Abstract
Vitamin B12 (cobalamin) can control phytoplankton development and community composition, with around half of microalgal species requiring this vitamin for growth. B12 dependency is determined by the absence of cobalamin-independent methionine synthase and is unrelated across lineages. Despite their important role in carbon and sulphur biogeochemistry, little is known about haptophytes utilization of vitamin B12 and their ability to cope with its limitation. Here we report the first evaluation of B12 auxotrophy among this lineage based on molecular data of 19 species from 9 families. We assume that all species encode only a B12-dependent methionine synthase, suggesting ubiquitous B12 auxotrophy in this phylum. We further address the effect of different B12 limitations on the molecular physiology of the model haptophyte Tisochrysis lutea. By coupling growth assays in batch and chemostat to cobalamin quantification and expression analyses, we propose that haptophytes use three strategies to cope with B12 limitation. Haptophytes may assimilate dissolved methionine, finely regulate genes involved in methionine cycle and B12 transport and/or limit B12 transport to the mitochondrion. Taken together, these results provide better understanding of B12 metabolism in haptophytes and represent valuable data for deciphering how B12-producing bacteria shape the structure and dynamics of this important phytoplankton community.
Similar content being viewed by others
Introduction
Vitamin B12, or cobalamin, can control phytoplankton growth1 and community composition in Polar Regions2,3,4 including the Southern Ocean5,6, and in some temperate coastal waters7. This organometallic cobalt-containing cofactor is only produced by certain species of archaea and bacteria. Cobalamin biosynthesis involves 30 enzymatic steps8,9,10 and eukaryotes, including algae, do not have the complete genetic equipment11,12. The metabolic need for cobalamin is relatively common among microalgae, with around 50% species being B12-auxotrophic11,12,13. Therefore, either through direct interactions11,14 or by cell lysis and release15, prokaryotes are the ultimate source of vitamin B12 for auxotrophic primary producers. Among phytoplankton species, haptophytes, whose origin has been dated around 830 million years ago16, are important contributors to global marine primary production, representing significant carbon sink in oceans17,18. These widespread eukaryotic microalgae are also one of the main producers of dimethylsulfoniopropionate (DMSP), the precursor of dimethyl sulfide (DMS), an important component of sulphur cycle that acts as a cloud condensation nuclei19,20. Thus, understanding how haptophytes acclimate to cobalamin limitation appears relevant for elucidating primary production and nutrient cycling processes in oceans.
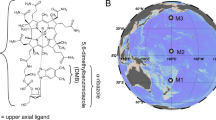
Within eukaryotes, vitamin B12 enables the activity of a relatively few number of enzymes: methionine synthase, class II ribonucleotide reductase (RNR II) and methylmalonyl-CoA-mutase (MMCM). Cobalamin has two active forms, methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl), permitting the activity of different enzymes. Methionine synthases are key enzymes for the production of proteins as they allow the conversion of 5-methyltetrahydrofolate and homocysteine into tetrahydrofolate and methionine. Whereas the first isoform of methionine synthase (METH, gene metH) needs MeCbl as cofactor and is encoded in all microalgae, the second isoform (METE, gene metE) does not need cobalamin, has a lower catalytic rate21 and is found in B12-independent species11,12,22. RNR II converts ribonucleotides into deoxyribonucleotides for DNA synthesis using MeCbl9 and MMCM (gene mmcm) is involved in the citric acid (TCA) cycle in the mitochondrion, where it converts methylmalonyl-CoA into succinyl-CoA with AdoCbl13. Nonetheless, species with these B12-dependent enzymes can grow without the vitamin if they possess the cobalamin-independent METE isoform. This suggests that B12-dependent reactions other than methionine synthesis are less critical for their development in cobalamin-deprived environments. In addition, accessory proteins CBLA and CBLB allow B12 transport of MeCbl and conversion into AdoCbl in the mitochondrion for MMCM activity23 (Fig. 1).
Schematic diagram of B12 utilization in eukaryotic C1 metabolism. B12 active forms methylcobalamine (MeCbl) and adenosylcobalamin (AdoCbl) catalyze different enzymatic reactions. B12-dependent METH uses MeCbl in the cytosol and B12-requiring MMCM needs AdoCbl in the mitochondrion. AdoHcyst, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; Hcyst, homocysteine; Met, methionine; TCA cycle, tricarboxylic acid cycle.
It has been proposed that loss of B12-independent methionine synthase arose multiple times in evolution12,13,24. A reason advanced would be that microalgae provided with a non-limiting supply of cobalamin would lost selective pressure on the energy-expensive25 METE and retain only METH. As an example, a recent work on Chlamydomonas reinhardtii grown with a source of B12 revealed a shift from cobalamin-independence to auxotrophy24. The conversion of methionine from homocysteine is essential in one-carbon metabolism as methionine undergoes several ways of use26. It is either assimilated into proteins, or converted by the enzyme methionine adenosyltransferase (MAT, gene metK) into S-adenosylmethionine (SAM), an important methyl donor and radical source22,27 (Fig. 1). There are many reactions involving SAM demethylation, such as DNA methylation, synthesis of vitamin B1 (thiamine)22,28 and DMSP biosynthesis29. SAM demethylation leads to the formation of S-adenosylhomocysteine (SAH) which is finally hydrolyzed to regenerate homocysteine by the S-adenosylhomocysteine hydrolase (SAHH, gene sahH). All these reactions from methionine production to homocysteine regeneration are described as the methionine cycle (Fig. 1).
Interestingly, the only way known for marine microalgae to produce DMSP implies both SAM demethylation and methionine transamination, which suggests that DMSP synthesis is an important sink of methionine29. The majority of DMSP production in the ocean is due to haptophytes and dinoflagellates and, as this molecule does not contain nitrogen, it is suggested that it acts in microalgae as a dissipating excess energy agent when sulphur assimilation exceeds nitrogen incorporation29. Numerous species from these lineages are considered to be cobalamin-dependent 11,13. Therefore, vitamin B12 may be particularly important in haptophytes and dinoflagellates cellular processes, especially in nitrogen-limited environments.
Previous studies based on culture assays showed that on the 22 haptophytes species tested, 8 were able to grow without B12 addition and were considered as B12-independent11,13. In absence of culture assay in truthful axenic condition and of molecular evidence for the presence of METE in these species, the cobalamin dependence of haptophytes lineage stays unclear. Moreover, the question of how haptophytes acclimate and regulate key metabolic enzymes in B12 limitation stays poorly documented. Considering that haptophytes are major contributors to nano and pico-plankton communities17,30 and play a significant role in organic matter cycling, deciphering B12 dependence and B12-associated metabolism of this lineage is of global importance.
Here, our analysis of genes metH and metE of 19 genome-sequenced or transcriptome-sequenced haptophyte species suggest that the auxotrophy for B12 is ubiquitous in the haptophyte lineage. In a second part, by using batch and continuous cultures in controlled photobioreactors, we investigated the effect of different levels of B12 limitation on the molecular physiology of the model haptophyte Tisochrysis lutea. Genes expression analyses showed that methionine cycle is finely regulated by B12 availability in the environment.
Results
Phylogenetic analysis of methionine synthase in haptophytes
A survey of methionine synthase isoforms in 19 haptophyte species based on transcriptomic and genomic datasets has been conducted (see Supplementary Table 1 on Supplementary Information for sequences details). All samples investigated contained the B12-dependent metH. The phylogeny of this phylum was reconstructed from this gene, with species from orders Coccolithales, Isochrysidales and Prymnesiales relevantly gathered (Fig. 2). This reconstruction was consistent with what is usually found for 18S sequence31, suggesting an absence of horizontal gene transfer. The cobalamin-independent isoform metE was not found in any of the samples, indicating that all 19 species are B12-auxotrophic (Fig. 2). The Marine Atlas of Tara Ocean Unigenes (MATOU) for eukaryotic data32 was also investigated by searching similar genomic and proteomic sequences of METE from Phaeodactylum tricornutum and Chlamydomonas reinhardtii. The MATOU database gather large-scale environmental metatranscriptomic and metagenomic information. Since no haptophyte sequence was retrieved in these large datasets, this reinforces the hypothesis of absence of B12-independent methionine synthase in the haptophyte lineage.
Considering that comprehensive genomic and transcriptomic data of Tisochrysis lutea (Isochrysidaceae) were available, it was taken as model species for molecular physiology analyses depending on cobalamin quotas. In silico searches in T. lutea (strain CCAP 927/14) genome31,33 allowed to identify several genes involved in vitamin B12 metabolism, conversion and transport. Gene metH coding for cobalamin-dependent methionine synthase was found and the presence of the related protein was confirmed in our proteomic dataset. The B12-independent methionine synthase was not found in our proteomic nor genomic data, suggesting B12 auxotrophy. Translated sequences of METH protein from other haptophytes were compared with the one of T. lutea when possible (see Supplementary Table 1 of Supplementary Information).
Assessment of B12 requirement of Tisochrysis lutea
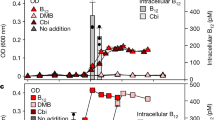
In order to validate biological dependency of T. lutea to vitamin B12, a growth assay was performed. The axenic microalgae were grown either in cobalamin-deprived medium, methionine adding or in complete medium. Cells grown with 40 ng L−1 cobalamin exhibited a maximal growth rate (μmax) of 0.35 ± 0.04 d−1 and a maximal biomass increase (ΔCmax) of 0.48 ± 0.02 arbitrary units (A.U.) (Fig. 3). Cobalamin-free cultures showed a growth rate five times lower and statistically significant (p = 8.11 10−8; two-tailed Student’s t test) with μmax = 0.07 ± 0.03 d−1 and ΔCmax = 0.05 ± 0.01 A.U. (Fig. 3). This was consistent with in silico analysis and clearly demonstrated T. lutea auxotrophy. The low growth observed for cobalamin-free cultures was due to the use of natural seawater which provided the cells with little naturally-present vitamin B12. Interestingly, microalgae grown with 0.50 mg L−1 methionine showed twice the growth of the negative control that was statistically significant (p = 4.04 10−4; two-tailed Student’s t test) with μmax = 0.17 ± 0.04 d−1 and ΔCmax = 0.16 ± 0.04 A.U. (Fig. 3), meaning that T. lutea is able to uptake and assimilate dissolved methionine and use it instead of cobalamin. The assimilation of dissolved free amino acids by marine microalgae is not well documented. This result confirmed that cobalamin is vital for methionine synthesis and that a lack of B12 may induce a lack of methionine.
B12-limited batch experiment
Cobalamin-limited batch culture in triplicate was set up to analyze expression of genes involved in vitamin B12 utilization, conversion and transport, and to compare their expression depending on cobalamin quota. Figure 4A presents the evolution of the average cell concentration against time and two sampling points for B12 and qPCR measurements. Figure 4B presents results for intracellular B12 measures, ranging from 20 ± 7 pg mg C−1 in early exponential phase to 8 ± 2 pg mg C−1 in late exponential phase, with a statistically significant two-fold decrease in intracellular cobalamin concentration due to vitamin starvation (p = 0.02; two-tailed Student’s t test). In their cobalamin-limited batch experiment, Cruz-Lopez et al.34 showed a two-fold decrease of B12 quota for the dinoflagellate Lingulodinium polyedrum34, which is consistent with our result.
Batch cultures of T. lutea in B12-limited medium. (A) Growth curve of T. lutea (means of three biological replicates ± one standard deviation), with gray arrows indicating sampling points for vitamin B12 content and qPCR analysis. (B) Boxplot of intracellular cobalamin content at two sampling points during exponential (Exp.) and stationary (Stat.) phase with bold line indicating median (n = 3 replicates).
The expression of genes metH, metK and sahH, involved in the methionine cycle, cblA, cblB and mmcm, involved in cobalamin transport, conversion and utilization in the mitochrondrion was followed during high cobalamin availability (early exponential phase) and cobalamin starvation (stationary phase). The expression of methionine cycle genes and mmcm did not show a clear trend (Fig. 5A–C,F; Supplementary Dataset 1; Supplementary Fig. 2 in Supplementary Information). In comparison, genes cblA and cblB were significantly repressed (p = 0.02 and p = 0.04; two-tailed Student’s t test) by 72-fold and 11-fold respectively (Supplementary Dataset 1; Supplementary Fig. 2). This finding suggests that B12 starvation decreases expression of genes involved in cobalamin transport and conversion. It must be pointed out that growth rate decrease at the end of the batch culture may lead to cellular processes influencing many biochemical pools. Therefore, the expression of genes analyzed here may be the result of a global physiological state not specifically related to B12 starvation. A more accurate approach using cobalamin-limited chemostat was thus undertaken to confirm the effect of different vitamin B12 status on genes expression.
Genes expression in batch cultures of T. lutea. Relative levels of expression of (A) METH, (B) METK, (C) SAHH, (D) CBLA, (E) CBLB and (F) MMCM genes. Values represent expression level at stationary phase divided by expression level at early exponential phase. Data are log2 normalized. Values are shown for each biological triplicate (1, 2 and 3). Bars indicate means of technical triplicate measurements and error bars represent one standard deviation (see Table 2 on Supplementary Information for primers).
Chemostat experiment in B12 limitation
Populations analysis
To accurately describe the effect of vitamin B12 status on the expression of genes involved in cobalamin use, a B12-limited chemostat experiment was implemented in controlled photobioreactors. Nitrogen-limited (N-limited) chemostats with the same dilution rate were taken as controls to verify whether the observed results were specific to B12 limitation or rather related to a more general physiological status. Effort was made to prevent any bacterial contamination throughout the duration of the experiments. Particulate carbon and nitrogen and cellular concentration (see Supplementary Fig. 3 in Supplementary Information) were monitored at high frequency and allowed to accurately describe culture phases. Biological duplicates exhibited similar trends during all the duration of the experiment (Fig. 6A,B). Based on stability of carbon and microalgal concentration, steady-state was reached in both chemostats at day 12 (Fig. 6A,B; Supplementary Fig. 3). At day 25, a spike of limiting nutrient (vitamin B12 or nitrates) resulted in an increase in carbon biomass in all cultures, confirming nutrient limitation during the steady-state phase (Fig. 6A,B). Samples were collected on days 14, 21, 25, 26 and 27 for B12 content and qPCR analyses. For a same dilution rate, carbon content was slightly higher in B12 limitation than in N-limited control chemostats. Based on N/C results, physiological status of N-limited chemostats were described: nitrogen limitation at steady-state; nutrient repletion one hour after nitrogen input during N/C increase and nutrient depletion 24 hours after nitrogen input, at N/C decrease.
Chemostat cultures of T. lutea. (A) Algal carbon concentration and (B) algal N:C ratio. Blue arrows represent nutrient spike, black arrows indicate sampling points (Ss 1, 2, 3, spike + 1 h and spike + 24 h). (C,D) Intracellular cobalamin content at three sampling points during steady-state (Ss 1, 2, 3), 1 and 24 hours after nutrient input. Bars indicate values for the two biological replicates, with error bars representing the range. Data are for B12-limited (black) and nitrogen-limited (gray) chemostats.
Intracellular B12 content
Intracellular cobalamin content was measured at different times in B12-limited chemostats and nitrogen-limited control cultures. Three samples were collected during steady-state. Mean cobalamin quota in B12-limited cultures prior to the cobalamin spike was 0.03 ± 0.02 pg μg C−1 (Fig. 6C). In comparison, B12 quota in N-limited chemostats was 1.73 ± 0.02 pg μg C−1, value 50 times greater than the one observed in B12-limited cultures (Fig. 6D). One hour after cobalamin spike, mean quota of B12-limited chemostats was multiplied by 8, reaching 0.24 ± 0.14 pg μg C−1, indicating an ability to quickly assimilate cobalamin (Fig. 6C). In N-limited cultures, B12 quota was on average nine times higher one hour after a spike of nitrogen (2.31 ± 0.03 pg μg C−1) relative to the one of B12-limited cultures after a cobalamin pulse (Fig. 6D). One day after nutrient spike, mean B12-limited chemostats quota dropped below steady-state value of 0.02 ± 0.01 pg μg C−1, suggesting rapid vitamin depletion (Fig. 6C), while after the nitrogen pulse the cobalamin quotas of the N-limited control cultures fell to 0.72 ± 0.17 pg μg C−1 (Fig. 6D), nearly 50 times higher than those of B12-limited cultures. This was likely attributable to the increase in cellular division, which was probably faster than vitamin acquisition. By combining B12 quotas and N/C ratio, physiological states for B12-limited chemostats were described: nutrient limitation at steady-state; nutrient repletion 1 hour after B12 input during N/C increase and nutrient depletion 24 hours after B12 input, at the end of N/C increase.
Molecular analyses
Genes expression analyses were carried out on samples collected at each physiological state to relate genes expression patterns to nutrient quotas. Genes for which expression was followed were the same as those of the batch experiment. Results presented for steady-state correspond to the third sample collected, just before nutrient spike.
As can be seen in Fig. 7G, at steady-state all genes involved in methionine cycle were more expressed in B12 limitation than in nitrogen limitation by 4-fold (metH) and 8-fold (metK and sahH) (Fig. 7G; Supplementary Dataset 2 and Supplementary Fig. 4 in Supplementary Information). Genes cblA and cblB were around 2-fold less expressed in cobalamin limitation than in nitrogen limitation, whereas mmcm was expressed almost at the same level (Fig. 7G).
Genes expression in chemostat cultures of T. lutea during steady-state (Ss 3), 1 and 24 hours after nutrient spike: relative expression level of (A) METH, (B) METK, (C) SAHH, (D) CBLA, (E) CBLB and (F) MMCM genes normalized by mean expression level of cobalamin-limited chemostats at repletion, one hour after nutrient input; (G) barplot representing genes expression levels at Ss 3 in cobalamin-limited cultures normalized by their mean expression level in N-limited cultures at Ss 3. Data are log2 normalized. Points and bars indicate means of technical triplicates for each biological duplicate (represented in black and grey) and error bars represent one standard deviation (see Table 2 on Supplementary Information for primers).
One hour after vitamin B12 adding in cobalamin-limited reactors, during repletion phase, the expression of methionine cycle genes decreased by a factor 2 to 4 (Fig. 7A–C) and that of cblA and cblB by a factor 2 (Fig. 7D,E). During subsequent nutrient depletion, their expression returned to that at steady-state (Fig. 7A–E; Supplementary Fig. 4). This pattern of expression reflects noticeably intracellular cobalamin rate contents (Fig. 6C), with methionine cycle genes overexpressed in B12-limited cells and repressed in B12-replete cells. Expression of mmcm seemed not to be affected by vitamin B12 spike (Fig. 7F; Supplementary Fig. 4).
One hour after nitrate spike in nitrogen-limited reactors, the expression of sahH showed a slight increase while the expression of metH, metK, cblA, cblB and mmcm did not seemed to be affected by nitrogen addition (see Supplementary Fig. 4 in Supplementary Information). These genes did not show clear changes of expression in the depletion phase (Supplementary Fig. 4 in Supplementary Information). A decoupling between nitrogen status and the decrease in gene expression could explain the absence of regulation 24 hours after nitrogen spike. Overall, methionine cycle genes showed a clear trend directly related to vitamin B12 quota, and did not respond in an evident way to nitrates spike. Genes encoding accessory proteins CBLA and CBLB also showed an explicit pattern of expression induced by cobalamin quota but were not influenced by nitrogen status. Gene mmcm did not show any clear regulation of expression during the experiment with its expression level being almost the same for all chemostats independently of the cultures physiological state.
Discussion
Haptophytes microalgae play an important role in carbon17,18 and sulphur cycling20 but their ability to respond to cobalamin variations is poorly known, despite the previously described impact of B12 limitation on phytoplankton growth and community composition2,3,35. The aim of this work was to (1) analyze B12 dependency of haptophytes based on molecular data and (2) provide insights into cobalamin molecular physiology of haptophytes by studying the model marine microalgae Tisochrysis lutea. This work combined bioinformatic searches, growth tests in batch and chemostat at different levels of B12 availability with the analyses of cobalamin quotas and expression of genes involved in B12 metabolism.
Nineteen haptophyte species across six orders and nine families were assessed for the presence of methionine synthase isoforms. All species investigated encoded the B12-dependent metH only. It has been observed that cobalamin auxotrophy is determined by the presence of metH and the absence of the cobalamin-independent methionine synthase metE11,12. Our results, mainly based on transcriptomic datasets, suggest that all of these haptophytes are cobalamin auxotrophs. No metE sequence of haptophytes was retrieved from the MATOU database, supporting the idea that species of this phylum are B12-requiring for growth. On the other hand, Croft et al.11 reported the occurrence of 8 haptophyte species among the 22 analyzed that did not require cobalamin11. It must be pointed out that 2 species over 22 were grown in their study and the remaining 20 were compiled from literature without any information about bacterial contamination. Recently, Helliwell et al. (2011) demonstrated the role of some bacteria so tightly attached to calcifying and non calcifying cells of E. huxleyi that they could not be disrupted with antibiotics, potentially providing the microalgae with vitamin B1212. Among 8 species considered as B12-independent 6 belong to Coccolithales. Strongly-attached, antibiotic-resilient bacteria may have been a B12 source for these species. The present paper is the first attempt to compile existing information based on molecular analyses for this phylum and, as no study found a species of Haptophyta phylum encoding metE nor a pseudogene, we assume that haptophytes are in majority cobalamin-dependent. This would be the first microalgae phylum gathering exclusively cobalamin auxotrophs, suggesting that their common ancestor did not encode metE, while in other phyla only certain species would have lost the B12-independent methionine synthase.
In order to explain the B12 molecular physiology of haptophytes, we selected the model species T. lutea for which comprehensive genomic and transcriptomic data were made available. Growth assays in natural seawater without cobalamin enrichment confirmed results of in silico approach as T. lutea was B12-limited two days after inoculation. Moreover, the absence of calcified coccoliths on the cells prevented presence of non detectable bacteria after strain purification. Adding methionine instead of B12 allowed T. lutea to develop, showing its ability to uptake and assimilate dissolved methionine to make up for cobalamin deprivation. This is consistent with another study11 demonstrating that the B12-dependent freshwater chlorophyte Lobomonas rostrata could be grown for several subcultures with METH products (i.e. methionine and folic acid). Our control without B12 exhibited 10 times lower maximal biomass compared with the control grown with 40 ng L−1 (24 pmol L−1) B12, suggesting around 4 ng L−1 (2.4 pmol L−1) cobalamin concentration in seawater. This is consistent with what was observed by Panzeca et al.36 and Suffridge et al.35 who estimated cobalamin concentrations ranging from 0.2 to 4 pmol L−1 in open oceans and 11 to 15 pmol L−1 in coastal ecosystems35,36 and indicating that vitamin B12 in natural seawater is limiting for this species. The maximal biomass obtained when T. lutea was grown with 500 g L−1 methionine was almost 3 times higher than the negative control but the concentration tested here was 625 times the maximal concentration found in seawater, that ranged from 0.27 ng L−1 offshore to 790 ng L−1 near the coast37. Recently, Suffridge et al.35 reported particulate methionine concentrations in seawater along a Mediterranean transect ranging from 0.30 to 3 ng L−1 35. These findings mean that methionine concentrations in natural environment are likely to be limiting for T. lutea development and support the idea that auxotrophic microalgae need to be supplemented with a readily available cobalamin source such as vitamin-producing bacteria14, cell lysate or B12-remodeling algae, that are able to convert the less bioavailable pseudocobalamin into a readily accessible vitamin B12 form38.
We investigated the molecular physiology of T. lutea in batch and chemostat by focusing on the expression dynamics of genes involved in vitamin B12 use, transport and conversion. Genes cblA and cblB, encoding proteins transporting cobalamin to the mitochondrion, were down-regulated under B12 starvation in batch and B12 limitation in chemostat compared with the N-limited controls. This suggests that when B12 is limiting, cobalamin-dependent activities in the mitochondrion are reduced, possibly in favor of other cellular processes. Methionine cycle genes metH, metK and sahH and B12-dependent mmcm were not clearly affected by cobalamin starvation in batch. This differs from the results of Bertrand et al.25,27 for the B12-requiring diatom Thalassiosira pseudonana, which exhibited an overexpression of methionine cycle genes in cobalamin starvation with respect to replete conditions25,27. As expression pattern in batch experiments could be the result of numerous cellular processes related to the absence of cell division, these results must be viewed with caution. To bypass this, we implemented cultures in chemostat.
In this experiment, methionine cycle genes, cblA and cblB exhibited dynamics remarkably mirroring cobalamin quotas, with an overexpression in B12 limitation and downregulation in B12 repletion. These results could mean that upregulation of metH, metK and sahH is needed in cobalamin-limited environments to maintain optimal biochemical kinetics for methionine production and SAM cycling. Interestingly, expression of mmcm, that catalyzes the conversion of succinyl-CoA to methylmalonyl-CoA in the mitochondrion with cobalamin as cofactor, remained identical independently of vitamin limitation in batch and chemostat. It has been suggested that B12-dependent MMCM is not vital for growth as not all cobalamin-requiring microalgae possess it12. Also, the reaction of MMCM is one of many entries in TCA cycle and there could be other mechanisms of regulation at this metabolic level which could explain the lack of modifications in mmcm expression. In the proteomic dataset from the N-limited chemostat described by Garnier et al.39, methionine cycle proteins and MMCM belonged to the top 400 highest accumulated proteins over the 4330 identified during steady-state. Proteins CBLA and CBLB were not detected in their experiment39. In general, our expression analysis trends are in accordance with their proteomic results, as genes cblA and cblB were the lowest expressed.
To our knowledge, this is the first time that an analysis of B12 molecular physiology of a microalgae has been conducted in chemostat with accurately described nutrient states. More notably, this is the first time that expression dynamics of methionine cycle genes and B12 transporters to the mitochondrion are correlated to slight changes in vitamin B12 status in a marine microalgae, with rapid response no later than one hour after nutrient amendment. This fast regulation has been reported for T. lutea genes coding for nitrate and nitrite transporters (TlNrt2.1 and TlNrt2.3) after addition of different nitrogen substrates40. This suggests that haptophytes hold quick acclimation mechanisms to nutrient availability that might explain their ecological success. The fact that these three methionine cycle genes, although not all coding for B12-dependent enzymes, are regulated in the same way raises the question of a common regulation system. Transcription factors are among major players in regulating gene expression, and some of them have already been described for T. lutea and related to oxidative stress response, triacylglycerol synthesis and photosynthesis41. Genes cblA and cblB were found to belong to a same group regulated by a shared transcription factor but methionine cycle genes were not gathered in a same module41. McRose et al.42 identified riboswitches affiliated with genes overexpressed in thiamine (vitamin B1) starvation in haptophytes microalgae42. Therefore, it is likely that such regulation mechanism would play a role in regulating, directly or not, cobalamin-related genes.
In conclusion, this is the first time that B12 dependency of haptophytes has been investigated. Based on 19 species surveyed, and since no haptophyte from the MATOU database was found encoding cobalamin-independent methionine synthase, we propose that haptophytes are cobalamin auxotrophs. Independence from vitamin B12 has been described as a mosaic pattern across evolution11,12,13 where Haptophyta would be the first microalgae phylum to gather only cobalamin-dependent species. The analysis of B12 molecular physiology of the model haptophyte species T. lutea has been undertaken. A controlled approach using chemostat cultures was performed to define precisely ecophysiological states, demonstrating the common assertion that this type of approach is of great interest when analyzing fine and rapid molecular changes in microorganisms43,44. Based on these results, we propose that haptophytes use different strategies to make up for cobalamin deprivation that include methionine assimilation, short-term regulation mechanisms in case of sudden B12 supply, such as cobalamin-producing bacteria excretion or cell lysis, and a preferential B12 allocation in the methionine cycle for METH activity. These results point out the importance of this cofactor in haptophytes cellular processes and represent a first attempt to understand the response of these ecologically important communities in vitamin B12-limited environments.
Methods
Sequence similarity search and validation
In silico analyses were realized by TBlastN and BlastP sequence similarity searches of the proteins on the new T. lutea genome31,33 with following entries (Uniprot): Chlamydomonas reinhardtii METH (A8HYR2) and METE (A8JH37), E. huxleyi METH (R1CGJ7), MAT from Escherichia coli (P0A817) and Arabidopsis thaliana (Q9SJL8), Homo sapiens and A. thaliana SAHH (P23526; O23255), Rattus norvegicus CBLA (D3ZNY3), Homo sapiens CBLB (Q96EY8) and MMCM (P22033), Propionibacterium freudenreichii subsp. shermanii MMCM (P11653). Homologous genes identified this way were searched again in T. lutea genome using TBlastX (expected threshold 1E-1). Conserved functional domains were identified by alignment of nucleic and proteic sequences in NCBI (METH: PFAM02574; METK: PFAM02773; SAHH: PFAM05221; CBLB: PFAM01923; MMCM: PFAM01642, PFAM02310 and PFAM08497).
Searches for methionine synthase isoforms in other haptophytes were firstly realized by BlastX of T. lutea METH on NCBI database, allowing to retrieve METH from Chrysochromulina sp. CCMP 291, Thalassiosira pseudonana CCMP 1335 and Phaeodactylum tricornutum CCAP 1055/1. The iMicrobe database was then queried, yielding sixteen haptophyte transcriptomes samples corresponding to 9 haptophyte families (see Table 1 in Supplementary Material for sequence references). TblastN of METE from C. reinhardtii (XP_001702934.1, NCBI) and P. tricornutum (B7G1X4, Uniprot); and METH from T. lutea were realized on the transcriptomes. Transcripts were translated into proteins using NCBI ORFfinder. Protein sequences were then aligned with METH from T. lutea and T. pseudonana and METE of P. tricornutum. Protein sequences were also verified by sequences alignments and conserved domains analyses. Identity and similarity with T. lutea sequences were estimated with LALIGN tool. Sequences alignment was conducted with MUSCLE (full mode) on the 21 METH sequences with T. pseudonana and P. tricornutum taken as outgroup. On total, 1206 positions were conserved on the 1508 initial (80%). Curation step was done with Gblocks tool, allowing gap positions within the final blocks and phylogenetic tree was realized with PhyML (100 bootstraps). Sequences homologous to genomic and proteic METE from P. tricornutum and C. reinhardtii were searched in the Marine Atlas of Tara Ocean Unigenes (MATOU) for eukaryotic data32 using BlastP and TBlastN (expected threshold 1E-1).
Algal strain and purification
To limit bacterial contamination, a purification step was carried out on Tisochrysis lutea CCAP 927/14 strain with an antibiotic treatment mix prepared following the method described by Cho et al.45. For the following experiments, an inoculum from purified T. lutea culture was transferred three times every 10 days. Ten percent volume were transfered each time in new Erlenmeyer flasks containing Conway medium enriched sterile seawater46 with B12 omitted in order for the cells to progressively run out their B12 quota. Axenicity was verified in all the experiments by epifluorescence microscopy and cytometric analysis using SYBR™ Green staining (Lonza, USA) and by plating on Marine Agar (BD Difco™, Becton Dickinson Company, USA). Petri dishes were then incubated 3 days at 25 °C before further observation. When no bacteria or colony were observed, strains were considered axenic.
Microalgal cultures
Microtiter plate growth assay
A growth assay was carried out to assess T. lutea’s cobalamin requirement and to investigate whether the microalgae can be grown with methionine, end product of METH activity. Two milliliters inoculum from the last cobalamin-limited batch were dispatched in test tubes (final concentration 1 106 cells mL−1) and enriched with Conway medium either containing 40 ng L−1 B12, or cobalamin free or cobalamin free enriched with 0.5 mg L−1 L-methionine (HPLC grade Sigma; >99% purity). Six replicates were inoculated for each condition in a microtiter plate that was incubated at 26 ± 1 °C and 90 μmol m−2 s−1. O.D.680 was monitored by spectrophotometry (Quant, BIO-TEK Instruments inc, USA).
Batch experiment
To identify modifications in T. lutea’s molecular physiology during vitamin B12 consumption, a cobalamin-limited batch experiment was first performed. Three 1-liter autoclaved glass bottles were inoculated at 2.5 106 cells mL−1 and enriched with Conway medium with 40 ng L−1 B12. Cultures were homogenized by filtered air bubbling (Midisart 0.2 m, Sartorius) and were placed at 27 ± 1 °C with a continuous irradiance of 180 μmol m−2 s−1 photons. Cellular concentration was followed by counting Lugol stained cells with Malassez haemocytometer. Samples for quantitative analysis of B12 and qPCR were taken at days 2 (early exponential phase) and 20 (stationary phase).
Chemostat experiment
Two inoculi were acclimatized at 27 ± 1 °C under a continuous irradiance of 180 μmol m−2 s−1 photons. After 10 days, they were divided into four autoclaved glass bottles filled with 4.5 L sterile seawater enriched with modified Conway medium, with a final concentration of either 40 ng L−1 B12 or 25 mg L−1 NaNO3 to ensure limitation in B12 or nitrogen (N) respectively. Bottles were set up in chemostat supplied with a continuous input of the media described above. The experiment was carried out in duplicate for each condition at 27 ± 1 °C with a continuous irradiance of 400 μmol m−2 s−1 photons and pH maintained at 8.2 by CO2 enrichment. Cultures were homogenized properly with a constant input of filtered air and a magnetic stirrer. Dilution rate (D) was adjusted at 0.5 d−1 and monitored daily by weighing system output. Populations were monitored by particulate carbon and nitrogen measurement and cell counting in Malassez haemocytometer. To confirm adequate nutrient limitation and to study physiological modifications after nutrient adding, discreet nutrient input was done at day 25 either with 25 g L−1 NaNO3 or 40 ng L−1 B12 for the N-limited and B12-limited chemostats respectively.
Biochemical analyses
Particulate carbon and nitrogen
Particulate organic nitrogen and carbon were measured by filtering 20 106 cells on 25 mm precombusted GF/F microfibers filters (0.7 m, Whatman, UK). Filters were then dried at 65 °C for at least 12 hours. Particulate organic nitrogen and carbon were analyzed with a CN elemental analyzer (Flash 2000, Thermo Fisher Scientific, Waltham, USA).
B12 measurements
Intracellular B12 quantification was assessed with an ELISA test kit (Immunolab, Germany) with a sensitivity of 0.3 ng mL−1. Zhu et al. showed that neither salinity nor dissolved organic matter do interfere with test quality47. This procedure allowed to measure the different chemical forms of vitamin B12 (cyanocobalamin, methylcobalamin, adenosylcobalamin and hydroxycobalamin) with a cross-reactivity of 98–100% among the chemical variants47. Cell pellets (80–150 106 cells) were resuspended in 100 μL PBS buffer (provided in the kit) and extraction was undertaken by boiling 15 minutes at 99 °C as previously described for microalgae48. Supernatant was collected after centrifugation (16 000 g, 5 minutes, 4 °C). Extracts were assayed following the method given in the kit, which provided cyanocobalamin solutions as standards. Absorbences at 450 and 620 nm (three technical replicates) were measured with a spectrophotometer (μQuant, BIO-TEK Instruments inc, USA). Standards and samples absorbency was defined as follows: O.D.450–O.D.620. Cobalamin concentration of samples was calculated using the calibration curve equation.
RNA extraction and RT-qPCR
Cell lysis was obtained by adding 1 ml Trizol (Life Technologies) and 200 μL chloroform to cell pellets of 300 106 cells. Samples were then purified with RNeasy™kit (Qiagen) following the provided protocol. RNA purity and concentration were verified using a spectrophotometer (Infinite 200 PRO) at 260 and 280 nm. Diluted samples of 250 ng μL−1 were treated with DNase (Promega) 1 hour at 37 °C. Reverse transcription was performed using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the provided protocol. Primer efficiency was quantified following protocol of Schmittgen et al.49 (see Table 2 on Supplementary Information for primers sequences). Primer specificity was estimated with a denaturation cycle at PCR end. PowerUp™SYBR™Green mix (Applied Biosystems) was used for RT-qPCR. Thermocycler (Mx3000P, Agilent) parameters were set as follows: 1 cycle of 15 minutes at 95 °C, 40 cycles of 30 seconds at 95 °C and 30 seconds at 60 °C. Six genes coding for 18S, actin, EF1, GAPDH, tubulin and ubiquitin were tested as housekeeping genes. As GAPDH exhibited low cycle threshold (Ct) variations with the same order of magnitude than target genes it has been selected as reference gene (see Fig. 1 of Supplementary Information). Gene expression was calculated by raising negative cycle threshold values of each pair of primers and dividing it by mean expression of reference gene. Raw data of genes expression is available in Supplementary Datasets 1 and 2 for the batch and chemostat experiments respectively.
References
Droop, M. R. A pelagic marine diatom requiring cobalamin. Journal of the Marine Biological Association of the United Kingdom 34, 229–231 (1955).
Bertrand, E. M. et al. Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnology and Oceanography 52, 1079–1093 (2007).
Koch, F. et al. The effect of vitamin B12 on phytoplankton growth and community structure in the Gulf of Alaska. Limnology and Oceanography 56, 1023–1034 (2011).
Moore, C. et al. Processes and patterns of oceanic nutrient limitation. Nature Geosciences 6, 701–710 (2013).
Panzeca, C. et al. B vitamins as regulators of phytoplankton dynamics. Eos Transactions American Geophysical Union 49, 594–596 (2006).
Gobler, C. J., Norman, C., Panzeca, C., Taylor, G. T. & Sañudo-Wilhelmy, S. A. Effect of B-vitamins (B1, B12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquatic Microbial Ecology 49, 181–194 (2007).
Sañudo-Wilhelmy, S. A., Gobler, C. J., Obkamichael, M. & Taylor, G. T. Regulation of phytoplankton dynamics by vitamin B12. Geophysical Research Letter 33, 1–4 (2006).
Raux, E., Schubert, H. L. & Warren, M. J. Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum. Cellular and Molecular Life Sciences 57, 1880–1893 (2000).
Martens, J.-H., Barg, H., Warren, M. J. & Jahn, D. Microbial production of vitamin B12. Applied Microbial Biotechnology (2002).
Warren, M. J., Raux, E., Schubert, H. L. & Escalante-Semeran, J. C. The biosynthesis of adenosylcobalamin (vitamin B12). Nature Product Reports 19, 390–412 (2002).
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J. & Smith, A. G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 483, 90–93 (2005).
Helliwell, E. K., Wheeler, G. L., Leptos, K. C., Goldstein, R. E. & Smith, A. G. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Trends in Genetics 29, 469–478 (2011).
Helliwell, E. K., Wheeler, G. L. & Smith, A. G. Widespread decay of vitamin-related pathways: coincidence or consequence? Trends in Genetics 29, 469–478 (2013).
Kazamia, E. et al. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environmental Microbiology 14, 1466–1476 (2012).
Droop, M. R. Vitamins, phytoplankton and bacteria: symbiosis or scavenging? Journal of Plankton Research 29, 107–113 (2007).
Liu, H., Aris-Brosou, S., Probert, I. & de Vargas, C. A time line of the environmental genetics of the haptophytes. Molecular Biology and Evolution 27, 161–176 (2010).
Liu, H. et al. Extreme diversity in noncalcifying haptophytes explains major pigment paradox in open oceans. Proceeding of the National Academy of Sciences 31, 1–6 (2009).
Jardillier, L., Zubkov, M. V., Pearman, J. & Scanlan, D. J. Significant CO12 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. The ISME Journal 4, 1180–1192 (2010).
Charlson, R. J., Lovelock, J. E., Andreae, M. O. & Warren, S. G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326, 655–661 (1987).
Malin, G. & Steinke, M. Dimethyl sulfide production: what is the contribution of the coccolithophores? In Coccolithophores: from molecular processes to global impact, 127–164 (Thierstein, H. R. & Young, J. R., 2004).
González, J. C., Banerjee, R. V., Huang, S., Sumner, J. S. & Matthews, R. G. Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Eschirichia coli: two solutions to the same chemical problem. Biochemistry 31, 6045–6056 (1992).
Bertrand, E. M. & Allen, A. E. Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Frontiers in Microbiology 3, 1–16 (2012).
Dobson, C. M. et al. Identification of the gene responsible for the cblA complementation group of vitamin B12-responsive methylmalonic acidemia based on analysis of prokaryotic gene arrangements. Proceedings of the National Academy of Sciences 99, 15554–15559 (2002).
Helliwell, K. E., Collins, S., Kazamia, E., Wheeler, G. L. & Smith, A. G. Fundamental shift in vitamin B12 eco-physiology of a model alga demonstrated by experimental evolution. The ISME Journal 9, 1446–1455 (2015).
Betrand, E. M. et al. Methionine synthase interreplacement in diatom cultures and communities: Implications for the persistence of B12 use by eukaryotic phytoplankton. Limnology and Oceanography 58, 1431–1450 (2013).
Banerjee, R. V. & Matthews, R. G. Cobalamin-dependent methionine synthase. The FASEB Journal 5, 1450–1459 (1990).
Bertrand, E. M. et al. Influence of cobalamin scarcity on diatom molecular physiology and identification of a cobalamin acquisition protein. Proceedings of the National Academy of Sciences 1762–1771 (2012).
Goyer, A. Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71, 1615–1624 (2010).
Stefels, J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. Journal of Sea Research 43, 183–197 (2000).
Thomsen, H., Buck, K. R. & Chavez, F. P. Haptophytes as components of marine phytoplankton. In The Haptophyte Algae, 187–208 (Clarendon Press, Oxford, 1994).
Carrier, G. C. et al. Draft genome and phenotypic characterization of Tisochrysis lutea strains. toward the production of domesticated strains with high added value. Algal Research 29, 1–11 (2018).
Villar, E. et al. The ocean gene atlas: exploring the biogeography of plankton genes online. Nucleic Acid Research 46, W289–W295 (2018).
Berthelier, J. et al. A transposable element annotation pipeline and expression analysis reveal potentially active elements in the microalgae Tisochrysis lutea. BMC Genomics 19 (2018).
Cruz-López, R., Maske, H., Yarimizu, K. & Holland, N. A. The B-vitamin mutualism between the dinoflagellate Lingulodinium polyedrum and the bacterium Dinoroseobacter shibae. Frontiers in Marine Science 5, 1–12 (2018).
Suffridge, C. P. et al. B vitamins and their congeners as potential drivers of microbial community composition in an oligotrophic marine ecosystem. Journal of Geophysical Research: Biogeosciences 123, 2890–2907 (2018).
Panzeca, C. et al. Distributions of dissolved vitamin B12 and Co in coastal and open-ocean environments. Estuarine, Coastal and Shelf Science 85, 223–230 (2009).
Sañudo-Wilhelmy, S. A. et al. Multiple B-vitamin depletion in large areas of the coastal ocean. Proceedings of the National Academy of Sciences 109, 14041–14045 (2010).
Helliwell, K. E. et al. Cyanobacteria and Eukaryotic Algae Use Different Chemical Variants of Vitamin B12. Current Biology 26, 999–1008 (2016).
Garnier, M. et al. Comparative proteomics reveals proteins impacted by nitrogen deprivation in wild-type and high lipid-accumulating mutant strains of Tisochrysis lutea. Journal of Proteomics 105, 107–120 (2014).
Charrier, A. et al. High-affinity nitrate/nitrite transporter genes (Nrt2) in Tisochrysis lutea: identification and expression analyses reveal some interesting specificities of haptophyta microalgae. Physiologia Plantarum 154, 572–590 (2015).
Thiriet-Rupert, S. et al. Identification of transcription factors involved in the phenotype of a domesticated oleaginous microalgae strain of Tisochrysis lutea. Algal Research 30, 59–72 (2018).
McRose, M. et al. Alternatives to vitamin B1 uptake revealed with discovery of riboswitches in multiple marine eukaryotic lineages. The ISME Journal 8, 2517–2529 (2014).
Hoskisson, P. A. & Hobbs, G. Continuous culture - making a comeback? Microbiology 151, 3153–3159 (2005).
Bull, A. T. The renaissance of continuous culture in the post-genomics age. Journal of Industrial Microbiology and Biotechnology 37, 993–1021 (2010).
Cho, J.-Y. et al. A procedure for axenic isolation of the marine microalga Isochrysis galbana from heavily contaminated mass cultures. Journal of Applied Phycology 14 (2002).
Walne, P. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. In Fishery Investigations, vol. 26, 62 (1970).
Zhu, Q., Aller, R. C. & Kaushik, A. Analysis of vitamin B12 in seawater and marine sediment porewater using ELISA. Limnology and Oceanography: Methods 9, 515–523 (2011).
Grant, M. A., Kazamia, E., Cicunta, P. & G., S. A. Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal-bacterial cocultures. The ISME Journal 8, 1418–1427 (2014).
Schmittgen, T. D., Jiang, J. M., Liu, Q. & Yang, L. Q. A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Research 32, 1–10 (2004).
Acknowledgements
This work was supported by the Atlantic MIcroalgae program consortium funded by Région Pays de la Loire. The authors wish to thank Ewa Lukomska for dedicated assistance for carbon and nitrogen measurements, Jean-Baptiste Bérard for kind help and support during the chemostat experiment, Bruno Saint-Jean and Gaël Bougaran for kind assistance and helpful comments on the results.
Author information
Authors and Affiliations
Contributions
C.N., S.J., F.M., R.K., D.G. and M.G. conceived the experiments. C.N. and S.J. conducted the experiments. C.N., S.J., F.M., R.K. and M.G. analyzed the results. C.N. wrote the manuscript and prepared the figures. All authors reviewed and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nef, C., Jung, S., Mairet, F. et al. How haptophytes microalgae mitigate vitamin B12 limitation. Sci Rep 9, 8417 (2019). https://doi.org/10.1038/s41598-019-44797-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44797-w
This article is cited by
-
Transcriptional insights into Chlorella sp. ABC-001: a comparative study of carbon fixation and lipid synthesis under different CO2 conditions
Biotechnology for Biofuels and Bioproducts (2023)
-
Microalgal Co-cultivation for Biofuel Production and Bioremediation: Current Status and Benefits
BioEnergy Research (2022)
-
Challenging microalgal vitamins for human health
Microbial Cell Factories (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.