Abstract
The aim of this retrospective study was to develop and validate a nomogram for predicting the risk of post-operative pulmonary infection (POI) in gastric cancer (GC) patients following radical gastrectomy. 2469 GC patients who underwent radical gastrectomy were enrolled, and randomly divided into the development and validation groups. The nomogram was constructed based on prognostic factors using logistic regression analysis, and was internally and crossly validated by bootstrap resampling and the validation dataset, respectively. Concordance index (C-index) value and calibration curve were used for estimating the predictive accuracy and discriminatory capability. Sixty-five (2.63%) patients developed POI within 30 days following surgery, with higher rates of requiring intensive care and longer post-operative hospital stays. The nomogram showed that open operation, chronic obstructive pulmonary disease (COPD), intra-operative blood transfusion, tumor located at upper and/or middle third and longer operation time (≥4 h) in a descending order were significant contributors to POI risk. The C-index value for the model was 0.756 (95% CI: 0.675−0.837), and calibration curves showed good agreement between nomogram predictions and actual observations. In conclusion, a nomogram based on these factors could accurately and simply provide a picture tool to predict the incidence of POI in GC patients undergoing radical gastrectomy.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related death according to the Global Cancer Statistics 20181. For GC patients, resection offers the only potential curative treatment to date. Although mortality and morbidity following radical gastrectomy for GC have reduced significantly with improvements of surgical techniques and peri-operative managements, post-operative pulmonary complications (PPCs), including post-operative pulmonary infection (POI), remain a clinically important event, especially among older or immuno-compromised patients2. With an incidence rate ranging from 1.1–12.3%3,4,5,6,7, PPCs have been identified to be associated with the largest attributable healthcare costs and hospital stays; even with increased peri-operative death8,9. Moreover, there is a growing body of evidence that post-operative infectious complications, including POI, is an adverse predictor for prognosis in various of cancer patients10,11,12,13,14,15. Thus, to reduce POI, it is essential to clarify the risk factors and to identify those patients at greatest risk of POI, and allow early intervention to reduce its incidence or improve post-operative clinical outcomes. Although several studies have investigated the risk factors for POI following gastrectomy for GC, their conclusions were usually based on a limited number of patients and have never been externally validated15,16.
Using a graphical calculator, nomograms have been used to build predictive models that serve as popular mathematical tools. There is a growing body of evidence that a nomogram can predict the metastatic probability, recurrence probability, overall survival, and disease risk for most types of cancer17. In addition, these nomograms have served as intuitive and simple tools for clinicians to reduce disease risk or improve prognosis by tailoring clinical treatments to the needs of individual patients. But to our knowledge, there has never been a study intended to develop a nomogram to predict the risk of POI following radical gastrectomy. Therefore, in this retrospective study, for the first time, we developed and cross-validated a novel nomogram to predict POI in GC patients following curative resection, using the database from a high volume tertiary hospital in China.
Patients and Methods
Patient population
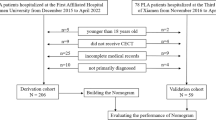
We reviewed the prospectively registered data of adult patients diagnosed with GC and underwent curative gastric resection in Hunan Cancer Hospital between November 2010 and July 2018. The inclusion and exclusion criteria are described in Figure 1. In total, 2469 consecutive patients were included in our study. Ethical approval was obtained by the Institutional Review Board of our hospital (N0.17 scientific research quick review in 2018). Every patient provided written informed consent for surgery and the use of their clinical data. To test the generalizability of the model, patients were randomly assigned to the development group (n = 1728) and the validation group (n = 741) at a ratio of 7:3.
Peri-operative management and surgical procedures
Prior to surgery, all patients were subjected to laboratory examination, abdominal computed tomography (CT), as well as X-ray and/or CT of the chest. The curative gastrectomy and lymphadenectomy was performed by qualified physicians according to the Japanese gastric cancer treatment guidelines18. As described in our previous study19, open procedures were usually performed for D2 or D2 + lymphadenectomy in patients with advanced GC, and laparoscopy or laparoscopy-assisted gastrectomy with D1 + α or β lymphadenectomy were performed for patients with early GC. To achieve R0 resection margins, combined multiorgan resection was performed in patients with a locally advanced tumor with potential invasion of nearby organs and in those with simultaneously occurring benign disease in other organs. All patients received a second- or third-generation cephalosporin for 3 to 5 days following surgery as prophylactic antibiotic treatment. Platinum and fluorouracil based regimens, such as S-1 plus oxaliplatin, were used for neoadjuvant chemotherapy, in some patients with stage cT3-4N + GC.
Data collection
The clinicopathological factors including baseline demographics, pre-operative laboratory measurements, intra-operative variables and pathological tumor characteristics (based on American Joint Committee on Cancer TNM staging system 7th edition) were evaluated. The primary outcome assessed was the development of POI within 30 days following surgery. The post-operative morbidity during the 30-day follow-up period was checked by the same physician during hospital stay. We sent an e-mail or letter or gave a call to patients who did not report for follow-up examinations. Given their little clinical significance of stage I complications, only Clavien-Dindo grade II or higher were regarded as having complications in the present study19.
Patients with lung infiltrate visible in chest X-ray and/or CT scan combined with respiratory symptoms, fever, leukopenia, or leukocytosis were diagnosed with POI, either following other complications (such as anastomotic leakage) or not, according to the criteria proposed by the United States Centers for Disease Control and Prevention20. The diagnosed with POI was checked by the researchers of the present study again by carefully reviewing the medical records of each patient.
Statistical analysis
We use univariate logistic analysis to analyze risk factors. All variables were entered into the multivariate logistic regression model through a backward step-down process performed using the Akaike information criterion stopping rule. The nomogram in our study was designed to give the risk probability of POI. Parameter estimates obtained from the above the multivariate logistic regression were used to construct nomogram scale, and only the variable with P value less than 0.05 were incorporated into the nomogram. Every variable in regression model can get a score on the top point line (0–100 points by default) in the nomogram plot thought drawing corresponding vertical line, then a total score can be get by adding every variable’s score. Last, we can obtain the predicted risk value through drawing a vertical line from the total score to the bottom risk line.
Model validation included bootstrap self-sample validation and cross validation. Bootstrap self-sample validation was performed by using 1000 bootstrap resamples of the development dataset. Source data was divided into two sets, the bigger set was used for developing the model while the smaller set was applied to the validation the constructed model, which called cross validation. The prognostic performance of the model was evaluated based the concordance index (C-index) value on a scale from 0 to 1 and 95% confidence interval (CI), as well as the area under the curve (AUC) for the receiver operator characteristic (ROC) plot. Generally, a C-index value > 0.70 indicated that the model was good for discrimination. The nomogram was calibrated by comparing the predicted rates of POI with the observed rates. All data management and statistical analyses were performed using the R software, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Clinicopathological characteristics and incidence of POI
The clinicopathological characteristics of the 2469 patients are shown in Table 1. The cohort included 1624 men (65.78%) and 845 women (34.22%), with a mean age as 55.44 years (range, 19–86). Of whom, 1888 patients (76.47%) were performed subtotal gastrectomy, and the remaining 581 cases underwent total gastrectomy. The mean operation time was 199.28 min (range, 70–584), and the mean estimated intra-operative blood loss was 203.41 mL (range, 30–2300). According to the 7th edition of the TNM staging system for GC, there were 649 (26.29%) stage I, 514 (20.82%) stage II, and 1306 (52.90%) stage III patients.
A total of 293 complications occurred in 220 patients of the entire cohort (8.91%), with pneumonia ranked as the second most common (n = 65, 2.63%), following surgical site infections (n = 116, 4.70%). With respect to the 65 patients who suffered from POI, the majority was managed with antibiotics and other conservative treatments (n = 50, 76.92%). Fifteen patients (23.08%) developed respiratory failure and needed intensive care unit care, which was significant more common than whose without POI (1.83%, P < 0.001). Moreover, 4 patients dead from respiratory failure and/or sepsis. The mortality rate was also significant higher in patients with POI than whose without POI (6.15% vs. 0.17%, P < 0.001). Additionally, patients with POI required longer post-operative hospital stays than those without POI (17.12 vs. 11.28 days, P < 0.001).
Prognostic nomogram for POI
The univariate logistic analyses showed that smoking, American Society of Anesthesiologist (ASA) score, chronic obstructive pulmonary disease (COPD), serum albumin level, pre-operative blood transfusion, operation procedure, extent of gastrectomy, combined multi-organ resection, operation time, amount of bleeding, intra-operative blood transfusion, tumor location, and tumor size were associated with POI (P < 0.05 for all, Table 1). Further multivariate logistic regression analyses showed that COPD, open operative procedure, operation time ≥4 h, intra-operative blood transfusion, and tumor located at middle third were independent risk factors for POI (Table 2). The prognostic nomogram for POI was constructed based on these five prognostic factors (Figure 2). The nomogram plot of GC patient outcomes showed that compared with laparoscopic procedure, open operation was the greatest contributing factor to the risk of POI. Other factors that significantly contributed to POI risk to a lesser degree were COPD, intra-operative blood transfusion, tumor located at upper and/or middle third and operation time ≥4 h in descending order.
Calibration and validation of the nomogram
Internal validation of the nomogram model yielded a C-index value of 0.756 (95% CI: 0.675−0.837), which was identical to the AUC of the ROC plot (Figure 3). The internal calibration curve showed good agreement between nomogram predictions and actual observations with the predictive probability ranging from 0 to 0.15 (Figure 4). Data for the validation group (n = 741) were used for cross validation of the nomogram model, which yielded a C-index value of 0.652 (95% CI: 0.602−0.703), which was lower than the index of the development group.
Discussion
In this retrospective study including a large cohort of GC patients who underwent curative gastrectomy from a single high-volume tertiary center in China, we found that the incidence of POI following radical gastrectomy for GC was 2.63%, which was similar to previous findings ranging from 1.1% to 3.6%3,4,7. Whereas some other studies reported that old patients (≥75 years) had a significant higher incidence of POI (5.1–13.3%), compared with that of patients aged 45 to 65 years16,21,22. Patients who suffered from POI had a significant higher rate of requiring intensive care and mortality rate, and longer post-operative hospital stays than those without POI. Given the significantly adverse influence of POI on post-operative recovery, surgeons should prioritize peri-operative managements to decrease its incidence. Although several studies have investigated the incidence and risk factors for POI following gastrectomy for GC, but the findings of these studies were usually based on a limited number of patients and limited variables, which may affect the adequate accuracy and power of the model7,23. Moreover, their conclusions have never been verified by other studies. Our logistic regression analysis found that open procedure, COPD, intra-operative blood transfusion, tumor located at middle third and longer operation time were significant prognosticators of POI. Thus these five parameters were incorporated into the nomogram for predicting POI. Further internal and cross validation showed good agreement between nomogram predictions and actual observations with the predictive probability. To our knowledge, this is the first time to develop and crossly validate a nomogram for predicting POI in GC patients who underwent radical gastrectomy. This nomogram may serve as intuitive and simple tool for clinicians to identify those who are at greatest risk of suffering from POI, and take some active medical interventions for its prevention.
Our nomogram showed that open operation procedure accounted for the largest contribution to the risk of POI. Compared with open surgery, minimally invasive surgery has been shown to decrease blood loss, reduce time to ambulation, and quicker recovery. A smaller incision in laparoscopic surgery could relieve pain from coughing after surgery. Severe pain often influences patients’ compliance with incentive spirometry and thus decreases effective discharge of sputum and increases the risk of POI. Severe pain also could increase requirement for narcotics, which further suppresses patients’ respiratory drives and increases atelectasis24. A resent meta-analysis has confirmed that laparoscopic gastrectomy was associated with a lower incidence of pneumonia in patients with one or more high-risk factors, such as age ≥70 years, BMI ≥ 30 kg/m2, compared with open surgery25. In this study, the proportion of laparoscopic or laparoscopy-assisted gastrectomy increased from 4.04% (43/1065) to 37.18% (522/1404) through the first 4 years (from November 2010 to December 2014) to the last 4 years (from January 2015 to July 2018, P < 0.001), with the incidence of POI decreased from 3.66% to 1.85% (P = 0.005). Laparoscopic surgery has been widely performed in general clinical practice to manage early stage GC. And to date, it has been proved to be safe and have comparable oncological results in highly selective patients with advanced GC performed by experienced surgeons18,26. Thus, laparoscopic surgery may serve as an option to reduce POI in selective GC patients, although further prospective studies are needed.
Then, the contributions of the COPD, intra-operative blood transfusion, tumor location, and operation time in the nomogram, though significant, were less than that of operation procedure. Patients with COPD often have reduced respiratory function. Smoking is the most common cause of COPD, with factors such as air pollution and genetics playing a smaller role. Long exposure of these toxic particles or gases can induce the release of inflammatory mediators, inhibit ciliary movement in the airway, reduce the activity of pulmonary surface-active substances, increase airway mucus secretion, and reduce the ability of bronchial mucosa clearance, which ultimately increase the incidence of post-operative pulmonary complications27. Li et al. reported that COPD were an independent risk factor for post-operative complications in elderly GC patients group (OR = 3.756)28. For those with high-risk of developing POI, such as COPD, pre-operative smoking cessation, lung expansion modalities including deep breathing exercises and cough, and selective nasogastric decompression might reduce its incidence and thus, should be performed as possible29.
Intra-operative blood transfusion has been shown to increase susceptibility to pulmonary infection7,16,21,28. Transfusion induced systemic inflammation and transfusion-related immunomodulation (TRIM) was accepted as the underlying mechanism19. With respect to transfusion time, our previous study has also found that intra-operative, rather than pre-operative or post-operative blood transfusion, was independent risk for post-operative infections following gastrectomy19. Possible explanation was intra-operative blood transfusion may act synergistically with surgical stress to induce immuno-suppression. Therefore, to reduce POI, there could be some room for consideration to perform blood transfusion pre-operatively or post-operatively rather than intra-operatively. Given the relatively low incidence of POI and only 65 patients who developed POI in the present study, we did not investigate the association of the transfusion timing with POI. Thus, the conclusions need further more investigations.
Patients with tumors located in the upper third of the stomach were most vulnerable to POI, followed in descending order by the middle and lower third. The diaphragm, the most important breathing muscle, lies above the stomach. Based on the anatomical position, the closer the tumor was to the diaphragm, the greater extent to which the diaphragm reflex was suppress during gastric resection, which lowered lung activity by limiting spontaneous sighs and deep breaths, reducing effective coughing to discharge the sputum, and increasing lung secretion retention, which could increase the risk of POI. In addition, upper third GC was usually diagnosed later and accompanied by greater lymph node metastasis and deeper infiltration, and No. 110 and 111 lymph node (lower thoracic para-esophageal node) was recommended to dissection in some patients with tumors invading the esophagus18. Total gastrectomy was commonly performed in these patients, which was a more complex procedure than distal subtotal gastrectomy, thus requiring longer operation time and was conformed to be associated with higher incidence of morbidity, which may also increase the risk of POI18,30.
Prolonged surgery was identified as an independent risk factor for POI in our study, which was echoed by a study including 25419 colectomies, in which a regression model demonstrated that each 60-min increase in operative time was associated with at least 13% increased odds of post-operative pulmonary complications24. Interestingly, despite a potential for prolonged surgery, a laparoscopic procedure decreased half the absolute risk of post-operative pulmonary complications. Thus, the authors concluded that when safe, laparoscopy should be performed as possible. Additionally, prolonged surgery probably means technical difficulty, such as overweight patients, extended lymphadenectomy or combined resection, which may also increase the risk of morbidity. Using ultrasonically activated coagulating shears, improving surgical experience and work volume may reduce the operative time, and thus probably decrease the risk of POI31,32.
Nomogram validation is highly essential for confirming the predictive performance and determining the generalizability of the nomogram to other GC patients33. We developed an internal validation method for our nomogram model, and found that the C-index value was 0.756 (95% CI: 0.675−0.837), which was identical to the AUC of the ROC plot. The calibration curve showed good agreement between the nomogram predictions and actual observations within a predictive probability range of 0 to 0.15, which indicated satisfactory and reliable nomogram performance. To our knowledge, this is the first report that POI risk nomogram have been developed using these parameters. Our nomogram allows surgeons to easily and accurately identify GC patients who are at high risk of POI, then to optimize treatment for POI prevention. From these significant factors for POI in the model, doctors are encouraged to perform laparoscopic surgery for selective patients if possible, especially for those with high-risk factors, such as COPD and upper third GC. Using ultrasonically activated coagulating shears and improving surgical experience to reduce operation time, performing blood transfusion pre-operatively instead of intra-operatively, may also decrease the risk of POI following gastrectomy for GC, which needs to be verified by further studies.
There are also some limitations of our findings. These include the retrospective nature and single-institution design of our study. Thus selective bias was impossible to avoid and the conclusion should be cited with appropriate interpretation. Second, the predictive factors involved in our nomogram were acquired from routine demographic and laboratory testing. Other important parameters, such as respiratory function testing and data on immune function, were not considered in the design of our nomogram. Third, the exact onset time of POI was not investigated in the present study, whereas early or late occurrence, “primary” POI or POI following other complications (such as anastomotic leakage) has been identified to be associated with different risk factors34. Fourth, Western GC patients usually have higher BMI, longer operation time and with different process of healthcare during hospitalization (such as nasogastric decompression and prophylactic antibiotic usage) and post-discharge compared with those in China, which may affect the applicability of the nomogram in Western patients. Last, but not the least, the sample size for the dataset used for nomogram cross validation was relatively small, and future prospective studies utilizing larger sample sizes are warranted to confirm our findings.
Conclusions
The GC nomogram for predicting the risk of POI has been established and validated. Our results confirmed that open operation, COPD, intra-operative blood transfusion, tumor located at upper and/or middle third and operation time ≥4 h were significant factors in the nomogram to predict POI. This makes it possible for physicians to choose some clinical activities to reduce post-operative pulmonary infection accurately and simply by using the picture tool of our nomogram.
References
Bray, F. et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 68, 394–424 (2018).
Kiuchi, J. et al. Putative Risk Factors for Postoperative Pneumonia Which Affects Poor Prognosis in Patients with Gastric Cancer. Int. J. Clin. Oncol. 21, 920–926 (2016).
Jung, M. R. et al. Definition and Classification of Complications of Gastrectomy for Gastric Cancer Based On the Accordion Severity Grading System. World J. Surg. 36, 2400–2411 (2012).
Kim, H. H. et al. Morbidity and Mortality of Laparoscopic Gastrectomy Versus Open Gastrectomy for Gastric Cancer: An Interim Report–A Phase III Multicenter, Prospective, Randomized Trial (KLASS Trial). Ann. Surg. 251, 417–420 (2010).
Sah, B. K. et al. Gastric Cancer Surgery and its Hazards: Post Operative Infection is the Most Important Complication. Hepatogastroenterology. 55, 2259–2263 (2008).
Dimick, J. B. et al. Hospital Costs Associated with Surgical Complications: A Report From the Private-Sector National Surgical Quality Improvement Program. J Am Coll Surg. 199, 531–537 (2004).
Mohri, Y., Tonouchi, H., Miki, C., Kobayashi, M. & Kusunoki, M. Incidence and Risk Factors for Hospital-Acquired Pneumonia After Surgery for Gastric Cancer: Results of Prospective Surveillance. World J. Surg. 32, 1045–1050 (2008).
Lawrence, V. A. et al. Incidence and Hospital Stay for Cardiac and Pulmonary Complications After Abdominal Surgery. J. Gen. Intern. Med. 10, 671–678 (1995).
Thompson, D., Makary, M. A., Dorman, T. Clinical and Economic Outcomes of Hospital Acquired Pneumonia in Intraabdominal Surgery Patients. 547–552 (2006).
Derogar, M., Orsini, N., Sadr-Azodi, O. & Lagergren, P. Influence of Major Postoperative Complications On Health-Related Quality of Life Among Long-Term Survivors of Esophageal Cancer Surgery. J. Clin. Oncol. 30, 1615–1619 (2012).
Baba, Y. et al. Prognostic Impact of Postoperative Complications in 502 Patients with Surgically Resected Esophageal Squamous Cell Carcinoma: A Retrospective Single-Institution Study. Ann. Surg. 264, 305–311 (2016).
Saeki, H. et al. Prognostic Significance of Postoperative Complications After Curative Resection for Patients with Esophageal Squamous Cell Carcinoma. Ann. Surg. 265, 527–533 (2017).
Artinyan, A. et al. Infectious Postoperative Complications Decrease Long-Term Survival in Patients Undergoing Curative Surgery for Colorectal Cancer: A Study of 12,075 Patients. Ann. Surg. 261, 497–505 (2015).
Tu, R. H. et al. Prognostic Significance of Postoperative Pneumonia After Curative Resection for Patients with Gastric Cancer. Cancer Medicine. 6, 2757–2765 (2017).
Suzuki, S. et al. Long-Term Impact of Postoperative Pneumonia After Curative Gastrectomy for Elderly Gastric Cancer Patients. Annals of Gastroenterological Surgery. 2, 72–78 (2018).
Miki, Y. et al. Risk Factors for Postoperative Pneumonia After Gastrectomy for Gastric Cancer. Surg. Today. 46, 552–556 (2016).
Xiao, H. F. et al. Development and Validation of Two Prognostic Nomograms for Predicting Survival in Patients with Non-Small Cell and Small Cell Lung Cancer. Oncotarget. 8, 64303–64316 (2017).
Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4) 1–19 (2017).
Xiao, H. et al. Impact of Peri-Operative Blood Transfusion On Post-Operative Infections After Radical Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis Focusing On the Timing, Amount of Transfusion and Role of Leukocyte Depletion. J Cancer Res Clin Oncol. 144, 1143–1154 (2018).
Horan, T. C., Andrus, M. & Dudeck, M. A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control. 36, 309–332 (2008).
Takeuchi, D. et al. Postoperative Complications in Elderly Patients with Gastric Cancer. J. Surg. Res. 198, 317–326 (2015).
Yamada, H. et al. Postoperative Complications in the Oldest Old Gastric Cancer Patients. Int. J. Surg. 11, 467–471 (2013).
Xiao Hua, Z. C. Y. O. Multivariate Analysis of Risk Factors for Pulmonary Infection After Radical Gastrectomy for Gastric Cancer. 3310–3313 (2014).
Owen, R. M. et al. Impact of Operative Duration On Postoperative Pulmonary Complications in Laparoscopic Versus Open Colectomy. Surg. Endosc. 27, 3555–3563 (2013).
Li, Z., Zhao, Y., Liu, Y., Yu, D. & Zhao, Q. Laparoscopic Versus Open Gastrectomy for High-Risk Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. Int. J. Surg. 65, 52–60 (2019).
Yu, J. et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 321, 1983–1992 (2019).
Shimizu, K., Nakata, M., Hirami, Y., Maeda, A. & Tanemoto, K. Recent Results Regarding the Clinical Impact of Smoking History On Postoperative Complications in Lung Cancer Patients. Interact Cardiovasc Thorac Surg. 7, 1001–1006 (2008).
Li, Y. et al. Clinicopathologic Characteristics of Elderly with Gastric Cancer, and the Risk Factors of Postoperative Complications. J. Invest. Surg. 30, 394–400 (2017).
Qaseem, A. et al. Risk Assessment for and Strategies to Reduce Perioperative Pulmonary Complications for Patients Undergoing Noncardiothoracic Surgery: A Guideline From the American College of Physicians. Ann. Intern. Med. 144, 575–580 (2006).
Martin, A. N. et al. Morbidity and Mortality After Gastrectomy: Identification of Modifiable Risk Factors. J. Gastrointest. Surg. 20, 1554–1564 (2016).
Sah, B. K. et al. Effect of Surgical Work Volume On Postoperative Complication: Superiority of Specialized Center in Gastric Cancer Treatment. Langenbecks Arch Surg. 394, 41–47 (2009).
Mohri, Y. et al. Ultrasonically Activated Shears in Gastrectomy for Large Gastric Cancers. Surg. Today. 37, 1060–1063 (2007).
Iasonos, A., Schrag, D., Raj, G. V. & Panageas, K. S. How to Build and Interpret a Nomogram for Cancer Prognosis. J. Clin. Oncol. 26, 1364–1370 (2008).
Yao, Z. et al. Analysis of Risk Factors of Pulmonary Infection in Patients Over 60 Years of Age After Radical Resection for Gastric Cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 22, 164–171 (2019).
Acknowledgements
This study was funded by the Natural Science Foundation of Hunan Province (No. 2018JJ6108) and the 2017 Annual Research Project of Health and Family Planning Commission of Hunan Province (No. B2017101).
Author information
Authors and Affiliations
Contributions
Haifan Xiao and Hua Xiao designed the study. Haifan Xiao, Hua Xiao, Huijun Zhou, Ke Liu, Xianzhen Liao, Shipeng Yan, Bin Yin and Yongzhong Ouyang collected and analyzed data. Haifan Xiao wrote the paper. Hua Xiao revised the paper. All authors confirmed that the content has not been published elsewhere and does not overlap with or duplicate their published work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, H., Zhou, H., Liu, K. et al. Development and validation of a prognostic nomogram for predicting post-operative pulmonary infection in gastric cancer patients following radical gastrectomy. Sci Rep 9, 14587 (2019). https://doi.org/10.1038/s41598-019-51227-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51227-4
This article is cited by
-
A nomogram to predict postoperative surgical site infection of adult patients who received orthopaedic surgery: a retrospective study
Scientific Reports (2023)
-
A nomogram for predicting postoperative pulmonary infection in esophageal cancer patients
BMC Pulmonary Medicine (2021)
-
A nomogram to predict postoperative infection for older hip fracture patients
Archives of Orthopaedic and Trauma Surgery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.