Abstract
MicroRNAs (miRNAs) are proposed as potential biomarkers for the diagnosis of numerous diseases. Here, we performed a meta-analysis to evaluate the utility of faecal miRNAs as a non-invasive tool in colorectal cancer (CRC) screening. A systematic literature search, according to predetermined criteria, in five databases identified 17 research articles including 6475, 783 and 5569 faecal-based miRNA tests in CRC, adenoma patients and healthy individuals, respectively. Sensitivity, specificity, positive/negative likelihood and diagnostic odds ratios, area under curve (AUC), summary receiver operator characteristic (sROC) curves, association of individual or combinations of miRNAs to cancer stage and location, subgroup, meta-regression and Deeks’ funnel plot asymmetry analyses were employed. Pooled miRNAs for CRC had an AUC of 0.811, with a sensitivity of 58.8% (95% confidence interval [CI]: 51.7–65.5%) and specificity of 84.8% (95% CI: 81.1–87.8%), whilst for colonic adenoma, it was 0.747, 57.3% (95% CI: 40.8–72.3%) and 76.1% (95% CI: 66.1–89.4%), respectively. The most reliable individual miRNA was miR-21, with an AUC of 0.843, sensitivity of 59.3% (95% CI: 26.3–85.6%) and specificity of 85.6% (95% CI: 72.2–93.2%). Paired stage analysis showed a better diagnostic accuracy in late stage CRC and sensitivity higher in distal than proximal CRC. In conclusion, faecal miR-21, miR-92a and their combination are promising non-invasive biomarkers for faecal-based CRC screening.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the second leading cancer-related cause of death in the United Kingdom (UK) and accounts for over 500,000 deaths annually worldwide1. The pathogenesis of CRC follows a protracted stepwise progression from benign colonic adenomas to malignant adenocarcinomas and distant metastasis. Patient survival inversely correlates to cancer stage during diagnosis, with up to 90% of deaths avertable if detected early2. However, CRC is often asymptomatic in its early stage and arises sporadically within the population, posing a challenge to the application of effective and timely treatments3. The mass screening of asymptomatic individuals for CRC utilising a non-invasive method is thus a high public health priority.
Under the National Health Service (NHS) Bowel Cancer Screening Program in the UK, currently, the faecal immunochemical test (FIT) is offered every two years to all asymptomatic men and women aged 60 to 744. The FIT, which examines faecal samples for hidden blood, is appealing because the costs are low, the test is widely available, and does not pose an immediate risk to the screened population5. Although the recent changes from Faecal Occult Blood Test (FOBT) to FIT has improved the screening power by specific targeting to human haemoglobin, the effectiveness of FIT is still restricted by its relatively low sensitivity, with about half of all malignant large bowel tumours and most polyps undetected. This is due to the intermittent nature of bleeding6 as well as degradation of haemoglobin in faeces7. Consequently, one in four CRC cases is only diagnosed at a late stage on emergency admission, resulting in poor prognosis8. Therefore, a more sensitive faecal-based non-invasive test is urgently needed.
miRNAs are a class of conserved endogenous, short non-coding RNAs with length of 18–24 nucleotides. miRNAs regulate gene expression through post-transcriptional processing by binding primarily to the 3′-untranslated region (3′UTR) of target mRNAs, resulting in mRNA degradation and/or translational repression9. Specific miRNAs (oncomiRs) through targeting tumour-suppressor genes have been found to be upregulated, while others targeting oncogenes are downregulated, in cancer. These alterations, through the regulation of intracellular signalling networks, induce cell proliferation, confer resistance to apoptosis and chemotherapy, and promote metastasis10. The expression of several miRNAs differs significantly between normal colonic tissues and CRC, and as colonocytes consistently exfoliate and shed into the lumen of the gastrointestinal (GI) tract, these changes in miRNA levels are represented in faecal specimens11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27. More recently, it was demonstrated that miRNAs are highly stable and detectable within samples throughout a 72 hour incubation period due to protection from ribonuclease degradation by exosomes28,29. Given that faeces contain genomic DNA and RNA derived from gut microbes, and miRNAs derived from blood cells released by tumours, the detection and utility of miRNAs for diagnostic purposes has been controversial. Therefore, this meta-analysis aims to assess the value of miRNAs as faecal-based biomarkers for CRC and colonic adenoma screening.
Results
Characteristics of selected studies
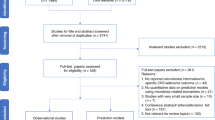
The initial literature search from five different databases yielded a total of 567 articles, of which 249 were excluded as duplicated records. Next, 165 articles were deemed irrelevant and excluded based on the title and abstract. The full-text of the remaining publications were screened, resulting in the inclusion of 17 publications (16 in English, 1 in Chinese) (Fig. 1). These publications contained 46 studies on CRC and 10 studies on adenomas, corresponding to 6475, 783 and 5569 faecal-based miRNA tests in CRC patients, adenoma and healthy controls, respectively (Tables 1 and 2). The clinical data and collection procedures are summarised in Suppl. Table 1A,B, and methods of miRNA extraction and quantification in Suppl. Table 2A,B.
Risk of bias
All included articles were evaluated for the risk of bias using the QUADAS-2 tool (Fig. 2)30. The major risk of bias in this study was in the index test, where 10 out of 17 publications had a high risk of bias due to the unclear or lack of statement regarding interpretation of index test results without knowledge of the results of the reference. Additionally, 14 out of 17 studies had an unclear risk of bias in the “Patient Selection” domain. This is due to a lack of detail on whether a consecutive or random sample of patients were enrolled. There was a low risk of bias in the reference standard, since all studies were histopathologically confirmed prior to the index test using either TNM or Dukes’ staging (Suppl. Table 1A,B). Concern about applicability in all domains was low.
Pooled diagnostic accuracy of faecal-based microRNA for colorectal cancer and adenoma
The detection accuracy of faecal-based miRNAs for CRC (Table 3) as well as colonic adenoma (Table 4) were pooled and analysed using the bivariate random effects model to evaluate the overall diagnostic measurements (Fig. 3). The DOR and log DOR were 8.32 (95% CI: 6.71–10.32) and 2.12 (95% CI: 1.90–2.33) for CRC and 5.31 (95% CI: 3.55–7.94) and 1.67 (95% CI: 1.27–2.07) for adenoma, respectively. The AUC value was 0.811 for the pooled CRC, and 0.747 for the pooled adenoma, respectively. The pooled studies of miRNAs in identification of CRC had a sensitivity of 58.8% (95% CI: 51.7–65.5%) and specificity of 84.8% (95% CI: 81.1–87.8%), whilst the pooled studies of miRNAs for identification of adenoma had a sensitivity of 57.3% (95% CI: 40.8–72.3%) and specificity of 76.1% (95% CI: 66.1–89.4%).
Diagnostic accuracy of pooled microRNAs in identification of colorectal cancer and colonic adenoma. Log Diagnostic Odds radios in (A) CRC was 2.12 (95% CI: 1.90–2.33) and (B) adenoma was 1.67 (95% CI: 1.27–2.07). (C) Summary receiver operating characteristic curves (SROC) for pooled miRNAs in CRC and colonic adenoma. The pooled miRNAs for CRC (n = 46) had a sensitivity of 58.8% (95% CI: 51.7–65.5%), specificity of 84.8% (95% CI: 81.1–87.8%) and AUC of 0.811. The pooled miRNAs for adenoma (n = 10) had a sensitivity of 57.3% (95% CI: 40.8–72.3%), specificity of 76.1% (95% CI: 66.1–89.4%) and AUC of 0.747. The number next to the dot/triangle corresponds to the study ID in Table 1 (Blue dots: CRC) or Table 2 (Red triangles: colonic adenoma). The circular regions (95% confidence contour) contain likely combinations of the mean value of sensitivity and specificity. Sen, sensitivity; Spe, specificity; SOP, summary operating point.
Relative quantitation has a higher detection accuracy in colorectal cancer screening
To investigate the potential of faecal-based miRNA in the non-invasive diagnosis of CRC, studies were subgrouped in different aspects to compare their detection accuracy (Table 3). For the individual/combination miRNA analysis, both presented a similar power of diagnostic accuracy. The individual miRNA analysis panel had a sensitivity of 53.5% (95% CI: 43.8–62.9%), specificity of 86.4% (95% CI: 81.8–89.9%), DOR of 7.71 (5.65–10.52), and log DOR of 2.04 (95% CI: 1.73–2.35). The combination of miRNAs had a sensitivity of 68.8% (95% CI: 63.0–74.0%), specificity of 81.6% (95% CI: 75.0–86.8%), DOR of 9.73 (95% CI: 7.51–12.60), and log DOR of 2.28 (95% CI: 2.02–2.53). The AUC value was 0.808 for the individual miRNAs, and 0.801 for the combination of miRNAs, respectively. The meta-regression analysis showed a significant effect on pooled sensitivity (Z-value: 2.310, P = 0.021) but not in specificity (Z-value: 1.28, P = 0.199) (Table 4). Comparing studies with large (n > 100) versus small size (n ≤ 100), there was a sensitivity of 53.4% (95% CI: 44.4–62.3%) versus 70.6% (95% CI: 64.2–76.3%), specificity of 86.3% (95% CI: 82.3–89.6%) versus 80.8% (95% CI: 72.3–87.1%), DOR of 7.83 (95% CI: 6.11–10.04) versus 10.16 (95% CI: 6.45–16.00) and AUC of 0.811 versus 0.801 respectively. The meta-regression analysis indicated that the sample size did not significantly affect the pooled specificity (Z-value: 1.601, P = 0.109), however it did affect the pooled sensitivity (z-value: 2.458, P = 0.014).
The quantitation methods of absolute versus relative qPCR for faecal-based miRNAs were compared. The pooled relative quantitation qPCR method exhibited a better diagnostic accuracy in CRC (Table 3), with a sensitivity of 55.5% (95% CI: 45.9–64.7%), specificity of 88.8% (95% CI: 85.2–91.6%), DOR of 10.738 (95% CI: 7.718–14.940), log DOR of 2.37 (95% CI: 2.04–2.70) and AUC of 0.846. By contrast, the pooled absolute quantitation qPCR method exhibited sensitivity, specificity, DOR, log DOR and AUC of 67.3% (95% CI: 62.3–71.9%), 69.2% (95% CI: 69.4–77.0%), 5.706 (95% CI: 4.937–6.594), 1.74 (95% CI: 1.06–1.89) and 0.763, respectively. The meta-regression analysis revealed that the qPCR relative quantitation method in CRC affected only specificity (Z-value = −4.317, P < 0.001) when compared to the absolute quantification approach. The pooled relative quantitation qPCR approach exhibited a DOR of 8.32 (95% CI: 5.55–12.48) and log DOR of 2.12 (95% CI: 1.71–2.52) with a specificity of 86.7% (95% CI: 67.6–95.3%) and sensitivity of 56.9% (95% CI: 31.4–79.2%), compared with the absolute quantification approach (Table 3 and Suppl. Fig. 1).
Subgroup meta-regression analysis in colonic adenoma was also performed, looking at differences in sample size, pooled individual/combination miRNAs and quantitation method (Table 4). The meta-regression indicated that a small sample size significantly affected both the specificity (Z-value: −2.011, P = 0.044) and sensitivity (Z-value: −1.309, P < 0.001). With respect to the pooled individual/combination miRNAs, meta-regression analysis did not show significant effects in both the pooled sensitivity (Z-value: −10.85, P = 0.853) and specificity (Z-value: −0.194, P = 0.846). For the qPCR relative quantitation method, a significant effect was observed in the sensitivity (Z-value = −3.356, P < 0.001) of pooled miRNAs.
Differences in detecting CRC depending on tumour stage and location
Meta-analysis on early versus late stage CRC as well as proximal versus distal CRC were performed to further evaluate the diagnostic ability of miRNAs. Pooled faecal miRNAs had a sensitivity of 57.0% (95% CI: 44.4–68.8%), specificity of 80.0% (95% CI: 71.1–86.7%), DOR of 5.58 (95% CI: 3.62–8.62) and log DOR of 1.72 (95% CI: 1.29–2.15) with respect to the diagnosis of early stage CRC, whilst in late stage CRC pooled miRNAs had a sensitivity of 62.1% (95% CI: 47.8–74.6%), specificity of 80.0% (95% CI: 71.1–86.7%), DOR of 6.70 (95% CI: 4.34–10.36) and log DOR of 1.90 (95% CI: 1.47–2.34) (Fig. 4A,B and Table 5). In proximal CRC, the pooled sensitivity, specificity, DOR, log DOR and AUC were 39.8% (95% CI: 21.8–61.0%), 82.4% (95% CI: 71.5–89.7%), 3.44 (95% CI: 2.53–4.66), 1.23 (95% CI: 0.93–1.54) and 0.719, respectively. For distal CRC, the pooled sensitivity, specificity, DOR, log DOR and AUC were 64.1% (95% CI: 43.9–80.3%), 81.9% (95% CI: 71.5–89.1%), 8.51 (95% CI: 4.97–14.57), 2.14 (95% CI: 1.60–2.68) and 0.818, respectively (Table 6 and Suppl. Fig. 2).
Diagnostic accuracy in early stage versus late stage and proximal versus distal colorectal cancer. (A) Summary receiver operating characteristic curves (SROC) for early (n = 11) and late (n = 11) stage CRC. (B) SROC for proximal (n = 9) and distal (n = 9) CRC. The number next to the blue dot/red triangle corresponds to the study ID in Table 1. The circular regions (95% confidence contour) contain likely combinations of the mean value of sensitivity and specificity. Sen = sensitivity; Spe = specificity SOP, summary operating point. #Early stage CRC includes TMN stages 0 + I + II or Dukes’ stage A + B; late stage CRC includes CRC stages III + IV or Dukes’ stage C + D. Proximal CRC is defined as from cecum to transverse colon, and distal CRC is defined as from the splenic flexure to the rectum.
The detection accuracy of individual microRNAs
Each individual miRNA reported by more than one research group was pooled for an accuracy estimation (Table 6). miR-21 was reported by five CRC and three colonic adenoma studies11,16,18,20,22. Pooled miR-21 in CRC had an AUC of 0.843, DOR of 9.28 (95% CI: 2.97–28.97) and log DOR of 2.23 (95% CI: 1.09–3.37) whilst pooled miR-21 in adenoma had an AUC of 0.771, DOR of 7.10 (96% CI: 1.99–25.34) and log DOR of 1.96 (96% CI: 1.96–3.23) (Fig. 5A and Table 6). The miR-21-related combination pool for CRC detection had an AUC of 0.843, with a DOR of 16.73 (95% CI: 7.00–39.94) and log DOR of 2.82 (95% CI: 1.95–3.69) from four different CRC studies. miR-92a was reported in four CRC and two adenoma studies11,20,21,22. The AUC, DOR and log DOR were 0.794, 8.57 (95% CI: 3.30–22.27) and 2.15 (95% CI: 1.19–3.10) for pooled miR-92a alone in the diagnosis of CRC, and 0.635, 0.467, 7.08 (95% CI: 1.43–34.97) and 1.96 (95% CI: 0.36–3.55) for pooled miR-92a alone in the diagnosis of colonic adenoma, respectively (Fig. 5B and Table 6).
Diagnostic accuracy in pooled miR-21 and miR-92a. (A) SROC for pooled miR-21 in the detection of CRC (n = 5) and colonic adenoma (n = 3). (B) SROC for pooled miR-92a in the detection of CRC (n = 4) and colonic adenoma (n = 2). The number next to the dot/triangle corresponding to the study ID in Table 1 (Blue dots: CRC) or Table 2 (Red triangles: colonic adenoma). Sen, sensitivity; Spe, specificity; SOP, summary operating point. The circular regions (95% confidence contour) contain likely combinations of the mean value of sensitivity and specificity.
The miR-92a-related combination pool for CRC was reported in five CRC studies, with AUC 0.791, DOR 10.47 (95% CI: 6.46–16.98) and log DOR 2.35 (95% CI: 1.87–2.83). The pooled miR-21 plus miR-92a combination exhibited an AUC of 0.837, with a specificity of 87.8% (95% CI: 79.5–93.0%), sensitivity of 53.7% (95% CI: 33.4–74.8%) and DOR of 2.19 (95% CI: 1.48–2.91). miR-20a, miR-106a, miR-135b and miR-223 were reported in two different articles, with an AUC of 0.797, 0.416, 0.798 and 0.777, DOR of 3.87 (95% CI: 1.75–8.55), 29.33 (95% CI: 7.74–111.11), 10.18 (95% CI: 4.91–21.09) and 14.69 (95% CI: 0.66–328.44), respectively (Table 6 and Suppl. Fig. 2).
Publication bias evaluation
The Deeks’ funnel plot asymmetry test was utilised to evaluate the potential publication bias from each included faecal-based miRNA study. The slope coefficient was associated with P = 0.03 in the pooled miRNAs in CRC, and P = 0.61 in pooled miRNAs in colonic adenoma studies, indicating that significant asymmetry was found in the CRC dataset (Fig. 6A) but not in the colonic adenoma dataset (Fig. 6B). The combination of CRC and colonic adenoma resulted in a P = 0.11 (Suppl. Fig. 3).
Discussion
To evaluate the diagnostic value of faecal-based miRNAs, data from 17 eligible publications, including 46 studies on miRNAs in CRC and 10 studies on colonic adenoma, corresponding to 6475, 783 and 5569 faecal-based miRNA tests in CRC patients, adenoma and healthy control volunteers, respectively, were subjected to meta-analysis. Our study reveals that pooled faecal-based miRNAs have a relatively high detection accuracy for CRC. However, the lack of consensus regarding the optimal quantitation method, data normalisation, and selection of control subjects, may present obstacles to clinical application.
qPCR-based studies were the subject of our meta-analysis analysis. This approach for miRNA level quantification is advantageous compared to others in that is fast and easily adoptable in a clinical setting. However, it comes with limitations that relate to the method for miRNA isolation and the selection of the appropriate reference/normalisation control. Although reference quantitation method demonstrated a better diagnostic accuracy compared to absolute quantitation, it is important to acknowledge that a variety of internal controls were used as references, including RUN6B(U6)11,15,16,17,18,24, miR-2423, miR-200b-3p19, miR-37812, miR-120227 and miR-425727. Increasing evidence suggests that RUN6B may not be a suitable endogenous control for miRNAs31,32 due to its rapid degradation in faeces20. miRNAs used as internal controls also have functions in the host cell, and their deregulation could interfere with the detection accuracy. For example, miR-24, a proposed tumour suppressor miRNA in CRC, controls cellular proliferation independently of p53 by targeting the 3’UTR of dihydrofolate reductase (DHFR) mRNA33,34. Deregulation of plasma miR-378 was also found in CRC patients35. miR-4257 has been reported to be down-regulated in bladder cancer cell lines and up-regulated in the plasma of patients with recurrence of non-small cell lung cancer (NSCLC)36,37. Peripheral levels of miR-1202 predicts and mediates the response to anti-depressants, specifically regulating the expression of metabotropic glutamate receptor-4 (GRM4) with levels correlating to changes in brain activity38,39,40. miR-1202 is deregulated in different types of cancers, such as breast cancer41, gastric cancer42 and clear cell papillary renal cell carcinoma43. Absolute quantitation was employed in several studies for faecal-based miRNA screening13,14,20,26 (Table 1), however, this necessitates a standard curve which depends on the quantification detection method and does not eliminate potential contamination by gut bacteria DNA/RNA14.
The combination of FIT and stool-based miRNA markers may increase detection accuracy to overcome this problem. A previous study indicated that the combination of miR-21 and miR-92a with FIT had a specificity of 96.8% and sensitivity of 78.4% while FIT alone only had a specificity of 98.4% and sensitivity of 66.7%22. A parameter that should be considered is the presence/absence of occult blood in samples as miRNAs expressed in blood cells may interfere with the assay, altering the levels of specific miRNAs. In an effort to assess the potential contribution of blood in faecal miRNA levels we have retrieved a list of circulating miRNAs44. Comparison showed that 8 miRNAs are detected in both blood and faecal specimens (Suppl. Fig. 4). This finding does not imply that blood cells are responsible for the alterations in the levels of these miRNAs, as their origin may as well be the tumour. Optimally, a controlled study including comparisons between samples positive and negative for FOBT/FIT could address the relative contribution. A more inclusive approach employing miRNA analyses and comparisons between matched blood, faecal specimens and tumours or colonic tissues would be most informative about the source of changes in miRNA levels. Furthermore, other colonic pathologies like inflammatory bowel diseases are characterised by deregulation of miRNAs detectable in tissues and serum45,46,47,48 and the presence of occult blood in faeces. A comprehensive analysis would include samples from different pathologies of the colon, assess and identify disease-specific miRNA signatures and their diagnostic/prognostic properties.
A clear conclusion on which quantitation method is more suitable cannot be drawn with the currently available data. The use of multiple internal controls or the geometric mean, using a multiplex screening method, such as microarrays or next generation sequencing, would provide the optimal means of normalisation. Alternatively, the NanoString nCounter technology enables profiling of around 800 molecular targets in one single reaction by utilising molecular “barcodes”. This approach normalises the data by using multiple targets, and more importantly quantifies multiple miRNAs which can be used simultaneously as biomarkers to improve detection accuracy. In addition, this platform overcomes the need for data processing and bioinformatic analysis expertise, as in the case of microarrays or high-throughput sequencing, thus may be easily utilised in a clinical setting46.
To evaluate the potential detection efficiency for each individual miRNA, individual miRNAs reported in more than one study were grouped to evaluate its detection accuracy. In this meta-analysis, miR-21 and miR-92a were the most commonly reported faecal-based miRNAs (Table 6). Numerous studies have characterised the functional roles of these two miRNAs in CRC pathogenesis and aggressiveness. Up-regulation of miR-21 and miR-92a promotes CRC cell migration, invasion and proliferation11,16,18,20,22, and inhibition of apoptosis49,50,51. Several significant targets of miR-21 are associated with CRC malignancy, such as phosphatase and tensin homolog (PTEN)49,52, programmed cell death protein 4 (PDCD4)53,54, and ras homolog gene family member B (RhoB)55. Among these, PTEN was reported frequently silenced in CRC by miR-21, resulting in PI3K/AKT pathway activation and induction of tumour formation49,52. Recently, a long non-coding RNA (LINC00312) suppressed in CRC was shown to regulate miR-21 levels through its function as a miRNA sponge, thereby regulating PTEN expression56. miR-92a has been shown to disrupt the expression of several tumour suppressors such as PTEN57,58, Dickkopf WNT Signalling Pathway Inhibitor 3 (DKK3)57, Kruppel-like factor 4 (KLF4)59 and mothers against decapentaplegic homolog 7 (SMAD7)60. Hence, miR-92a activates the PI3K/AKT, WNT/β-catenin and BMP/Smad pathways and enhances tumorigenesis. Subject to this analysis five studies reported the use of miR-21 in the identification of CRC, and three studies reported its use in identification of adenomas11,16,18,20,22. Four studies reported the utility of miR-92a in the identification of CRC, and two studies in identification of adenomas11,17,20,22. miR-21 had a better detection accuracy range compared with miR-92a, with a DOR of 9.28 (95% CI: 2.97–28.97) and summary AUC of 0.843. Panels including a combination of either miR-21 or miR-92a, as well as panels including both miR-21 and miR-92a demonstrated a small improvement in detection (Fig. 5 and Table 6). However, due to the small number of published studies, with each having wide confidence intervals, a direct comparison between two faecal-based miRNAs may not be accurate. Additional data are needed to limit potential errors.
The FOBT or FIT, have limited sensitivity for detecting proximal compared with distal CRC61,62. This is due to the degradation of haemoglobin. Hence, tumour location analysis for faecal-based miRNA detection was also considered and reported by several studies – with none of them reporting a statistical difference. In this study, the results between pooled miRNAs for proximal and distal CRC reveal differences associated with tumour location, with an AUC of 0.719 versus 0.818, and DOR of 3.44 (95% CI: 2.53–4.66) versus 8.51 (95% CI: 4.97–14.57) (Fig. 4B and Table 5).
Our study is characterised by many strengths but should be interpreted in the context of specific shortcomings. Firstly, subgroup analysis suggested that the combination of faecal miRNAs exhibited a good accuracy for CRC and colonic adenoma patients screening (Tables 3, 4 and Fig. 3). However, certain combinations of miRNAs may not significantly improve the detection accuracy. For example, the panel containing miR-223, miR-92a, miR-16 and miR-106b had a sensitivity of 73.9%, specificity of 82.2% and AUC of 0.8413, whereas the combination of miR-18a and miR-135b only had a sensitivity of 66%, specificity of 72% and AUC of 0.7526. Therefore, an optimal miRNA combination panel should be prioritised. Secondly, the majority of studies were performed in East Asia (Hong Kong, Taiwan, China, Japan and Singapore) (Table 1) with only one study in the USA, Europe and the Middle East, making it unclear whether the ethnic background of participants has an influence on the expression of miRNAs in CRC. Thirdly, due to the high cost of colonoscopy, the majority of test subjects were recruited from the corresponding clinics. This may result in a degree of bias, since the subjects are not representative of the general population. Last but not least, the publication bias analysis revealed that pooled miRNAs in CRC have a significant asymmetry (P = 0.03). This may be due to file-drawer effects, bias from the studies with small same sizes, lack of clarity in reporting the results for some publications, or the level of detail provided being lower than the one required for our analysis. Consequently, some studies were excluded, resulting in a possible bias in our meta-analysis (Fig. 6A).
In conclusion, faecal-based miRNAs show a relatively high accuracy for the non-invasive detection of colonic adenomas and CRC in the studied population. The use of a panel of miRNAs as biomarkers may result in a higher CRC detection rate, while the combination of miRNA biomarkers with FOBT or FIT may increase the detection accuracy. Large, ideally multi-centre, double-blinded randomised controlled trials are needed to establish the value of miRNAs as biomarkers in CRC screening within the general population.
Methods
Overview
The study protocol followed the Cochrane Handbook for Diagnostic Test Accuracy Review63 and the Preferred Reporting Items in Systematic Reviews and the Meta-Analysis statement (PRISMA)64. Investigators of each of the original studies obtained approval from their local ethics committee and had written, informed patient consent.
Literature search strategy
The search strategy was designed to identify any studies describing the diagnostic value of faecal-based miRNA for CRC and colonic adenoma patients. After an initial search for articles in PubMed, assessments of key terms within the title and abstract were conducted. A full systematic search using the established key terms was adopted for the following databases: PubMed, Ovid Embase, The Cochrane Library, Scopus and Web of Science. The search terms used were “miRNA OR microRNA OR miR” AND “colorectal cancer OR colorectal tumor OR colorectal adenocarcinoma OR colorectal carcinoma OR colorectal neoplasm OR colon cancer OR colonic adenoma OR colonic adenocarcinoma OR stomach cancer OR rectal cancer OR CRC” AND “stool OR feces”. The search formulas are available as supplementary data (Supplementary data 1). Manual searching of related citations and reference lists was undertaken. Book chapters, letters to editors, commentaries, editorials, patents, and non-peer reviewed articles were excluded. Two investigators independently screened the search results, initially through articles’ title and abstract. The filtered candidate articles were then scrutinised independently through full-text reading. Discrepancies were resolved through discussion between the two investigators.
Study selection criteria
All research articles in any language published up to November 17, 2017 were eligible for inclusion. An electronic data extraction form was developed, and pre-tested, with data extracted by two researchers. Eligible studies included in this meta-analysis adhered to the following criteria: (1) studies evaluated the diagnostic value of miRNAs for detecting human CRC or colonic adenomas; (2) all CRC patients involved in the study had been confirmed by histology; (3) studies contained data on miRNAs’ sensitivity, specificity, and sample size to enable reconstruction of the diagnostic 2 × 2 contingency table. Exclusion criteria were set as follows: (1) duplicated studies, the later ones were excluded; (2) publications that were unrelated to the diagnostic value of miRNAs for CRC; (3) incomplete data reporting. The detection accuracy of miRNAs between proximal (from cecum to transverse colon) and distal (from splenic flexure to rectum) CRC, as well as between early (CRC stages 0 + I + II or Dukes’ stages A + B) and late (CRC stages III + IV or Dukes’ stages C + D) stage CRC were evaluated separately if investigators reported the location and stage. Each individual miRNA and miRNA combinations were grouped together if found in more than two studies.
Risk of bias
The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) was utilised to assess the quality of included publications, evaluating four key domains (“Patient Selection”, “Index Test”, “Reference Standard”, and “Flow and Timing”) in two categories (risk of bias and applicability of diagnostic accuracy studies)30. Each category in all publications was judged as low, high or unclear based on the assessment criteria provided. Assessment of each included study was performed by two investigators, with disagreements resolved by consensus after discussion.
Data synthesis
A meta-analysis of diagnostic test accuracy was conducted on faecal-based non-invasive miRNA tests through a bivariate random effects modelling approach. The bivariate model accounts for the correlation between the studies’ sensitivity and specificity in two different levels. The first level represents a variability between sensitivity and specificity within one study; the second level represents the heterogeneity in diagnostic performance of the index test across the testing studies. Random effects meta-analysis methods were applied in our study as heterogeneity is presumed to exist.
Statistical analyses in this study were performed using the statistical package mada version 0.4.8 in R (version 3.4.3) to implement the bivariate normal approach of Reitsma et al.65. Sensitivity, specificity, diagnostic odds ratio (DOR), log DOR, positive likelihood ratio (+LR) and negative likelihood ratio (–LR) were calculated along with their 95% confidence interval (95% CI) based on the random effects model (DerSimonian and Laird method) with continuity correction66,67. Summary receiver operating characteristic (sROC) curves, area under the curve (AUC) and partial AUC were also utilised to examine the pooled faecal-based miRNAs in CRC, adenoma and the subgroups. Potential sources of heterogeneity were investigated using subgroup and bivariate meta-regression (restricted maximum likelihood (REML) estimators) analysis. The Deeks’ funnel plot asymmetry test was examined using the midas package in Stata (version 12).
Interpretation of diagnostic test accuracy statistics
The AUC was interpreted in four-grades: >0.97, excellent; 0.93–0.96, very good; 0.75–0.92, good; < 0.75, not accurate68. The values of –LR and +LR were also divided into four categories. The –LR values < 0.1, 0.1–0.2, 0.2–0.5 and >0.5 were identified as large, moderate, small and not meaningful decreases in probability, respectively69. The +LR values >10, 5–10, 2–5 and <2 were classified as large, moderate, small and not meaningful increases in probability, respectively69. P < 0.05 was considered statistically significant.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA. Cancer J. Clin. 65, 87–108 (2015).
Smith, R. A. et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001–testing for early lung cancer detection. CA. Cancer J. Clin. 51, 38–75 (2001).
Yau, T. O. Precision treatment in colorectal cancer: Now and the future. JGH Open, https://doi.org/10.1002/jgh3.12153 (2019).
Halloran, S. P. Bowel cancer screening. Surgery (Oxford) 27(9), 397–400 (2009).
Logan, R. F. A. et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 61, 1439–1446 (2012).
Simon, J. B. Should all people over the age of 50 have regular fecal occult-blood tests? Postpone population screening until problems are solved. N. Engl. J. Med. 338, 1151–2; discussion 1154-5 (1998).
Doubeni, C. A. et al. Fecal Immunochemical Test (FIT) for Colon Cancer Screening: Variable Performance with Ambient Temperature. J. Am. Board Fam. Med. 29, 672–681 (2016).
Mayor, S. One in four cases of bowel cancer in England are diagnosed only after emergency admission. BMJ 345, e7117 (2012).
Liz, J. & Esteller, M. lncRNAs and microRNAs with a role in cancer development. Biochim. Biophys. Acta - Gene Regul. Mech. 1859, 169–176 (2016).
Esquela-Kerscher, A. & Slack, F. J. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–69 (2006).
Koga, Y. et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev. Res. (Phila). 3, 1435–42 (2010).
Kalimutho, M. et al. Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. Journal of Gastroenterology 46, 1391–1402 (2011).
Chang, P.-Y. et al. MicroRNA-223 and microRNA-92a in stool and plasma samples act as complementary biomarkers to increase colorectal cancer detection. Oncotarget 7, 10663–75 (2016).
Yau, T. O. et al. microRNA-20a in human faeces as a non-invasive biomarker for colorectal cancer. Oncotarget 7, 1559–68 (2016).
Zhu, Y. et al. Fecal miR-29a and miR-224 as the noninvasive biomarkers for colorectal cancer. Cancer Biomark. 16, 259–264 (2015).
Liu, H. et al. MicroRNA-21 and microRNA-146a identification in stool and its clinical significance in colorectal neoplasms. Int. J. Clin. Exp. Med. 9, 164441–16449 (2016).
XUE, Y. et al. [Values of fecal microRNA-141, −17-3p and -92a-3p in the diagnosis and prognostic evaluation of colorectal cancer]. Tumor 36, 901–907 (2016).
Bastaminejad, S. et al. Investigation of MicroRNA-21 Expression Levels in Serum and Stool as a Potential Non-Invasive Biomarker for Diagnosis of Colorectal Cancer. Iran. Biomed. J. 21, 106–113 (2017).
Wu, C. W. et al. Novel Approach to Fecal Occult Blood Testing by Assay of Erythrocyte-Specific microRNA Markers. Dig. Dis. Sci. 62, 1985–1994 (2017).
Wu, C. W. et al. Detection of miR−92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut 61, 739–45 (2012).
Kuriyama, S. et al. Fecal MicroRNA Assays as a Marker for Colorectal Cancer Screening. Gastroenterology 142, S-770 (2012).
Kanaoka, S. et al. Potential Usefulness of Fecal Immunochemical Test Plus Fecal MicroRNA Assay As a Marker for Colorectal Cancer Screening. Gastroenterology 144, S-599-S-600 (2013).
Koga, Y. et al. Fecal miR-106a is a useful marker for colorectal cancer patients with false-negative results in immunochemical fecal occult blood test. Cancer Epidemiol. Biomarkers Prev. 22, 1844–1852 (2013).
Zhao, H. J. et al. MiR-194 deregulation contributes to colorectal carcinogenesis via targeting AKT2 pathway. Theranostics 4, 1193–1208 (2014).
Wu, C. W. et al. Identification of microRNA-135b in stool as a potential noninvasive biomarker for colorectal cancer and adenoma. Clin. Cancer Res. 20, 2994–3002 (2014).
Yau, T. O. et al. microRNA-221 and microRNA-18a identification in stool as potential biomarkers for the non-invasive diagnosis of colorectal carcinoma. Br. J. Cancer 111, 1765–1771 (2014).
Phua, L. C. et al. Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol. Rep. 32, 97–104 (2014).
Hunter, M. P. et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 3, e3694 (2008).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 105, 10513–8 (2008).
Whiting, P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–36 (2011).
Benz, F. et al. U6 is unsuitable for normalization of serum miRNA levels in patients with sepsis or liver fibrosis. Exp. Mol. Med. 45, e42 (2013).
Xiang, M. et al. U6 is not a suitable endogenous control for the quantification of circulating microRNAs. Biochem. Biophys. Res. Commun. 454, 210–214 (2014).
Gao, Y. et al. Down-regulation of miR-24-3p in colorectal cancer is associated with malignant behavior. Med. Oncol. 32, 362 (2015).
Mishra, P. J. et al. MiR-24 tumor suppressor activity is regulated independent of p53 and through a target site polymorphism. PLoS One 4, e8445 (2009).
Zanutto, S. et al. Circulating miR-378 in plasma: a reliable, haemolysis-independent biomarker for colorectal cancer. Br. J. Cancer 110, 1001–1007 (2014).
Dejima, H., Iinuma, H., Kanaoka, R., Matsutani, N. & Kawamura, M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol. Lett. 13, 1256–1263 (2017).
Xu, X., Wang, X., Fu, B., Meng, L. & Lang, B. Differentially expressed genes and microRNAs in bladder carcinoma cell line 5637 and T24 detected by RNA sequencing. Int. J. Clin. Exp. Pathol. 8, 12678–87 (2015).
Lopez, J. P. et al. Co-Variation of Peripheral Levels of miR-1202 and Brain Activity and Connectivity During Antidepressant Treatment. Neuropsychopharmacology 42, 2043–2051 (2017).
Lopez, J. P. et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat. Med. 20, 764–8 (2014).
Fiori, L. M. et al. Investigation of miR-1202, miR-135a, and miR-16 in Major Depressive Disorder and Antidepressant Response. Int. J. Neuropsychopharmacol, https://doi.org/10.1093/ijnp/pyx034 (2017).
Hamam, R. et al. microRNA expression profiling on individual breast cancer patients identifies novel panel of circulating microRNA for early detection. Sci. Rep. 6, 25997 (2016).
Tokuhisa, M. et al. Exosomal miRNAs from Peritoneum Lavage Fluid as Potential Prognostic Biomarkers of Peritoneal Metastasis in Gastric Cancer. PLoS One 10, e0130472 (2015).
Munari, E. et al. Clear cell papillary renal cell carcinoma: micro-RNA expression profiling and comparison with clear cell renal cell carcinoma and papillary renal cell carcinoma. Hum. Pathol. 45, 1130–8 (2014).
Toiyama, Y., Okugawa, Y., Fleshman, J., Richard Boland, C. & Goel, A. MicroRNAs as potential liquid biopsy biomarkers in colorectal cancer: A systematic review. Biochim. Biophys. acta. Rev. cancer 1870, 274–282 (2018).
Oikonomopoulos, A., Polytarchou, C., Joshi, S., Hommes, D. W. & Iliopoulos, D. Identification of Circulating MicroRNA Signatures in Crohn’s Disease Using the Nanostring nCounter Technology. Inflamm. Bowel Dis. 22, 2063–9 (2016).
Polytarchou, C. et al. Assessment of Circulating MicroRNAs for the Diagnosis and Disease Activity Evaluation in Patients with Ulcerative Colitis by Using the Nanostring Technology. Inflamm. Bowel Dis. 21, 2533–9 (2015).
Schönauen, K. et al. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 24, 1547–1557 (2018).
Polytarchou, C. et al. MicroRNA214 Is Associated With Progression of Ulcerative Colitis, and Inhibition Reduces Development of Colitis and Colitis-Associated Cancer in Mice. Gastroenterology 149, 981–92.e11 (2015).
Wu, Y. et al. MicroRNA-21 (Mir-21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell. Physiol. Biochem. 43, 945–958 (2017).
Feng, Y.-H. et al. MicroRNA-21-mediated regulation of Sprouty2 protein expression enhances the cytotoxic effect of 5-fluorouracil and metformin in colon cancer cells. Int. J. Mol. Med. 29, 920–6 (2012).
Ahmadi, S., Sharifi, M. & Salehi, R. Locked nucleic acid inhibits miR-92a-3p in human colorectal cancer, induces apoptosis and inhibits cell proliferation. Cancer Gene Ther. 23, 199–205 (2016).
Xiong, Y. et al. Correlation of over-expressions of miR-21 and Notch-1 in human colorectal cancer with clinical stages. Life Sci. 106, 19–24 (2014).
Peacock, O. et al. Inflammation and MiR-21 pathways functionally interact to downregulate PDCD4 in colorectal cancer. PLoS One 9, e110267 (2014).
Saxena, A., Shoeb, M., Ramana, K. V. & Srivastava, S. K. Aldose reductase inhibition suppresses colon cancer cell viability by modulating microRNA-21 mediated programmed cell death 4 (PDCD4) expression. Eur. J. Cancer 49, 3311–9 (2013).
Liu, M. et al. miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett. 585, 2998–3005 (2011).
Li, G., Wang, C., Wang, Y., Xu, B. & Zhang, W. LINC00312 represses proliferation and metastasis of colorectal cancer cells by regulation of miR-21. J. Cell. Mol. Med. 22, 5565–5572 (2018).
Zhang, G.-J. et al. MiR-92a promotes stem cell-like properties by activating Wnt/β-catenin signaling in colorectal cancer. Oncotarget 8, 101760–101770 (2017).
Ke, T.-W., Wei, P.-L., Yeh, K.-T., Chen, W. T.-L. & Cheng, Y.-W. MiR-92a Promotes Cell Metastasis of Colorectal Cancer Through PTEN-Mediated PI3K/AKT Pathway. Ann. Surg. Oncol. 22, 2649–55 (2015).
Lv, H. et al. MicroRNA-92a Promotes Colorectal Cancer Cell Growth and Migration by Inhibiting KLF4. Oncol. Res. 23, 283–290 (2016).
Chen, E. et al. MiR-92a promotes tumorigenesis of colorectal cancer, a transcriptomic and functional based study. Biomed. Pharmacother. 106, 1370–1377 (2018).
Strul, H. Fecal occult blood test for colorectal cancer screening. Ann. Oncol. 13, 51–56 (2002).
Hirai, H. W. et al. Systematic review with meta-analysis: faecal occult blood tests show lower colorectal cancer detection rates in the proximal colon in colonoscopy-verified diagnostic studies. Aliment. Pharmacol. Ther. 43, 755–764 (2016).
Leeflang, M. M. G., Deeks, J. J., Takwoingi, Y. & Macaskill, P. Cochrane diagnostic test accuracy reviews. Syst. Rev. 2, 82 (2013).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6, e1000097 (2009).
Reitsma, J. B. et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 58, 982–990 (2005).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–88 (1986).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 45, 139–145 (2015).
Jones, C. M. & Athanasiou, T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann. Thorac. Surg. 79, 16–20 (2005).
Grimes, D. A. & Schulz, K. F. Refining clinical diagnosis with likelihood ratios. Lancet 365, 1500–1505 (2005).
Acknowledgements
This study was partly supported by a grant from the Litwin Initiative at the Crohn’s and Colitis Foundation and NTU QR funds.
Author information
Authors and Affiliations
Contributions
T.O.Y. and C.M.T. contributed to the design of the study; E.K.H. designed and performed the literature search. T.O.Y. performed the data analysis and wrote the manuscript; B.D. reviewed the data analysis, C.M.T., E.K.H., B.D. and C.P. wrote and reviewed the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yau, T.O., Tang, CM., Harriss, E.K. et al. Faecal microRNAs as a non-invasive tool in the diagnosis of colonic adenomas and colorectal cancer: A meta-analysis. Sci Rep 9, 9491 (2019). https://doi.org/10.1038/s41598-019-45570-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45570-9
This article is cited by
-
Rebound increase in microRNA levels at the end of 5-FU-based therapy in colorectal cancer patients
Scientific Reports (2023)
-
Fecal DNA methylation markers for detecting stages of colorectal cancer and its precursors: a systematic review
Clinical Epigenetics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.