernberg, T., Fredriksen, S., Norderhaug, K. M. & Pedersen, M. F. Arctic kelp forests: Diversity, resilience and future. Glob. Planet. Change 172, 1–14 (2019).
Harley, C. D. G. et al. Effects of climate change on global seaweed communities. J. Phycol. 48, 1064–1078 (2012).
Hepburn, C. et al. Diversity of carbon use strategies in a kelp forest community: implications for a high CO ocean. Glob. Chang. Biol. 17, 2488–2497 (2011).
Kuffner, I. B., Andersson, A. J., Jokiel, P. L., Ku’ulei, S. R. & Mackenzie, F. T. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114 (2008).
Raven, J. A., Giordano, M., Beardall, J. & Maberly, S. C. Algal evolution in relation to atmospheric CO : carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci 367, 493–507 (2012).
Wernberg, T. et al. Seaweed communities in retreat from ocean warming. Curr. Biol. 21, 1828–1832 (2011).
Siegert, M. J. et al. The Antarctic Peninsula under a 1.5 °C global warming scenario. Front. Environ. Sci. 7, 102 (2019).
Turner, J. et al. Antarctic climate change during the last 50 years. Int. J. Climatol. 25, 279–294 (2005).
Schoenrock, K. M., Schram, J. B., Amsler, C. D., McClintock, J. B. & Angus, R. A. Climate change impacts on overstory Desmarestia spp. from the western Antarctic Peninsula. Mar. Biol. 162, 377–389 (2015).
Schoenrock, K. M. et al. Climate change confers a potential advantage to fleshy Antarctic crustose macroalgae over calcified species. J. Exp. Mar. Biol. Ecol. 474, 58–66 (2016).
Kortsch, S. et al. Climate-driven regime shifts in Arctic marine benthos. Proc. Natl. Acad. Sci. USA 109, 14052–14057 (2012).
Bartsch, I. et al. Changes in kelp forest biomass and depth distribution in Kongsfjorden, Svalbard, between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biol. 39, 2021–2036 (2016).
Mystikou, A. et al. Seaweed biodiversity in the south-western Antarctic Peninsula: surveying macroalgal community composition in the Adelaide Island/Marguerite Bay region over a 35-year time span. Polar Biol. 37, 1607–1619 (2014).
Griffiths, H. J. Antarctic marine biodiversity - What do we know about the distribution of life in the Southern Ocean? PLoS One 5, e11683 (2010).
Griffiths, H. J., Danis, B. & Clarke, A. Quantifying Antarctic marine biodiversity: The SCAR-MarBIN data portal. Deep Sea Res. Part II Top. Stud. Oceanogr 58, 18–29 (2011).
Rückamp, M., Braun, M., Suckro, S. & Blindow, N. Observed glacial changes on the King George Island ice cap, Antarctica, in the last decade. Glob. Planet. Change 79, 99–109 (2011).
Ferron, F. A., Simões, J. C., Aquino, F. E. & Setzer, A. W. Air temperature time series for King George Island, Antarctica. Pesqui. Antart. Bras. 4, 155–169 (2004).
Chung, H. Flora and community structure of benthic marine algae in Maxwell Bay, Antarctica Ph. D Thesis, Seoul National University, (1996).
Chung, H., Oh, Y. S., Lee, I. K. & Kim, D. Y. Macroalgal vegetation of Maxwell Bay in King George Island, Antarctica. Korean J. Phycol. 9, 47–58 (1994).
Dayton, P. K. Polar Benthos In Polar Oceanography: Chemistry, Biology, and Geology (ed. Smith, W. O.) 631–685 (Academic Press, 1990).
Scherrer, K. J. N. et al. Mechanistic model identifies increasing light availability due to sea ice reductions as cause for increasing macroalgae cover in the Arctic. Limnol. Oceanogr. 64, 330–341 (2019).
Klöser, H., Mercuri, G., Laturnus, F., Quartino, M. L. & Wiencke, C. On the competitive balance of macroalgae at Potter Cove (King George Island, South Shetlands). Polar Biol. 14, 11–16 (1994).
Waters, J. M. et al. Australia’s marine biogeography revisited: Back to the future? Austral. Ecol. 35, 988–992 (2010).
Wernberg, T. & Goldberg, N. Short-term temporal dynamics of algal species in a subtidal kelp bed in relation to changes in environmental conditions and canopy biomass. Estuar. Coast. Shelf Sci. 76, 265–272 (2008).
Smykla, J., Wołek, J. & Barcikowski, A. Zonation of vegetation related to penguin rookeries on King George Island, Maritime Antarctic. Arct. Antarct. Alp. Res. 39, 143–151 (2007).
Eriksson, B. K., Johansson, G. & Snoeijs, P. Long-term changes in the macroalgal vegetation of the inner Gullmar Fjord, Swedish Skagerrak coast. J. Phycol. 38, 284–296 (2002).
Wootton, J. T. Direct and indirect effects of nutrients on intertidal community structure: variable consequences of seabird guano. J. Exp. Mar. Biol. Ecol. 151, 139–153 (1991).
Zmudczyńska-Skarbek, K., Balazy, P. & Kuklinski, P. An assessment of seabird influence on Arctic coastal benthic communities. J. Mar. Syst. 144, 48–56 (2015).
Wiencke, C., Amsler, C. D. & Clayton, M. N. Macroalgae In Biogeographic atlas of the Southern Ocean (eds Claude De Broyer et al.) 66-73 (Scientific Committee on Antarctic Research Cambridge, 2014).
Wiencke, C. & Clayton, M. N. Antarctic Seaweeds In Synopses of the Antarctic Benthos Vol. 9 (ed Johann-Wolfgang Wägele) 239 (Koeltz Scientific Books, 2002).
Wulff, A. et al. Biodiversity, biogeography and zonation of marine benthic micro- and macroalgae in the Arctic and Antarctic. Bot. Mar. 52, 491–507 (2009).
Oliveira, E. C., Absher, T. M., Pellizzari, F. M. & Oliveira, M. C. The seaweed flora of Admiralty Bay, King George Island, Antarctic. Polar Biol. 32, 1639–1647 (2009).
Klöser, H., Quartino, M. L. & Wiencke, C. Distribution of macroalgae and macroalgal communities in gradients of physical conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 333, 1–17 (1996).
Pellizzari, F. et al. Diversity and spatial distribution of seaweeds in the South Shetland Islands, Antarctica: an updated database for environmental monitoring under climate change scenarios. Polar Biol. 40, 1671–1685 (2017) .
Valdivia, N. et al. Up, down, and all around: scale-dependent spatial variation in rocky-shore communities of Fildes Peninsula, King George Island, Antarctica. PLoS One 9, e100714 (2014).
Zielinnski, K. Bottom macroalgae of the Admiralty Bay (King George Island, South Shetlands, Antarctica). Pol. Polar Res. 11, 95–131 (1990).
Sahade, R. et al. Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv. 1, e1500050 (2015).
May, R. M. Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature 269, 471 (1977).
Sutherland, J. P. Multiple stable points in natural communities. Am. Nat. 108, 859–873 (1974).
Beisner, B. E., Haydon, D. T. & Cuddington, K. Alternative stable states in ecology. Front. Ecol. Environ. 1, 376–382 (2003).
von der Meden, C. E. O. et al. Long-term change in epibenthic assemblages at the Prince Edward Islands: a comparison between 1988 and 2013. Polar Biol. 40, 2171–2185 (2017).
Yoon, H. I., Han, M. W., Park, B.-K., Oh, J.-K. & Chang, S.-K. Glaciomarine sedimentation and palaeo-glacial setting of Maxwell Bay and its tributary embayment, Marian Cove, South Shetland Islands, West Antarctica. Mar Geol 140, 265–282 (1997).
Lim, C. H. Modelling waves and currents in Potter Cove, King George Island, Antarctica PhD Thesis. BIS der Universität Oldenburg (2014).
Chung, H. et al. Species composition and biomass distribution of benthic macroalgae in Maxwell Bay, King George Island, Antarctica. Korean J. Polar Rec. 11, 1–12 (2000).
Clarke, K. & Warwick, R. Change in marine communities: anapproach to statistical analysis and interpretation. 2nd edn, 127 (PRIMER-E Ltd, Plymouth, 2001).
Heaven, C. S. & Scrosati, R. A. Benthic community composition across gradients of intertidal elevation, wave exposure, and ice scour in Atlantic Canada. Mar. Ecol. Prog. Ser. 369, 13–23 (2008).
Lasiak, T. Multivariate comparisons of rocky infratidal macrofaunal assemblages from replicate exploited and non-exploited localities on the Transkei coast of South Africa. Mar. Ecol. Prog. Ser. 167, 15–23 (1998).
Dolbeth, M. et al. Anthropogenic and natural disturbance effects on a macrobenthic estuarine community over a 10-year period. Mar. Pollut. Bull. 54, 576–585 (2007).
Yu, O. H. et al. Spatial variation in macrobenthic communities affected by the thermal discharge volumes of a nuclear power plant on the East Coast of Korea. Ocean Polar Res 35, 299–312 (2013).
Macleod, C. K., Moltschaniwskyj, N. A. & Crawford, C. M. Ecological and functional changes associated with long-term recovery from organic enrichment. Mar. Ecol. Prog. Ser. 365, 17–24 (2008).
Shannon, C. E. & Weaver, W. The mathematical theory of communication. 125 (University Illinois Press, 1949).
Acknowledgements
This work was supported a grant from the Basic Research Program (PE19070 and PE20120) of the Korea Polar Research Institute. We thank the crews of King Sejong Station for their assistance in SCUBA diving and field surveys during the years of 2016–2018. Authors also thank to Prof. Emer. Robert DeWreede, University of British Columbia, for critical reading and comments.
Author information
Authors and Affiliations
Contributions
Y.W.K., H.-G.C., J.H.K., conceived the study design and conceived ideas; Y.W.K., D.S.L., collected samples; Y.W.K., H.-G.C., D.S.L., J.H.K., analyzed the data; Y.W.K., H.-G.C., J.H.K., contributed to interpretation of results; Y.W.K., H.-G.C., J.H.K., wrote the manuscript. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ko, Y.W., Choi, HG., Lee, D.S. et al. 30 years revisit survey for long-term changes in the Antarctic subtidal algal assemblage. Sci Rep 10, 8481 (2020). https://doi.org/10.1038/s41598-020-65039-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65039-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.