Abstract
In this study, juvenile Manchurian trout, Brachymystax lenok (initial weight: 6.43 ± 0.02 g, mean ± SE) were received for nine weeks with five types of diets prepared by gradually replacing the proportion of fish oil (FO) with linseed oil (LO) from 0% (LO0) to 25% (LO25), 50% (LO50), 75% (LO75), and 100% (LO100). The eicosapentaenoic (EPA) and docosahexaenoic (DHA) composition decreased with increasing inclusion level of LO (P < 0.05). With increasing LO inclusion level, triglyceride (TAG) content of serum increased significantly, however, there was a decrease in high-density lipoprotein cholesterol (HDL) (P < 0.05). LO substitution of FO up-regulated the gene expression level of lipid metabolism-related genes Fatty Acid Desaturases 6 (FAD6), Acetyl-Coa Carboxylase (ACCα), Sterol Regulatory Element Binding Protein 1 (SREBP-1), and Sterol O- Acyl Transferase 2 (SOAT2), and down-regulated the gene expression level of Peroxisome Proliferator-Activated Receptor a (PPARα) (P < 0.05). The SOD activities of both serum and liver in LO100 were significantly lower than in LO25 (P < 0.05). The CAT activity of the liver in LO100 was significantly lower than in LO0 and LO25 (P < 0.05). This study indicates that the Manchurian trout may have the ability to synthesize LC-PUFAs from ALA, and an appropriate LO in substitution of FO (<75%) could improve both the lipid metabolism and the oxidation resistance.
Similar content being viewed by others
Introduction
Fish oil (FO) is an ideal lipid source in the fish diet since it is rich in highly unsaturated fatty acids (HUFAs), particularly eicosapentaenoic (EPA) and docosahexaenoic (DHA)1,2. Both EPA and DHA play important roles in accelerating fish growth and neural development, regulating the fish metabolism, and improving the immunity of the organism3,4. However, with the reduction of the global fish meal and fish oil production, finding suitable alternative lipid sources to replace fish oil has become a hot research topic in fish nutrition5. Linseed oil (LO) contains an abundance of α-linolenic acid (ALA, C18:3n-3), which is one of the essential fatty acids for freshwater fish. In general, freshwater fish have the ability to synthesize long chain polyunsaturated fatty acids (LC-PUFAs), utilizing ALA; thus, LO has become a potential high-quality substitution oil source6,7. Previous studies have shown that FO substitution by a suitable content of LO in the feed would improve the diet conversion rate of fish as well as fish health6,7,8,9,10,11,12.
However, it has been reported that the absorption of polyunsaturated fatty acids (PUFAs) in fish diet (especially EPA and DHA) will increase the oxidative stress (OS) of the entire organism; furthermore, liver cells might be damaged due to lipid peroxidation, which affects normal physiological function13. Addition of a specific proportion of vegetable oil (VO) to the diet reduces the fish’s peroxidation in liver14. Therefore, it is of great significance to study the influence of LO substitution of FO on oxidation resistance in fish. Superoxide dismutase (SOD) and catalase (CAT) are the main antioxidant enzymes of the oxidation resistance defense system in fish15. Thus, SOD and CAT are common indexes for the oxidation resistance16,17.
LO, as a substitution for FO, modulates fish lipid metabolism. The fish’s digestion and absorption of lipids as well as the biological synthesis and decomposition of fatty acids are associated with enzymes related to the fat metabolism and relevant transcription regulators1. Fatty acid desaturase 6 (FAD6) controls the synthesis of highly unsaturated fatty acids, and acetyl-CoA carboxylase (ACCα) is the rate-limiting enzyme catalyzing the synthesis of fatty acid18. Sterol O-acyl transferase 2 (SOAT2) is mainly involved in the absorption of cholesterol and the assembly of lipoprotein19. The sterol regulatory element binding protein-1 (SREBP-1) plays a central role in the control of the fatty acid entry into cholesterol ester20,21, and peroxisome proliferators-activated receptors (PPARα) mainly regulate the β-oxidative decomposition of fatty acids22.
The Manchurian trout (Brachymystax lenok) is a cold-water salmonid fish, which is mainly distributed throughout eastern Siberia, Mongolia, Kazakhstan, Korea, and China. Because it has relatively high nutritious and economic value, it is cultured widely in the northern areas of China23. Earlier studies showed that an appropriate lipid level in the diet was favorable for the growth of juvenile Manchurian trout24. However, the lipid metabolism and oxidative status of Manchurian trout following LO substitution of FO has not been reported to date. Therefore, the aim of this study was to investigate the influence of FO replacement by LO on both the lipid metabolism and oxidative status of juvenile Manchurian trout by substituting FO with different levels of LO. The appropriate level of LO as FO substitute was comprehensively estimated.
Materials and Methods
Ethical standards
All procedures and protocols were approved by the Inner Mongolia University for Nationalities in accordance with national and international guidelines for the care and use of animals in experimentation (2017-IMUN-016).
Experimental diets
Five groups of diets were prepared with iso-protein (approximately 39.5%) and iso-lipid (approximately 18.5%) using fish meal and soybean meal as the main protein source and FO and LO as the main lipid sources, as shown in Table 1. In each dietary group, the proportion of LO was gradually increased as substitution of FO, with substitution levels of 0% (LO0), 25% (LO25), 50% (LO50), 75% (LO75), and 100% (LO100). Alcohol was used to conduct the degreasing treatment to fish meal used in this experiment to reduce the effects of fish oil in fish meal on the experiment25. The raw material of diets was mixed and ground, trace components were mixed and added according to the scale up principle, and finally, the respective lipid was uniformly mixed into the diet. The mixture was extruded as a particle diet (diameter of 1.5 mm) using a DS32-II type two-screw extruder (Jinan Saixin Puffing Machinery Ltd.) after water addition. The mixture was then dried at 50 °C, and stored at −20 °C until future use. Raw materials and rough components are shown in Table 1 and the fatty acid composition of diets is shown in Table 2.
Procedure of the feeding experiment
The Manchurian trout used in this experiment were purchased from the cold-water fish farm of Fengcheng City (Dandong, Liaoning Province, China). The culture experiment was conducted at the Cold Water Fish Culture Laboratory of Inner Mongolia University for Nationalities. Prior to the trial, all Manchurian trout were temporarily cultured for two weeks in an indoor cold-water fish cycling system. During this temporary culture period, Manchurian trout were fed a commercial diet. Prior to the trial, Manchurian trout abstained from eating for 24 hours. Healthy Manchurian trout (average body weight: 6.43 ± 0.02 g, mean ± SE) were selected and randomly stocked into 15 buckets (150 L/bucket, triplicate per diet group), with 20 fish per bucket. Trout were fed until visual satiety twice per day (07:00 and 17:30) with one of the five diets for nine consecutive weeks. A cycling water system was employed with a flow velocity of 1.5 L/min, a water temperature of 15 ± 2 °C, and a dissolved oxygen level >7.5 mg/L.
Sample collection
At the beginning of the trial, five fish were anaesthetized using MS-222 (100 mg/L), homogenized in a blender and stored at −20 °C for whole-body fatty acid composition analysis. At the end of the trial, fish were anaesthetized, weighted, and counted in each bucket 24 h after feeding. Four fish per bucket were randomly selected and their blood was taken from the tail vein, injected in a centrifuge tube and allowed to clot at 4 °C for 4. Then, the blood was centrifuged for 15 min at 3000 g and 4 °C. The serum was stored in centrifuge tubes and stored at −80 °C for the analysis of blood biochemical index and antioxidant enzyme activity. After taking blood samples, the liver samples (four fish per bucket) were immediately stripped, placed into cryopreservation tubes, and immediately stored in liquid nitrogen at −80 °C for analysis of the metabolism index, oxidative status, and gene expression. In addition, three fish per bucket were selected and homogenized in a blender for the analysis of whole fish crude composition and fatty acid composition. The samples were stored at −20 °C until analysis.
Proximate composition and fatty acid composition analysis
The AOAC standard method26 was adopted for a biochemical composition analysis of both diet and whole fish. All chemical analyses were performed in triplicate. The dry matter was analyzed after drying to a constant weight in a baking oven at 105 °C. The Kjeldahl method (Kjeltec TM 8400, FOSS, Sweden) was adopted to analyze crude protein (total nitrogen × 6.25). The ash content was burnt in a Muffle furnace at 550 °C for 16 h after which, it was measured. The lipids in samples were extracted according to the method of Folch et al. (chloroform/mathanol, 2:1,v/v)27. Fatty acid methyl esters (FAME) were prepared by the methanol-benzene-acetyl chloride method according to Sukhija and Palmquist28. Then, the obtained FAME were analyzed with a Agilent 6890 gas chromatograph (Agilent, USA). The sample (150 mg of dry mass) was added to a hydrolysis tube with 4 ml chloroacetyl methanol solution (chloroacetyl methanol = 1 + 10) and 1 ml C11:0 (1.0 mg/ml) internal standard solution. Then, 1 mL n-hexane was added and reacted in a water bath at 80 °C for 2 h. After cooling, 5 ml of 7% potassium carbonate was added, shaken until an even mixture was obtained, and centrifuged at 1000 r/min for 5 min. Then, the mixture was put into the sample vial through a 0.2 μm filter membrane, and detected by gas chromatograph (Agilent, USA). The chromatographic column was DB-23 (60.0 m × 250 μm × 0.25 μm). He was used as carrier gas and the flow rate was 2.0 ml/min. The shunt ratio was 30:1, the inlet temperature was 260 °C, and the injection volume was 1 ul. The detector was FID and the temperature was 270 °C. The FAs were identified using standard mixtures of methylesters (Supelco 37 component FAME mix), and the FA composition was determined by C11:0.
Analysis of antioxidant index
The liver samples were homogenized in 10 volumes (w/v) of ice-cold physiological saline and centrifuged at 2500 g for 10 min at 4 °C. Then, the resultant supernatant was conserved until use. Total antioxidant capacity (T-AOC), SOD, and CAT indexes in serum and liver samples were measured based on the relevant toolkits (A015-2-1, A001-3-2 and A007-1-1, Nanjing Jiancheng Bioengineering Ltd., China) according to specification. Corresponding to the 50% inhibition rate of SOD in 1 ml of reaction solution per mL of serum or per mg of histone was defined as one unit of SOD activity. The amount of 1 mol of H2O2 decomposed per mL of serum or per mg of tissue protein per second was defined as one unit of CAT activity.
Analysis of blood biochemical index
Serum biochemical index triglyceride (TAG), total cholesterol (TCHO), high density lipid protein cholesterol (HDL-C), and low-density lipid protein cholesterol (LDL-C) were measured according to the relevant toolkits (A110-2-1, A111-2-1, A112-2-1, and A113-2-1, Nanjing Jiancheng Bioengineering Ltd., China) according to specification.
Real time quantitative PCR
The total RNA in liver was isolated using RNAiso Plus (Takara, Dalian, China), 1.2% agarose gel electrophoresis was used to detect the RNA quality; the RNA concentration was examined using a spectrophotometer at 260 and 280 nm (NanoDrop 2000, Thermo Scientific, USA). According to specifications, 1000 ng RNA was reversed to cDNA via the reverse transcription toolkit (Takara, Dalian, China) following RNA purification. The real time quantitative PCR (RT-qPCR) primers of the lipid metabolism-related gene and the antioxidant gene are shown in Table 3. Expression of the β-actin gene is stable and was thus used as RT-qPCR reference gene. To conduct quantitative analysis, the SYBR Premix Ex TaqTMII toolkit (Takara, Dalian, China) was used based on the specification of StepOne Plus Real-Time PCR to prepare a standard curve of both the target gene and reference gene as well as to measure the amplification efficiency of both target gene and reference gene. The relative expression levels were normalized to reference gene and calculated using a variation of the Livak and Shmittgen method29 corrected for variation in amplification efficiency, as described by Fleige and Pfaffl30.
Statistical analysis
All experimental data are presented as average values ± standard error (means ± SE) and analyzed by one-way analysis of variance (ANOVA) in SPSS19.0. Shapiro-Wilk and Levene tests were used to confirm normality and homogeneity of variance, respectively. Differences between average values were compared using Tukey’s test, and P < 0.05 indicates a significant difference.
Results
Growth performance and whole-body composition
The growth performance of fish has been reported previously31. Briefly, both the final weight (22.33 ± 0.35 g, mean ± SE) and weight gain for the LO100 diet were significantly lower than for the other diets (P < 0.05). The feed intake of fish in the LO75 (2.34 ± 0.03%/day, mean ± SE) and the LO100 (2.44 ± 0.02%/day, mean ± SE) diets were significantly higher than for other diets (P < 0.05). The feed conversion rate of the LO100 diet was significantly higher than for other diets (P < 0.05). LO substitution of FO significantly influenced body composition of Manchurian trout as shown in Table 4. With increasing inclusion level of LO in the diet, the protein content of the fish decreased significantly (P < 0.05). The lipid content of the fish in LO25 was highest, and was significantly higher than that in LO50, LO75, and LO100 (P < 0.05). No significant differences were found in dry matter and ash contents of fish in all diets (P > 0.05).
Whole fish fatty acid composition
The whole fish fatty acid composition changed in response to the different diets (Table 5). The SFA composition in LO75 and LO100 were significantly higher than in other diets (P < 0.05). The MUFA composition increased with increased inclusion level of LO. The n-6 PUFA composition in LO100 was significantly higher than that of other diets except for LO75 (P < 0.05). The ALA composition increased with the increase of LO inclusion level (P < 0.05). With increased inclusion level of LO in diets, the n-3 LC- PUFA (EPA + DHA) composition decreased significantly (P < 0.05).
Blood biochemical index
The influence of LO as substitution of FO on serum biochemical index of juvenile Manchurian trout is shown in Table 6. The TAG content significantly increased with increased inclusion level of LO (P < 0.05). Compared to LO0, the TCHO content significantly increased in LO25 and LO50 while it decreased in the remaining diets. HDL-C activity showed an initial increase, which was followed by a decrease with increasing LO inclusion level; furthermore, the HDL-C activity in LO25 and LO50 was significantly higher than that in LO0 (P < 0.05). LDL-C activity among all groups did not show different changes except LO25, which was significantly lower than for other diets (P < 0.05).
Antioxidant index
The influence of LO substitution of FO on antioxidant index of serum and liver of Manchurian trout is shown in Table 7. T-AOC activity of serum in LO25 and LO50 were significantly higher than in other diets (P < 0.05). T-AOC activity of the liver gradually decreased, while it was significantly higher than in other diets in LO0 and LO25 (P < 0.05). The SOD activities of serum and liver in LO100 were significantly lower than in LO25 (P < 0.05). The CAT activity of serum among all different diets were not significantly different (P > 0.05). The CAT activity in the liver in LO100 was significantly lower than in LO0 and LO25 (P < 0.05).
Expression of lipid metabolism-related genes
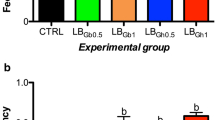
The influence of LO substitution of FO on the expression of lipid metabolism-related genes of the Manchurian trout is shown in Fig. 1. With increasing LO inclusion level of FO, the expression level of the FAD6 gene gradually increased, and the levels of LO50, LO75, and LO100 were significantly higher than those of other groups (P < 0.05). The expression level of the ACCα gene showed an increasing trend with increasing LO inclusion level, and those in LO50, LO75, and LO100 groups were significantly higher than those of the LO0 and LO25 groups (P < 0.05). The expression level of the SOAT2 gene in LO0 was significantly lower than that of other diets (P < 0.05). The expression level of the SREBP-1 gene in LO0 was significantly lower than that of other diets except LO25 (P < 0.05). The expression level of the regulator PPARα gene gradually decreased with increasing LO inclusion level, and levels of LO50, LO75, and LO100 groups were significantly lower than that in LO0 (P < 0.05).
Expression of liver lipid metabolic-related genes (FAD6, ACCα, SREBP-1, SOAT2, and PPARα) in Manchurian trout fed with a gradient linseed oil diet. Values are means ± SE from three treatments of fish (n = 3) with four fish per treatment. Means in each bar that share the different superscript letters indicate significant differences as determined by Tukey’s test (P < 0.05).
Expression of antioxidant gene
The expression level of the SOD gene of the liver in LO0 was significantly higher than that in LO100 (P < 0.05) (Fig. 2). The expression level of the CAT gene was not different among the diets (P > 0.05).
Liver antioxidant gene expressions (SOD and CAT) in Manchurian trout fed with a gradient linseed oil diet. Values are means ± SE from three treatments of fish (n = 3) with four fish per treatment. Means in each bar that share the different superscript letters indicate significant differences as determined by Tukey’s test (P < 0.05).
Discussion
ALA has an important physiological function, since it is an essential fatty acid for freshwater fish. LO is one of the best vegetable oil sources with which to substitute FO since LO contains a large amount of ALA6,7. In this study, the whole fish protein content gradually decreased after LO replaced FO, where the lipid content was highest at a substitution level of 25% and gradually decreased after that, which was consistent with the results of the Atlantic salmon (Salmo salar) and the turbot (Scophthalmus maximus)7,32. The tissue fatty acid composition of fish was modified by the diets8. Similar results were obtained in the present experiment, where the fatty acid composition of whole fish reflected the FA profile of the diet they received. The ALA composition of whole fish increased with the increase of ALA composition in diets. Studies showed that the VO substitution of FO decreased the n-3 LC-PUFA8,11,33. In the present experiment, with increased inclusion level of LO in diets (decreased inclusion level of FO), n-3 LC-PUFA (EPA + DHA) decreased significantly.
The LO substitution of FO changed the serum lipid metabolism of Manchurian trout. TAG and TCHO levels reflect the status of an organism’s fat metabolism. In this experiment, the TAG content in serum increased significantly with increasing LO inclusion level (n-3 HUFA content gradually decreased), which was consistent with the results reported by Lemaire et al.34. In this experiment, with increasing inclusion level of LO, TCHO showed a decreasing trend. Increasing VO levels in aquatic feeds usually reduces dietary cholesterol content and increases phytosterol levels35. Similar dietary modifications have been reported to induce a decrease in plasma cholesterol and LDL-C in fish36,37,38,39,40. Therefore, lower plasma cholesterol levels in LO rich diets were likely promoted by variations in the dietary cholesterol supply or by the presence of physterol, which interferes with cholesterol absorption. HDL-C played an important role in the regulation of the process of cholesterol transportation from extrahepatic tissues to the liver to be metabolized, and a relatively high level of HDL-C is a feature of wellness of an organism. In this experiment, the HDL-C level in a low substitution treatment (<75%) showed a higher value, indicating that a low LO substitution level (<75%) was favorable for fish metabolism, which was consistent with the results of the turbot7.
LO substitution has a specific impact on the synthesis of highly unsaturated fatty acids. FAD6 is the first rate-limiting enzyme of n-3 LC-PUFA synthesis1. In this experiment, the expression level of the FAD6 gene increased with increasing LO inclusion level in the diet, which was consistent with the results reported for the silver barb (Puntius gonionotus)11 as well as the Atlantic salmon32,41. LO mainly consists of C18:3n-3, and freshwater fish can convert C18:3n-3 in the diet to HUFA under the effect of FAD6, desaturase, and elongase to compensate for a HUFA deficiency in the diet. Acetyl-CoA carboxylase (ACCα) is the rate-limiting enzyme catalyzing the synthesis of long-chain fatty acids18. In this experiment, with increasing LO inclusion level, the expression level of the ACCα gene increased, thus promoting fatty acid synthesis and increasing the TAG content in serum. SOAT2 is the main cholesterol esterase of the liver and the small intestine19. LO substitution of FO affected the synthesis of cholesterol of juvenile Manchurian trout. With increasing LO inclusion level, the expression level of the SOAT2 gene increased, which means that the β–oxidation was increased. This was similar to the results for the turbot by Wang et al.7. SREBP-1 regulates the synthesis of fatty acid and lipid matter20,21. The expression level of the SREBP-1 gene showed an increasing trend with increasing inclusion of LO in the diets, which was similar to the results reported by De Tonnac et al.42 and Li et al.43. The n-3 HUFA in diet could decrease the expression level of the SREBP-1 gene to down-regulate the gene of synthesizing fatty acid, e.g., the expression of ACCα18. PPARα is an essential regulator, regulating both the decomposition and metabolism of fat, and the β-oxidation of fatty acid is mainly realized via PPARα regulation22. In this experiment, LO substitution of FO reduced the expression level of the PPARα gene, which was consistent with previous studies13,43 suggesting weakened fish β-oxidation ability.
LO substitution of FO changed the fish’s oxidative status. Oxidative stress happens when excess free radicals are generated in the body, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). When the oxidation degree exceeds the removal of oxides, cells and tissues are damaged44. SOD and CAT are among the primary antioxidant enzymes involved in fish antioxidant defense system15. SOD is the first enzyme that responds to oxygen radicals, thus preventing the initialization of the chain reaction triggered by superoxide radicals45.
In this experiment, SOD activity in serum and liver increased at low LO inclusion levels and decreased in response to high LO inclusion levels. These results showed that the fish’s oxidation resistance did not decrease but rather increased after partial LO substitution of FO, while the oxidation resistance of fish was significantly inhibited after complete substitution. T-AOC is a representation of the overall level of various large and small antioxidant molecules as well as enzymes in a system. In this experiment, T-AOC activity in the serum was highest in LO25 and T-AOC in liver was highest in LO0, indicating that a low LO inclusion proportion was favorable for improving the total oxidation resistance of fish. When the LO inclusion level was too high, the overall oxidation resistance of fish decreased, which has negative effects.
In this experiment, the expression level of SOD and CAT genes in the liver changed in response to increasing LO substitution of FO. CAT removes hydrogen peroxide in the organism by decomposing it into molecule oxygen and water to protect cells from damage by hydrogen peroxide; CAT is therefore, one of the essential enzymes in the biological defense system46,47. Although, the expression levels of the CAT gene were not significantly different, the expression levels of the CAT gene at substitution levels of 75% and 100% were lower compared to the LO0 without substitution. These results show that the body was vulnerable to hydrogen peroxide when the level of LO replacement in the diet exceeded 75%. The expression level of SOD in the liver significantly decreased in LO100 compared with other diets in this experiment. The results showed that LO substitution of FO, at high level (100%), decreased the oxidation resistance of fish. In this study, the expression levels of SOD and CAT genes in the liver were basically consistent with the changes of SOD and CAT activities in both serum and liver of fish. This suggested that SOD and CAT expression levels in the liver of fish might be correlated with SOD and CAT activities in both serum and liver of fish. T-AOC activity was highest in LO0 diet (100% FO) in liver, but in serum, it was higher for LO25 and LO50 diets. The mechanism of tissue difference of T-AOC activity between serum and liver requires further study.
In summary, the Manchurian trout (Brachymystax lenok) may have the ability to synthesize LC-PUFAs from ALA. LO substitution of FO at an appropriate proportion in the diet (<75%) improved both lipid metabolism and the oxidative status of juvenile Manchurian trout.
References
Tocher, D. R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107 (2015).
Tech, C. Y. & Ng, W. K. The implications of substituting dietary fish oil with vegetable oils on the growth performance, fillet fatty acid profile and modulation of the fatty acid elongase, desaturase and oxidation activities of red hybrid tilapia, Oreochromis sp. Aquaculture 465, 311–322 (2016).
Mourente, G., Dick, J. R., Bell, J. G. & Tocher, D. R. Effect of partial substitution of dietary fish oil by vegetable oils on desaturation and β-oxidation of [1-14C] 18:3n-3 (LNA) and [1-14C] 20:5n-3 (EPA) in hepatocytes and enterocytes of European sea bass (Dicentrarchus labrax L.). Aquaculture 248, 173–186 (2005).
Bureau, D. P., Hua, K. & Harris, A. M. The effect of dietary lipid and long-chain n-3 PUFA levels on growth, energy utilization, carcass quality, and immune function of rainbow trout Oncorhynchus mykiss. J. World Aquacult. Soc. 39, 1–21 (2008).
Tacon, A. G. J. & Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture 285, 146–158 (2008).
Bell, J. G., Henderson, R. J., Tocher, D. R. & Sargent, J. R. Replacement of dietary fish oil with increasing levels of linseed oil: modification of flesh fatty acid compositions in Atlantic salmon (Salmo salar) using a fish oil finishing diet. Lipids 39, 223–232 (2004).
Wang, Q., He, G. & Mai, K. Modulation of lipid metabolism, immune parameters, and hepatic transferrin expression in juvenile turbot (Scophthalmus maximus L.) by increasing dietary linseed oil levels. Aquaculture 464, 489–496 (2016).
Menoyo, D., López-Bote, C. J., Obach, A. & Bautista, J. M. Effect of dietary fish oil substitution with linseed oil on the performance, tissue fatty acid profile, metabolism, and oxidative stability of Atlantic salmon. J. Anim. Sci. 83, 2853–2862 (2005).
Turchini, G. M. & Francis, D. S. Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil- or linseed oil-based diets. Brit. J. Nutr. 102, 69–81 (2009).
Li, F. J., Lin, X., Lin, S. M., Chen, W. Y. & Guan, Y. Effects of dietary fish oil substitution with linseed oil on growth, muscle fatty acid and metabolism of tilapia (Oreochromis niloticus). Aquacult. Nutr. 22, 499–508 (2016).
Nayak, M., Saha, A., Pradhan, A., Samanta, M. & Giri, S. S. Dietary fish oil replacement by linseed oil: Effect on growth, nutrient utilization, tissue fatty acid composition and desaturase gene expression in silver barb (Puntius gonionotus) fingerlings. Comp. Biochem. Physiol. B Biochem Mol. Biol. 205, 1–12 (2017).
Liu, C. et al. Effects of totally replacing dietary fish oil by linseed oil or soybean oil on juvenile hybrid sturgeon, Acipenser baeri Brandt ♀ × A. schrenckii Brandt ♂. Aquacult. Nutr. 24, 184–194 (2018).
Jin, M. et al. Regulation of growth, tissue fatty acid composition, biochemical parameters and lipid related genes expression by different dietary lipid sources in juvenile black seabream, Acanthopagrus schlegelii. Aquaculture 479, 25–37 (2017).
Bellagamba, F. et al. Assessment of oxidatively generated DNA damage in rainbow trout (Oncorhychus mykiss) fed with different lipid sources. Aquaculture 317, 124–132 (2011).
Radovanović, T. B. et al. Superoxide dismutase and catalase activities in the liver and muscle of barbel (Barbus barbus) and its intestinal parasite (Pomphoryinchus laevis) from the Danube river. Serbia. Arch Biol Sci 62, 97–105 (2010).
Zuo, R. T., Ai, Q. H., Mai, K. S. & Xu, W. Effects of conjugated linoleic acid on growth, non-specific immunity, antioxidant capacity, lipid deposition and related gene expression in juvenile large yellow croaker (Larmichthys crocea) fed soya bean oil based diets. Brit. J. Nutr. 110, 1220–1232 (2013).
Martínez-Álvarez, R. M., Morales, A. E. & Sanz, A. Antioxidant defenses in fish: biotic and abiotic factors. Rev. Fish Biol. Fisher. 15, 75–88 (2005).
Ntambi, J. M. & Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 43, 91–104 (2004).
Lopez, A. M., Posey, K. S. & Turley, S. D. Deletion of sterol O-acyltransferase 2 (SOAT2) function in mice deficient in lysosomal acid lipase (LAL) dramatically reduces esterified cholesterol sequestration in the small intestine and liver. Biochem. Bioph. Res. Co. 454, 162–166 (2014).
Minghetti, M., Leaver, M. J. & Tocher, D. R. Transcriptional control mechanisms of genes of lipid and fatty acid metabolism in the Atlantic salmon (Salmo salar L.) established cell line, SHK-1. BBA-Mol. Cell Biol. L. 1811, 194–202 (2011).
Zheng, J. L. et al. Molecular cloning and expression pattern of 11 genes involved in lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Gene 531, 53–63 (2013).
Hashimoto, T. et al. Defect in peroxisome proliferator-activated receptor a-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J. Biol. Chem. 275, 28918–28928 (2000).
Zhang, J.M. Heilongjiang province ichthyography. Heilongjiang Science and Technology Press, Harbin, pp 52–54 (in Chinese) (1995).
Chang, J., Niu, H. X., Jia, Y. D., Li, S. G. & Xu, G. F. Effects of dietary lipid levels on growth, feed utilization, digestive tract enzyme activity and lipid deposition of juvenile Manchurian trout, Brachymystax lenok (Pallas). Aquacult. Nutr. 24, 694–701 (2017).
Zuo, R. et al. Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans). Fish Shellfish Immun. 32, 249–258 (2012).
AOAC. Official methods of analysis of the association official analytical chemists. AOAC international. 16th edn. AOAC, Arllington VA (1995).
Folch, J., Lees, M. & Sloane-Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Sukhija, P. S. & Palmquist, D. L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agr. Food Chem. 36, 1202–1206 (1988).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408 (2001).
Fleige, S. & Pfaffl, M. W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 27, 126–139 (2006).
Yu, J. et al. Effect of variation in the dietary ratio of linseed oil to fish oil on growth, body composition, tissues fatty acid composition, flesh nutritional value and immune indices in Manchurian trout, Brachymystax lenok. Aquacult. Nutr. 25, 377–387 (2018).
Leaver, M. J. et al. Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar). BMC Genomics 9, 1–15 (2008).
Turchini, G. M., Francis, D. S., Senadheer, S. P. S. D., Thanuthong, T. & De Silva, S. S. Fish oil replacement with different vegetable oils in Murray cod: evidence of an “omega-3 sparing effect” by other dietary fatty acids. Aquaculture 315, 250–259 (2011).
Lemaire, P. et al. Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (cholesterol, triglycerides) of sea-bass (Dicentrarchus labrax). Aquaculture 93, 63–75 (1991).
Liland, N. S. et al. High levels of dietary phytosterols affect lipid metabolism and increase liver and plasma TAG in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 110, 1958–1967 (2013).
Gilman, C. I., Leusch, F. D., Breckenridge, W. C. & MacLatchy, D. L. Effects of a phytosterol mixture on male fish plasma lipoprotein fractions and testis P450scc activity. Gen. Comp. Endocr. 130, 172–184 (2003).
Richard, N., Kaushik, S., Larroquet, L., Panserat, S. & Corraze, G. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Brit. J. Nutr. 96, 299–309 (2006).
Richard, N., Mourente, G., Kaushik, S. & Corraze, G. Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrarchus labrax L.). Aquaculture 261, 1077–1087 (2006).
Morais, S. et al. Diet × genotype interactions in hepatic cholesterol and lipoprotein metabolism in Atlantic salmon (Salmo salar) in response to replacement of dietary fish oil with vegetable oil. Brit. J. Nutr. 106, 1457–1469 (2011).
Castro, C. et al. Regulation of glucose and lipid metabolism by dietary carbohydrate and lipid source in gilthead sea bream juveniles. Br. J. Nutr. 116, 19–34 (2016).
Zheng, X. Z., Tocher, D. R., Dickson, C. A., Bell, J. G. & Teale, A. J. Highly unsaturated fatty acid synthesis in vertebrates: New insights with the cloning and characterisation of a Δ6 desaturase of Atlantic salmon. Lipids 40, 13–24 (2005).
De Tonnac, A., Labussière, E., Vincent, A. & Mourot, J. Effect of α-linolenic acid and DHA intake on lipogenesis and gene expression involved in fatty acid metabolism in growing-finishing pigs. Brit. J. Nutr. 116, 7–18 (2016).
Li, C., Liu, P., Ji, H., Huang, J. & Zhang, W. Dietary n - 3 highly unsaturated fatty acids affects the biological and serum biochemical parameters, tissue fatty acid profile, antioxidation status and expression of lipid-metabolism-related genes in grass carp, Ctenopharyngodon idellus. Aquacult. Nutr. 3, 373–383 (2015).
Kohen, R. & Nyska, A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30, 620–650 (2002).
Ross, S. W., Dalton, D. A., Kramer, S. & Christensen, B. L. Physiological (antioxidant) responses of estuarine fishes to variability in dissolved oxygen. Comp. Biochem. Phys. C Toxicol Pharmacol 130, 289–303 (2001).
Bhagat, J., Ingole, B. S. & Singh, N. Glutathione s-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: A review. I. S. J. 13, 336–349 (2016).
Yu, H. et al. Regulation of dietary glutamine on the growth, intestinal function, immunity and antioxidant capacity of sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immun. 50, 56–65 (2016).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 31260638, 31360640, and 31460692) and the Natural Science Foundation of Inner Mongolia (2018LH03015).
Author information
Authors and Affiliations
Contributions
Ying Han and Huaxin Niu designed the experiments. Jianhua Yu performed the experiment. Jianhua Yu analyzed the data and wrote the main manuscript. Ying Han and Shuguo Li critically revised the content. Jie Chang and Zongfu Hu improved the language of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, J., Li, S., Niu, H. et al. Influence of dietary linseed oil as substitution of fish oil on whole fish fatty acid composition, lipid metabolism and oxidative status of juvenile Manchurian trout, Brachymystax lenok. Sci Rep 9, 13846 (2019). https://doi.org/10.1038/s41598-019-50243-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50243-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.