Abstract
Trigeminal neuralgia (TN), a sudden, needle-like pain in the distribution area of the trigeminal nerve, can seriously affect the physical and mental health of patients. In chronic pain conditions including TN, increased levels of brain-derived neurotrophic factor (BDNF) may enhance pain transmission. This study compares the effect of palmatine administration on the expression of BDNF and its receptor TrkB (tropomyosin receptor kinase B) in trigeminal ganglion cells of Sprague-Dawley rats in a sham versus TN model group. Within 14 days of surgery, the mechanical allodynia threshold of the TN group was significantly lower than that of the sham group, while the TN + palmatine group had a higher mechanical pain sensitivity threshold than the TN group (p < 0.05). Real-time quantitative PCR, immunohistochemistry, and immunofluorescence showed that BDNF and TrkB expression in the TN group was higher than that in the sham group, while palmatine treatment could reverse these changes. Western blotting showed that palmatine treatment could reduce the elevated phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) in TN rats. Thus, the BDNF/TrkB pathway may be involved in the pain transmission process of TN, and palmatine treatment may reduce pain transmission by inhibiting the BDNF/TrkB pathway and suppressing ERK1/2 phosphorylation.
Similar content being viewed by others
Introduction
Trigeminal neuralgia (TN) is a sharp, sudden pain in the trigeminal nerve region, the largest pair of nerves in the brain. TN causes intractable facial pain, which can seriously affect the patient’s quality of life1. Currently, treating TN is challenging. Pharmacological treatments, including carbamazepine, oxcarbazepine, and other anticonvulsants and antipsychotics, have achieved varying degrees of success. However, their side effects and potential loss in efficacy over time has prompted the search for other drugs that can achieve more comprehensive and long-lasting pain control2. Furthermore, carbamazepine and oxcarbazepine confer a significant risk of side effects and complications3. Indeed, both are prohibited to primary TN patients with an atrioventricular block4. Moreover long-term use of these medications can cause significant side effects such as drowsiness, rashes, and tremors5. Some TN patients may end up needing surgery, particularly if pharmacotherapy is ineffective or they become resistant to the drugs. Surgical treatments include microvascular decompression, gamma knife radio surgery treatment (GKRS), stereotactic radio surgery, and other invasive approaches6,7,8. Unfortunately, invasive treatments for TN can cause severe, diverse, and unpredictable side effects. For example, GKRS treatment for TN has a 50% chance of failure or recurrence, and the expensive surgery increases psychological and living burden of patients9. Thus, we need to further understand the molecular mechanism of TN to find new drug targets for the prevention and treatment of this debilitating disease.
Brain-derived neurotrophic factor (BDNF) is an important molecule for neurogenesis and neuronal survival10,11. Neuronal activity induces the synthesis and release of BDNF, which then recruits and activates cohesive proteins involved in various cellular signalling pathways12. Many studies have confirmed that BDNF and its tropomyosin receptor kinase B (TrkB) are associated with chronic pain transmission13. Recently, after repeated dural stimulation for trigeminal allodynia, some scholars discovered an increased expression of BDNF and TrkB in the trigeminal nucleus caudalis14,15. Hence, we speculate that the BDNF-TrkB signalling may be involved in the development of TN.
Palmatine is an alkaloid derived from dried rhizomes, a Chinese plant, and has been used to treat infectious diseases in China for thousands of years16,17. Palmatine has many therapeutic effects, has long-term presence in the bloodstream, and penetrates the blood-brain barrier18,19. For instance, palmatine may inhibit growth and the invasion of cancer, suppress inflammatory cytokines, and alleviate comorbid diabetic neuropathic pain and depression20,21,22. The study aimed to investigate whether palmatine treatment can affect the expression of BDNF/TrkB in the trigeminal ganglion (TG) of TN model rats and its possible molecular mechanism, which may provide new insights into the prevention and treatment of TN.
Results
Effects of palmatine on trigeminal neuropathic mechanical allodynia
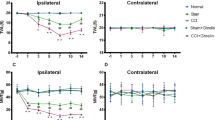
The pain threshold in the TN group was significantly decreased in comparison with that in the sham group from the 3rd to 14th day after operation [d1: p = 0.128, F (3,44) = 1.996; d3: p < 0.05, F (3,44) = 4.945; d5: p < 0.01, F (3.44) = 66.551; d7: p < 0.01, F (3.44) = 164.307; d9: p < 0.01, F (3.44) = 193.993; d11: p < 0.01, F (3.44) =198.512; d13: p < 0.01, F (3.44) = 152.246. ANOVA was used]. We found that palmatine treatment noticeably reduced the pain sensitivity of TN rats for 2 weeks. See Fig. 1.
Mechanical withdrawal threshold (MWT). Three to fourteen days after the operation, the pain threshold in the TN group significantly decreased compared with the shams. Palmatine treatment noticeably reduced pain sensitivity of TN rats. Data are presented as mean ± SD, n = 12. *p < 0.05 vs sham group; **p < 0.01 vs sham group; ##p < 0.01 vs TN group.
Palmatine reduced the expression levels of BDNF/TrkB in the TG of TN rats
Through qPCR analysis, we detected the mRNA expression of BDNF/TrkB in the TG of each group. These results showed that the expression of BDNF and TrkB mRNA in the TG of TN rats was higher than those in the sham group. The expression of BDNF and TrkB mRNA in TN + palmatine group was significantly decreased by palmatine treatment compared to the TN group. [BDNF: p < 0.01, F (3,20) = 49.115; TrkB: p < 0.01, F (3,20) = 35.660] see Fig. 2.
Western blotting was used to measure the levels of BDNF/TrkB protein in the TG. The results showed that the protein expression of BDNF and TrkB in the TN group was higher than that in the sham group. The protein expression of BDNF/TrkB in the TN + palmatine group was lower than the TN group [BDNF: p < 0.01, F (3,20) =19.171; TrkB: p < 0.01, F (3,20) =113.892; see Fig. 3].
Immunohistochemistry was used to detect BDNF and TrkB immunoreactivities in the TG. The results showed that the expression of BDNF/TrkB receptor in the TG of TN group rats was higher than that in the sham rats, but palmatine treatment could reduce this change [BDNF: p < 0.01, F (3,20) = 87.760; TrkB: p < 0.01, F (3,20) = 37.211] (see Fig. 4).
Immunohistochemistry analysis. The integrated optical density (IOD)/Area of BDNF and TrkB staining in the TG of TN rats were higher than the shams. Palmatine treatment noticeably reduced these changes. Arrows indicate immunostained cells. Scale bar: 100 μm. Data are presented as mean ± SD, n = 6. **p < 0.01 vs sham group; ##p < 0.01 vs TN group.
Using immunofluorescence we also detected the immunoreactivities of the BDNF/TrkB. The co-expression of the BDNF or TrkB receptor and glial fibrillary acidic portein (GFAP) in the 4 groups on the 14th day is shown in Fig. 5. Because there was little co-expression of GFAP with either BDNF or TrkB receptor, we confirmed that the BDNF and TrkB receptor were expressed mainly in TG neurons [BDNF: p < 0.01, F (3,20) = 72.024; TrkB: p < 0.05, F (3,20) = 51.027] (see Fig. 5).
Immunofluorescences analysis. BDNF or TrkB receptor expression were shown in the TG. There was little GFAP co-expression with BDNF or TrkB receptor. Arrows indicate neuroglia and neurons emitting green and red fluorescence, respectively. Scale bar: 100 μm. Data are presented as mean ± SD, n = 6. **p < 0.01 vs sham group; ##p < 0.01 vs TN group.
These results indicate that palmatine treatment can reduce the levels of BDNF/TrkB in the TG of TN rats.
Effects of palmatine on the ERK1/2 and phosphorylation of ERK1/2 in the TG of TN rats
Through a Western blot analysis, we detected ERK1/2 and its phosphorylation in the TG. The integrated optical density (IOD)/Area ratio of ERK1/2 to β-actin showed no significant differences in the four groups (p > 0.05). Meanwhile, the IOD/Area ratio of p-ERK1/2 to ERK1/2 in the TN group were higher than in the sham group, but the IOD/Area ratio of p-ERK1/2 to ERK1/2 in the TN + palmatine group was significantly reduced compared to the TN group [ERK/β-actin: p > 0.05, F (3,20) = 0.375; p-ERK/ERK: p < 0.01, F (3,20) = 684.695] (See Fig. 6).
Western blot analysis. Integrated optical density (IOD) ratio of ERK1/2 to β-actin showed no significant differences between the groups. The IOD ratio of p-ERK1/2 to ERK1/2 in the TN group was higher than the shams. Palmatine treatment significantly reduced this phosphorylation. Data are presented as mean ± SD, n = 6. **p < 0.01 vs sham group; ##p < 0.01 vs TN group.
Discussion
Although TN, a severe facial pain disorder, has been studied for decades, neither medicines nor surgical treatments can reach satisfactory effects23. The molecular mechanism of TN remains unclear and needs further investigation. The TG contains the primary sensory neurons of the head and face for pain transmission. Thus, the involved molecules in the TG have become a hot topic in TN research.
BDNF is a neurotrophin involved in the growth, differentiation and apoptosis of neurons24. Current research confirms that BDNF is related to pain. For instance, BDNF has been shown to be involved in the pathogenesis of cancer-induced pain, diabetic neuropathic pain and inflammatory pain25,26,27. However, reports on the effects of BDNF on TN are currently rare28; therefore, further studies on their role in TN would be useful.
In our experiment, we found that the TN group had an increased BDNF/TrkB expression in the TG. Thus, we presume that this increase of BDNF/TrkB may have promoted TN and may be involved in the sensitization process. Furthermore, our immunofluorescence results showed that BDNF/TrkB were co-expressed in the neurons of the TG, and that BDNF and GFAP showed less co-localization in the TG, which suggested that the neurons might have released BDNF through autocrine secretion. Indeed, it has been reported that BDNF is released by TG’s neurons through autocrine activity10,29. However, the autocrine secretion of BDNF in TG requires further research to obtain more direct evidence.
Palmatine is an isoquinoline alkaloid extracted from the dried rhizome of Radix stephaniae30. It has been used clinically to treat bacterial and viral infections in China for thousands of years31,32. In our lab, we have found that palmatine relieved hyperalgesia and depression of diabetic rats21. Currently on PubMed, however, there are no studies on the therapeutic effect of palmatine on TN. Our research shows that palmatine significantly alleviates the mechanical allodynia of the TN group, suggesting that palmatine may be a potential drug to treat TN. In the TN + palmatine group, the expression of BDNF and TrkB receptor had decreased significantly compared to the TN group. Therefore, we speculate that the effects of palmatine may be related to blockage of the BDNF/TrkB pathway. Between the sham group and sham + palmatine group, the results showed no differences, which demonstrated that palmatine dose used was safe and had few adverse reactions in this experiment.
Some findings suggest that there is a TrkB-ERK/Akt pathway and BDNF-ERK signalling in the cell, and that the activation of the BDNF/TrkB pathway may increase the levels of ERK1/2 and p-ERK1/233,34,35. Our study also shows that the expression of p-ERK1/2 in the TG of TN rats was remarkably higher than that in the sham rat, but palmatine administration could reduce this expression to comparable levels. These results suggest that palmatine can exert an analgesic effect on TN, potentially through the downregulation of the ERK1/2 pathway. Thus, it is tempting to speculate that the role of ERK phosphorylation in the TG is related to the BDNF/TrkB-mediated TN pain transmission, and that palmatine administration may affect the phosphorylation of ERK in TN rats. There are multiple pathways in pain transmission besides ERK1/2 pathway, therefore, there might be other involved pathways which need further research in the future.
Conclusion
Our experiments have demonstrated that palmatine administration increases the mechanical pain threshold of TN rats by reducing the expressions of BDNF and the TrkB receptor and by inhibiting the ERK1/2 pathway in TG. These findings suggest that palmatine and its analgesic mechanism deserve further study as a potential pharmacotherapy in the treatment of TN and other chronic pain conditions.
Materials and Methods
TN pain animal model
Healthy Sprague-Dawley rats (male, 180–220 g) were obtained from the Department of Animal Science, Nanchang University of Traditional Chinese Medicine. The procedures were approved by the Animal Care and Use Committee of Nanchang University Medical School. The animals had free access to food and water in a room with a comfortable temperature of 20 °C to 25 °C. The International Association for the Study of Pain (IASP)’s ethical guidelines for pain research in animals were followed36.
These rats were randomly divided into 4 groups: sham, TN, sham + palmatine, and TN + palmatine. For the TN group, the right infraorbital nerve was exposed and loosely ligated through a previously described method, but for the sham group, the right infraorbital nerve was exposed but not ligated36. For the sham + palmatine group and the TN + palmatine group, 10% palmatine was administered through an intraperitoneal injection of 20 mg/kg a day for 2 weeks.
Evaluation of mechanical allodynia
Mechanical allodynia was measured as described in detail previously in our published paper36. The mechanical nociceptive threshold was evaluated using electronic pain meter (BME-404 NO.E5489, Institute of Biomedical Engineering, CAMS & PUMC). The stimulation site was the facial area overlying the infraorbital nerve, i.e., the whisker portion in the centre of the nasal region and the surrounding hairy skin. The threshold of mechanical stimulation intensity of the right side of the rat’s face was measured 1, 3, 5, 7, 9, 11, and 13 days after the operation. Each rat was stimulated 6 times at 1-minute intervals. The electronic mechanical pain tester transmitted the stimulation pressure to the computer’s corresponding software (BME-Tactile), which automatically calculated the mechanical pain threshold of the camp. Animal positive reaction performance included: (1) rapid retreat, dodging and turning, or rats to avoid facial irritation, curling up to the cage wall, or hiding the head and face under the body; (2) aggressive behaviours by biting; (3) scratching the face and repetitive scratching in the same location for at least 3 consecutive times. Any 1 or more of the above 3 reactions were considered to be positive for the stimulation test, i.e., the stimulation threshold was an effective threshold.
Quantitative real-time PCR
Total RNA was isolated from the right trigeminal ganglion using the TRIzol Total RNA Reagent (TransGen Biotech Company). Reverse transcription to cDNA was performed according to the kit instructions (EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix, TransGen Biotech). The primers were designed with Primer Express 3.0 Software (Applied Biosystems) and the sequences were as follows: BDNF: Forward: 5′-CCTCTGCTCTTTCTGCTGGA-3′, Reverse: 5′-GCTGTGACCCACTCGCTAAT-3′;TrkB: Forward: 5′-TGGAGGAAGGGAAGTCTGTG-3′, Reverse: 5′-AGTGGTGGTCTGAGGTTGGA-3′; β-actin: Forward: 5′-AAGATCCTGACCGAGCGTGG-3′, Reverse: 5′-CAGCACTGTGTTGGCATAGAGG-3′. The quantification of gene expression was obtained using the ABI PRISM ® 7500 Sequence Detection System (Applied Biosystems Inc., Foster City, USA).
Immunohistochemistry
On the 14th day, the rats were anesthetized with 10% chloral hydrate (Shanghai Macklin Biochemical Co., Ltd). We then cut the skin and the diaphragm under the costal margin of the rat, exposed the heart, flushed the blood with 0.9% saline, and infused 500 ml of 4% paraformaldehyde (PFA) until the body became stiff. Next, the rat was decapitated, the right trigeminal ganglion was removed and fixed in 4% PFA for 24 hours before being dehydrated for 24 hours. Next, the TG was embedded and cut into 10-μm-thick sections, naturally dried, and stored in a −20 °C freezer. The slices were then incubated in 3% H2O2 for 10 minutes at 37 °C. To block endogenous peroxidase activity and nonspecific antigen binding, the processed slices were incubated with 10% goat serum for 30 minutes at 37 °C. The sections were then washed in PBS and incubated with rabbit anti-BDNF or anti-TrkB (BDNF 1:50 diluted in PBS; Abcam company; TrkB 1:100, Abcam company) overnight at 4 °C. After washing 3 times with PBS, the sections were incubated with biotinylated goat anti-rabbit secondary antibodies (Beijing Zhongshan Biotech Co., China) for 30 minutes at 37 °C. After washing with PBS, the samples were stained with DAB kit (Beijing Zhongshan Biotech Co., China) for 3–5 minutes. Immunohistochemical results were obtained by inverted fluorescence microscope (Olympus, Tokyo, Japan). After immunohistochemistry, Image-Pro Plus 6.0 software was used to analyse the changes in the levels of BDNF/TrkB determined with integrated optical density (IOD) in the ganglia. The background level was determined by averaging the optical density of 10 random areas21.
Immunofluorescence
Immunofluorescence was then studied. The TG iced-slices were washed in PBS and incubated in a blocking solution containing 3% bovine serum albumin (BSA) in PBS with 0.3% Triton X-100 (Solarbio Company, Beijing, China) for 30 minutes at room temperature. In 4 °C refrigerator primary antibodies (mouse anti-GFAP, Abcam Company, 1:200; rabbit anti-BDNF, Abcam, 1:500) were incubated overnight. The samples were washed using PBS and the secondary antibody (goat anti-rabbit TRITC, Jackson ImmunoResearch Inc., West Grove, PA, USA, 1:200; goat anti-mouse FITC, Beijing Zhongshan Biotech Co., 1:200) was added for 45 minutes at 37 °C. Controls omitted the primary antibody. A fluorescence microscope (Olympus, Japan) and Image-Pro Plus 6.0 software were used to detect the immunofluorescence intensity of BDNF or GFAP expression. The co-expression steps of TrkB and GFAP were the same as mentioned above, except for the different dilution ratio (TrkB 1:1000, GFAP 1:200)27.
Western blotting experiment
After anaesthetizing the rats with 10% chloral hydrate (Shanghai Xinya Medical Company, Shanghai, China), the TG tissues were taken out and homogenized with a detergent lysis buffer containing phenylmethanesulfonyl fluoride (PMSF). The lysate was then transferred to a 1.5 ml centrifuge tube with a pipette and centrifuged at 12000 rpm for 5 minutes at 4 °C. The supernatant was collected and placed at −20 °C. The total protein in the supernatant was measured using the Lowry method. The protein was diluted with a sample buffer and at 95 °C for 10 minutes. SDS-polyacrylamide gel (12%) electrophoresis was used to separate the same amount of proteins (20 μg), and then transferred onto a nitrocellulose (NC) membrane in a Bio-Rad electrophoresis device. Primary antibodies (rabbit polyclonal anti-BDNF, Abcam; TrkB, Abcam; ERK1/2 or p- ERK1/2, Cell Signaling Technology; and β-actin, Advanced Immunochemicals) were used. The secondary antibody, goat anti-rabbit IgG, was purchased from Beijing Zhongshan Biotech. Using a Bio-Rad system, the labelled proteins were then imaged with an enhanced chemiluminescence. The optical density of the target proteins band were calculated using Image-Pro Plus software after normalizing to each β-actin band36.
Statistical analysis
The data were analysed using the SPSS software (version 21.0). All results are represented as the mean ± SEM. Statistical significance was analysed with a one-way analysis of variance (ANOVA), and was followed, when appropriate, by Fisher’s post hoc test for multiple comparisons. Results were considered significant when p < 0.05.
Ethical approval
The Animal Care and Ethics Committee of Nanchang University approved the procedures of this research. The IASP’s ethical guidelines for the study of pain in animals were followed.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Lota, A. S. & Dubrey, S. W. Intractable pain from trigeminal neuralgia. Br. J. Hosp. Med. (Lond) 73, 230–231 (2012).
Di Stefano, G., Truini, A. & Cruccu, G. Current and Innovative Pharmacological Options to Treat Typical and Atypical Trigeminal Neuralgia. Drugs 78, 1433–1442, https://doi.org/10.1007/s40265-018-0964-9 (2018).
Maarbjerg, S., Di Stefano, G., Bendtsen, L. & Cruccu, G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia: an international journal of headache 37, 648–657, https://doi.org/10.1177/0333102416687280 (2017).
Marin-Gracia, M. et al. Lacosamide associated with high-degree block in a patient with trigeminal neuralgia. Revista de neurologia 66, 189–192 (2018).
Obermann, M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord 3, 107–115, https://doi.org/10.1177/1756285609359317 (2010).
Ariai, M. S., Mallory, G. W. & Pollock, B. E. Outcomes After Microvascular Decompression for Patients with Trigeminal Neuralgia and Suspected Multiple Sclerosis. World neurosurgery, https://doi.org/10.1016/j.wneu.2013.09.027 (2013).
Ryu, J., Lee, S. H., Choi, S. K. & Lim, Y. J. Gamma knife radiosurgery for trigeminal schwannoma: a 20-year experience with long-term treatment outcome. Journal of neuro-oncology 140, 89–97, https://doi.org/10.1007/s11060-018-2934-1 (2018).
Holland, M. T. et al. Stereotactic radio surgery and radio frequency rhizotomy for trigeminal neuralgia in multiple sclerosis: A single institution experience. Clinical neurology and neurosurgery 162, 80–84, https://doi.org/10.1016/j.clineuro.2017.09.004 (2017).
Patra, D. P. et al. Repeat Gamma Knife radiosurgery versus microvascular decompression following failure of GKRS in trigeminal neuralgia: a systematic review and meta-analysis. Journal of neurosurgery, 1–10, https://doi.org/10.3171/2018.5.JNS18583 (2018).
Acheson, A. et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374, 450–453, https://doi.org/10.1038/374450a0 (1995).
Boulanger, L. & Poo, M. M. Gating of BDNF-induced synaptic potentiation by cAMP. Science 284, 1982–1984 (1999).
Briz, V., Liu, Y., Zhu, G., Bi, X. & Baudry, M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. The Journal of cell biology 210, 1225–1237, https://doi.org/10.1083/jcb.201504092 (2015).
Obata, K. & Noguchi, K. BDNF in sensory neurons and chronic pain. Neurosci Res. 55, 1–10, https://doi.org/10.1016/j.neures.2006.01.005 (2006).
Kooshki, R. et al. Orexin-A inhibits capsaicin-induced changes in cyclooxygenase-2 and brain-derived neurotrophic factor expression in trigeminal nucleus caudalis of rats. The Korean journal of pain 31, 174–182, https://doi.org/10.3344/kjp.2018.31.3.174 (2018).
Liu, C. et al. P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Molecular pain 14, 1744806918795930, https://doi.org/10.1177/1744806918795930 (2018).
Zhang, X. J. et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacological research 137, 34–46, https://doi.org/10.1016/j.phrs.2018.09.010 (2018).
Wang, L. et al. Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. Journal of natural medicines 71, 257–264, https://doi.org/10.1007/s11418-016-1057-2 (2017).
Gao, Y., Hu, S., Zhang, M., Li, L. & Lin, Y. Simultaneous determination of four alkaloids in mice plasma and brain by LC–MS/MS for pharmacokinetic studies after administration of Corydalis Rhizoma and Yuanhu Zhitong extracts. J Journal of Pharmaceutical and Biomedical Analysis. 92, 6–12 (2014).
Tarabasz, D. & Kukula-Koch, W. Palmatine: A review of pharmacological properties and pharmacokinetics. Phytotherapy research: PTR, https://doi.org/10.1002/ptr.6504 (2019).
Hambright, H. G., Batth, I. S., Xie, J., Ghosh, R. & Kumar, A. P. Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFκB/FLIP %J Molecular carcinogenesis. 54, 1227–1234 (2015).
Shen, Y. et al. Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain research bulletin 139, 56–66, https://doi.org/10.1016/j.brainresbull.2018.02.005 (2018).
Ma, W. K. et al. Palmatine from Mahonia bealei attenuates gut tumorigenesis in ApcMin/+ mice via inhibition of inflammatory cytokines. Molecular medicine reports 14, 491–498, https://doi.org/10.3892/mmr.2016.5285 (2016).
Sanchez-Larsen, A. et al. Assessment of efficacy and safety of eslicarbazepine acetate for the treatment of trigeminal neuralgia. European journal of pain 22, 1080–1087, https://doi.org/10.1002/ejp.1192 (2018).
Conover, J. C. et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature 375, 235–238, https://doi.org/10.1038/375235a0 (1995).
Bao, Y. et al. PAR2-mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Molecular pain 10, 28, https://doi.org/10.1186/1744-8069-10-28 (2014).
Lin, Y. T., Ro, L. S., Wang, H. L. & Chen, J. C. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. Journal of neuroinflammation 8, 126, https://doi.org/10.1186/1742-2094-8-126 (2011).
Ge, H. et al. Dihydromyricetin affects BDNF levels in the nervous system in rats with comorbid diabetic neuropathic pain and depression. Scientific reports 9, 14619, https://doi.org/10.1038/s41598-019-51124-w (2019).
Luo, D., Luo, L., Lin, R., Lin, L. & Lin, Q. Brain-derived neurotrophic factor and Glial cell line-derived neurotrophic factor expressions in the trigeminal root entry zone and trigeminal ganglion neurons of a trigeminal neuralgia rat model. Anatomical record, https://doi.org/10.1002/ar.24364 (2020).
Harward, S. C. et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 538, 99–103, https://doi.org/10.1038/nature19766 (2016).
He, S. M. et al. Identification and Characterization of Genes Involved in Benzylisoquinoline Alkaloid Biosynthesis in Coptis Species. Frontiers in plant science 9, 731, https://doi.org/10.3389/fpls.2018.00731 (2018).
Mai, C. T. et al. Palmatine attenuated dextran sulfate sodium (DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Molecular immunology 105, 76–85, https://doi.org/10.1016/j.molimm.2018.10.015 (2019).
Rong, Q. et al. In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori. BMC complementary and alternative medicine 16, 331, https://doi.org/10.1186/s12906-016-1310-y (2016).
Kajiya, M. et al. BDNF mimetic compound LM22A-4 regulates cementoblast differentiation via the TrkB-ERK/Akt signaling cascade. International immunopharmacology 19, 245–252, https://doi.org/10.1016/j.intimp.2014.01.028 (2014).
Ohta, K. I. et al. Prolonged maternal separation attenuates BDNF-ERK signaling correlated with spine formation in the hippocampus during early brain development. Journal of neurochemistry 141, 179–194, https://doi.org/10.1111/jnc.13977 (2017).
Ortega, J. A. & Alcantara, S. BDNF/MAPK/ERK-induced BMP7 expression in the developing cerebral cortex induces premature radial glia differentiation and impairs neuronal migration. Cerebral cortex 20, 2132–2144, https://doi.org/10.1093/cercor/bhp275 (2010).
Xiong, W. et al. Effects of long non-coding RNA uc.48+ on pain transmission in trigeminal neuralgia. Brain research bulletin 147, 92–100, https://doi.org/10.1016/j.brainresbull.2019.02.009 (2019).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81460106, 81860199), and the grants of Provincial Science and Technology Development of General Projects (No. 20192BBG70021) and Health Commission of Jiangxi Province (No. 20191070).
Author information
Authors and Affiliations
Contributions
Lijuan Liu, Lingkun He, Cancan. Yin, Ruoyu Huang, Wenhao Shen, Huixiang Ge, Mengyun Sun and Shujuan Li contributed to the experimental design, operation and analysis; Lijuan Liu, Wei Xiong and Yun Gao contributed to conception, interpretation, drafting and critically revising manuscript. All authors gave final approval and agree to be accountable for all aspects of the work’s integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, L., He, L., Yin, C. et al. Effects of palmatine on BDNF/TrkB-mediated trigeminal neuralgia. Sci Rep 10, 4998 (2020). https://doi.org/10.1038/s41598-020-61969-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61969-1
This article is cited by
-
Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review
Pain and Therapy (2024)
-
Genome-wide Analysis of Histone H3 Lysine 27 Trimethylation Profiles in Sciatic Nerve of Chronic Constriction Injury Rats
Neurochemical Research (2023)
-
The protective effect of Palmatine on depressive like behavior by modulating microglia polarization in LPS-induced mice
Neurochemical Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.