Abstract
Animals can respond to dynamic environments through phenological plasticity of life history events; however, changes in one part of the annual cycle can diminish the success of subsequent life history events. Our aims were to determine the associations between reproduction and moult phenology across years and to quantify phenological plasticity across varying environmental conditions. We conducted demographic surveys of 4,252 flipper-tagged Weddell seals (Leptonychotes weddellii) in the Ross Sea, Antarctica during four austral summers. At each sighting, seals were assigned a moult code based on the visible presence of new fur and the start date of each animal’s moult was back-calculated. Reproductive success and parturition dates were obtained for the breeding season prior to and following the moult. We found that successful reproduction delayed moult by 16 days relative to non-parturient females. Phenology of the intervening moult was indicative of previous reproductive dynamics but not predictive of subsequent reproductive outcomes. Across years, moult phenology varied by about two weeks and covaried strongly with sea ice break-out timing for all reproductive categories. Our findings suggest these polar mammals have some flexibility within the annual cycle that allows adjustment of moult phenology to fluctuating environmental conditions without compromising future reproductive success.
Similar content being viewed by others
Introduction
Animals can respond to dynamic environments through phenological plasticity of life history events1,2. However, changes in one part of the annual cycle can impact subsequent processes (i.e. carry-over effects3,4;) and these shifts can in turn diminish future success in foraging, breeding, or survival5,6,7. Among most vertebrates, it is uncommon for moult and reproduction to overlap due to the high energetic costs of both8,9,10. Because peak food availability11,12 and suitable climate13 are important to the success of both life history events14,15, an adaptive balance exists between breeding and moulting phenology16. Thus, it is important to study phenological variation in the larger context of annual cycles17 and survival14,18,19 in order for the ecological implications of dynamic environments and perturbations to be understood20,21.
Documenting moult progression is a prerequisite for understanding the carry-over effects of phenological disruptions; however, the moult is poorly understood relative to other life history events22. To date, the role of the moult as an intermediate life history event between two breeding seasons has been studied almost exclusively in birds15,23. In these studies, reproductive success has been found to delay moult relative to sexually mature but non-parturient conspecifics13,14,24,25,26,27. Given the apparent role of reproductive and stress hormones in delaying moult onset28, later reproduction and lower resource availability likely result in later moulting across birds and mammals.
In mammals, moult initiation and completion are difficult to identify because follicle growth precedes visible hair loss29,30 and the extent of hair loss can be difficult to determine. As a result, mammalian moult studies have been restricted to basic descriptions of where and approximately when moult occurs and how it influences animal behavior31,32. In seals (family Phocidae), some evidence suggests that moult is delayed in reproductively successful individuals33,34,35,36,37; however, the effects of reproduction on moult have primarily been established at the population level or in a limited number of captive animals. Thus, the drivers and consequences of moult duration and phenology in individuals are unclear.
In this study, we use free-ranging Weddell seals as a model species to evaluate the moult as an intermediate life history event between two reproduction events. Specifically, we address three aims: (1) to describe the duration and phenology of the moult across age, sex, reproductive categories, and environmental conditions; (2) to analyse the relationship between reproductive phenology (October-November, Year 1) and subsequent moulting phenology (January–March, Year 1) in paturient females; and (3) to understand the relationship between moulting phenology and reproductive outcomes in the following season (October-November, Year 2). As the most southern breeding mammal, Weddell seals have highly constrained annual cycles and serve as a useful model for understanding mammalian moult cycles. Further, the ages and reproductive histories of most individuals are known due to a 45-year demographic study38,39.
Methods
Research activities were approved by National Marine Fisheries Service Marine Mammal permit #17411, University of Alaska IACUC protocols #419971 and #854089 and the Antarctic Conservation Act permit #2014-003 and were carried out in accordance with guidelines for handling marine mammals.
Field methods
In 2013–2017, we conducted semi-weekly surveys of Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica (77°S, 165°E). Each seal was approached and its flipper tag identification number, age class, and sex were recorded along with a qualitative moult code based on the visible presence of new fur (Fig. 1): code 0 - moult had not begun, no new fur visible; code 1- head moulted and/or a thin stripe of new fur visible along the spine; code 2 - head completely moulted and connected to a wide swath of new fur along the spine; code 3 - only small patches of unmoulted fur remained laterally between the front and rear flippers; and code 4 - fully moulted, no old fur visible. If the moult code could not be assigned because the animal was wet, covered in snow, or laying so that dorsal pelage was not visible, moult state was noted as unknown. Ages and sexes were obtained for tagged individuals based on a long-term demographic study38,39,40. Year is given as the austral summer each seal was observed moulting (e.g., 2013 is the 2013–14 austral summer season, including October 2013–December 2013 pupping and December 2013–March 2014 moulting) (Table 1). We use sea ice break-out date as a proxy for the timing of food availability because the annual ice break-out triggers a phytoplankton bloom that enhances productivity for seals via trophic linkages41. For each year, ice break-out date was obtained from satellite imagery using methods described in Beltran42.
Adult females were assigned a reproductive category for the pupping period preceding the moult season in which they were observed (October–December, hereafter Year 1 pupping period) and the pupping period following the moult (hereafter Year 2 pupping period). If adult females were observed with a dependent pup, they were considered to have pupped successfully (hereafter, attendant parturients). Each colony was visited every two to three days throughout the reproductive season, which allowed pupping success and precise birth dates of new-born pups (those with visible umbilical cord stumps) to be determined for many adult females per year. Using the pupping date distribution for new-borns to estimate the quantiles of pupping dates for each year, we categorized attendant parturient females as Early-, Mid-, or Late-Parturients (Table 2, Fig. 2). Alternatively, we categorized females who did not give birth (Attendant Juvenile Females, Attendant Non-Parturients) or who were not observed during the reproductive season (Non-Attendant Adult Females, Non-Attendant Juvenile Females). Because male breeding behavior is difficult to assess, we did not attempt to link breeding and moulting phenology in males. Due to logistical constraints in 2013, pupping quartiles calculations were modified slightly (see Supplemental Material).
Based on individual sightings, adult female Weddell seals were assigned a phenology category for three life history events: reproduction in Year 1, moulting in Year 1, and reproduction in Year 2. Early, mid, and late correspond to the distribution 0–25%ile, 25–75%ile, and 75–100%ile, respectively; Non-Parturient had previously pupped but were not parturient in a given year; and Non-Attendant had been seen during the Year 1 pupping and moulting season but were not seen during the Year 2 pupping season.
Analytical methods
Estimating moult stage durations
Although an existing R package “moult”43 is available for analyses of moult dynamics, it does not allow for individual random effects in moult duration. Therefore, we developed custom functions in R (R Development Core Team 2017, version 3.3.2) to estimate the duration of moult based on subsequent sightings of a series of moult codes in each individual. Moult stage durations τn were calculated as the amount of time that passed between the observed moult stage (n) and the moult stages preceding (n − 1) and following (n + 1), using a midpoint approach (see Fig. 3). The moult codes 0, 1, 2, 3, and 4 represent each of the five stages and were used to calculate moult durations τn for codes n = 1, 2, 3 as follows:
where First is the first sighting in a given moult code n, and Last is the last sighting in a given moult code n. For instance, the moult stage 1 duration τ1 is the difference between [the temporal midpoint of the first code 2 sighting and the last code 1 sighting] and [the temporal midpoint of the first code 1 sighting and the last code 0 sighting during the Jan/Feb moult season]. Stage durations were calculated for all seals that were observed in three consecutive moult codes (e.g. codes 0, 1, 2 in the case of τ1), and the distributions of those durations was tested for normality using Lilliefors tests. Moult stage durations did not differ across years, sexes, or reproductive histories (unpaired t-test, p > 0.05 for each stage); thus, data were combined to calculate the mean and standard deviation τn for the duration of each stage. Total moult duration Τ was then calculated by summing τ1, τ2, and τ3 (Fig. 3). We acknowledge that the existence of a negative co-variance between the duration of τn and τn+1 results in a conservative estimate of Τ. Moult duration values are presented as mean μ ± standard deviation σ (see Supplemental Materials for σ equations).
Moult sighting data from a theoretical animal with moult code n shown as numbers along the date axis. Each sighting is represented as a grey circle with moult code n shown. Mean moult stage durations τn were used to back-calculate a start date for each individual when the animal was not observed in a moult code n. A glossary of parameters is shown in the top panel.
Estimating moult initiation dates
Of the 4252 unique seal-year combinations that were observed during the study, 1208 were observed in both moult codes 0 and 1 (i.e., beginning of moult was known to occur between two set dates. For these individuals, we estimated the moult initiation date as the temporal midpoint between the last code 0 sighting and the first code 1 sighting). Of the remaining individuals, 681 were first observed in moult code 1, 681 in moult code 2, 444 in moult code 3, and 749 in moult code 4. To include the animals in our moult phenology analysis that had not been observed at moult initiation, we back-calculated moult initiation dates for each remaining animal based on their moult code k at first sighting Firstk. Estimating the beginning of a stage required that we first estimate the mean difference Δn between [the midpoint of the first code n + 1 sighting and the last code n sighting] and [the subsequent code n sighting] (Fig. 3), using:
for all seals that were observed in two consecutive moult codes (e.g. codes 0, 1 to calculate Δ1). Using a Lilliefors test, the Δn distributions were found to be normal. This resulted in average difference Δn values of 5.4 ± 3.9 days (n = 347 animals) for Δ1, 4.5 ± 2.9 days (n = 347 animals) for Δ2, 4.3 ± 2.9 days (n = 226 animals) for Δ3, 5.1 ± 3.7 days (n = 213 animals) for Δ4 (Table 3). Finally, to back-calculate an initiation date for each animal based on their moult code k at first sighting Firstk, we subtracted the difference value Δk and the sum of the stage durations τ for each moult stage n in which the animal was not observed:
To control for inter-annual variation in moult timing, individuals were assigned to moult categories based on the moult initiation date relative to the initiation dates of the other animals in the year of sighting::
-
“Early-Moulters”, who initiated moulting before the 25th percentile of the moult initiation dates;
-
“Middle-Moulters”, who initiated moulting between (or on) the 25th and 75th percentile of the moult initiation dates; or
-
“Late-Moulters”, who initiated moult after the 75th percentile of the moult initiation dates.
Drivers of moult phenology
To evaluate relationships between moult phenology and sex, year, and reproductive category, we constructed biologically plausible models and then selected the best models using an information-theoretic approach (Table S4). Mixed-effects models were constructed using the package lme4 and selected using AIC44,45 in R (R Core Team 2017). The global model was Date_init~Repro_cat*Year*Age + (1|ID) where 1|ID is the random effect of individual and Repro_cat is a combined sex/reproductive history category that includes males and females. Age differed by reproductive category (mean ages for Juvenile Females = 4.45 years old (yo), Males = 8.73 yo, Attendant Parturients = 14.03 yo, Attendant Non-Parturient = 15.12 yo; ANOVA, Tukey HSD post-hoc, p < 0.05 for all, except Attendant Parturients:Attendant Non-Parturient p > 0.05). However, the model AIC was higher when Age was included in the global model (Table S4); as a result, all ages within a single reproductive category were combined for the remaining analyses. For parturient females, the relationship between Year 2 pup birth date and Year 1 pup birth date (with and without the added factor of Year 2 moult initiation date) was also examined using a linear mixed-effects model with year as a fixed effect and individual as a random effect using the package lme4 in R. Finally, a multinomial logistic regression of Year 2 reproductive success as a function of Year 1 reproductive category plus Year 1 moult category was examined using the package mgcv in R.
Interactions between pupping success/phenology and moult phenology
For sexually mature females, we calculated three sets of transition probabilities:
-
1)
From all Year 1 pupping categories into each moulting category (Table S1; transition probabilities 26% Early-Moulters, 48% Mid-Moulters, and 26% Late-Moulters).
-
2)
From all Year 1 moulting categories into Year 2 pupping categories (Table S2; expected transition probabilities 24% Attendant Non-Parturient, 16% Attendant Early-Parturients, 32% Attendant Mid-Parturients, 17% Attendant Late-Parturients, 12% Non-Attendant Adult Females)
-
3)
From all from Year 1 pupping categories into Year 2 pupping categories (Table S3; expected transition probabilities 22% Attendant Non-Parturient, 16% Attendant Early-Parturients, 31% Attendant Mid-Parturients, 16% Attendant Late-Parturients, 14% Non-Attendant Adult Females).
These “expected” transition probabilities (null hypothesis; seals transition from one category to another with equal probabilities) were compared against the “observed” transition probabilities using a Markov simulation on 10,000 multinomial draws. P-values were adjusted to account for table-wide Type I errors using a Bonferonni-type correction46 (see Supplemental Material).
Results
Demography of moulting animals
Survey frequencies and counts for each study year are provided in Table 1. We observed 2% of all animals during all four study years, 11% during three years, 25% during two years, and all other animals during only one study year (62%). Tagged animals (all ages and sexes) were observed an average of 2.1 ± 1.4 times within a moulting season (minimum 1, maximum 15, median 2, mode 1). Of the 4252 seals seen during the moult, 63% of animals had been seen during the lactation period several weeks earlier. For females, the composition of Juvenile Females, Attendant Parturients, and Attendant Non-Parturient seen during the moulting period stayed relatively consistent within and across years, averaging 23%, 51%, and 26%, respectively.
Moult duration
Moult stage durations were 10.2 ± 5.3 days for τ1 (stage 1), 9.4 ± 4.0 days for τ2 (stage 2), and 9.6 ± 3.8 days for τ3 (stage 3) (Table 3). Using these average stage durations, the entire visible moult duration Τ was 29.2 ± 7.7 (mean ± standard deviation) days for Weddell seals. Using Equation 5, animals first seen in moult codes 1 (i.e. k = 1), 2, 3, and 4 had σDate_init values of 3.90 days (n = 1007), 6.04 days (n = 503), 7.25 days (n = 251), and 8.50 days (n = 369), respectively. Note that animals with only sightings at code 0 were excluded (n = 182) such that the total number of animals assigned initiation dates was 2130.
Links between pupping phenology and moulting phenology in one season
Moult initiation date ranged from Dec 09 to Feb 28 with a mean start date of January 15 ± 13.5 (SD) days, although the variance was generally smaller within each reproductive category. Based on the lowest AIC value and Akaike weight, the best mixed-effects model included the interaction between Repro_cat and Year (Table S4). Thus, the wide range (81 days) of moult initiation dates likely resulted from influences of year and reproductive categories.
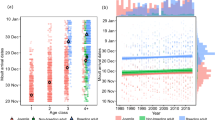
The resulting transition probabilities from pupping categories to moulting categories in each year are provided in Table S1. Attendant Non-Parturient had the earliest average moult initiation dates (range December 28 to January 12 across study years) followed by Juvenile Females (January 04 to January 15), Males (January 09 to January 24), and females that had given birth (Attendant Parturients; January 15 to January 29) (Fig. 4). Given that eight of fifteen transition outcomes differ significantly from expected, the data strongly suggest that moult phenology is not independent from pupping phenology in a given year (Table S1) (Fig. 5). Specifically, the Early-Moult category is more likely to be comprised of Attendant Non-Parturient (28% greater than expected) than Attendant Early-, Mid-, or Late- Parturients (13%, 13%, and 15% less than expected, respectively). Animals in the Mid-Moult category were disproportionately composed of animals that had been Non-Attendant during the previous pupping period (14% greater than expected). Animals that pupped contributed significantly more than expected to the Late-Moult category, with Attendant Late-Parturients (46%) contributing more than Attendant Mid-Parturients (39%), or Attendant Early-Parturients (36%). For Attendant Parturients, the moult initiation date was significantly related to when the pup was born (linear mixed effects model, R2 = 0.24). Thus, moult phenology is delayed in Attendant Parturients relative to Attendant Non-Parturients, and there is a direct relationship between date of pupping and moult onset.
Moult initiation dates across reproductive categories and years (panels; 2013 is the 2013 austral summer including the December 2013 – February 2014 moult) for Attendant individuals. Within each year, different letters denote significantly different moult initiation dates across reproductive categories (Tukey’s HSD, p < 0.05). During all study years, sexually mature females that did not produce a pup (Non-Parturients) moulted earlier than all other reproductive categories. On the contrary, sexually mature females that produced a pup (Parturients) tended to moult later than sexually immature females (Juvenile Females, significant difference in 2013, 2015, 2016), and Males (significant difference in 2013, 2016).
Proportion of seals in each moult category in Year 1 comprised of different reproductive categories from Year 1. Moult phenology was not independent of pupping phenology: the Early-Moulters category was predominated by Attendant Non-Parturient, whereas the Late-Moulters category was predominated by Attendant Parturients.
Inter-annual variation in ice dynamics, moult phenology, and colony attendance
Among study years, the moult was earliest in 2013 (mean moult initiation date January 08 ± 13 days) and latest in 2016 (January 22 ± 14 days) with moult onset during the two intermediate years occurring in between (January 11 ± 11 days in 2014 and January 14 ± 10 days in 2015) (Fig. 4, Table 1). These inter-annual differences in moult initiation date were supported by the raw survey data. In the first survey of 2013, 33% of observed seals had yet to begin moulting (moult code 0) and 12% had already completed the moult (moult code 4), whereas in a 2016 survey on that same date, 47% of seals had yet to begin moulting, and only 1% had completed the moult. The one exception was Attendant Parturients, in which moult initiation began significantly earlier in 2014 (January 15) than 2013 (January 19) and 2014 (January 18) (Fig. 4, Table 1, TukeyHSD on ANOVA, p < 0.05). While the 2016 moult surveys extended later than other years (Table 1), this would not have impacted moult initiation dates, as most seals seen after February 13–15 (final survey dates of 2013, 2014, and 2015) had started to moult (and thus moult start date would have already been detected prior to the end of surveys). Indeed, removing the 2016 sightings after February 15 still resulted in significantly later 2016 moult start dates as compared to other years for all reproductive categories. Sea ice break-out date varied by 33 days across the study years and both ice break-out and moult initiation dates were later in 2016 than other years (Tale 1). The within-year moult phenology variance in our study may be artificially high because we do not control for some factors known to affect moult start (e.g., circulating hormone concentrations47, body condition36).
Links between pupping and moulting phenology in one season and pupping in the next
For sexually mature females, Year 2 pupping categories (Pupping, Skip-Pupping, or Non-Attendant) were significantly related to moult initiation date in Year 1 (chi-square test, χ2 = 18.923, p = 0.0153): Early-Moulters contributed 4% less than expected to the Non-Attendant-Parous category whereas Late-Moulters showed the opposite trend, contributing 6% more than expected to Non-Attendant-Parous (Table S2). However, results also indicate that moult phenology reflects previous reproduction dynamics rather than driving future reproduction dynamics, and that the Year 2 pupping categories were more strongly related to the Year 1 pupping categories, than by the dates of the intervening moult (chi-square test, χ2 = 130.52, p < 0.0001, Table S3). Further, Year 1 moult timing did not help explain whether animals became Parturient, Non-Parturient, or Non-Attendant in Year 2 (multinomial logistic regression, AIC without moult = 1339, AIC with additive effect of moult = 1340). In general, individuals in a given pupping category in Year 1 were likely to remain in the same reproductive phenology category in Year 2 (Table S3) and there was a strong relationship between Y1 and Y2 birthdates (Y2PupDate = 0.63(±SE 0.06)*Y1PupDate-23.64, R2 = 0.292). When added into a multiple regression, moult timing was not a significant explanatory factor in Y2 birthdates (P = 0.173). Thus, contrary to initial expectations, Year 2 pupping success and phenology are driven by Year 1 reproductive dynamics rather than the intervening moult phenology.
Discussion
Reproductive history affects moult phenology
Within sexually mature individuals, we found that post-parturient females and males moulted later than non-parturient females. Within parturient females, we found that later birth was associated with later moult (see Table S1). Moult initiation is likely delayed in these groups relative to sexually immature and non-parturient individuals by the elevated circulating cortisol48 and prolactin49 levels during lactation and elevated testosterone during breeding47,50. Energetics may also contribute to carryover effects between reproduction and moult; specifically, reduced body condition following offspring care may delay moult onset until individuals regain enough energy stores by foraging. A similar phenomenon has been noted in several species of seals33,34,35,36,37,51, birds13,14,24,25,26,27, and terrestrial mammals52,53,54.
The energetic implications of late moult onset in parturient individuals is unclear. Because epidermal cells have a minimum temperature threshold for mitotic division55, mismatches between moult phenology and ambient conditions could lead to higher temperature differentials and consequently higher heat loss. In our study, the moult initiation date of Attendant Non-Parturient (January 06 ± 12 days) aligned with the warmest air temperatures of the year (January 03–0556); in contrast, Attendant Parturients initiated moult on average 16 days later (in up to 5 °C colder temperatures) than Attendant Non-Parturients (see Table 1). Thus, by moulting later, parturient individuals may experience increased moult costs and require additional prey resources. These costs, in addition to the high energetic costs of lactation57, may result in Parturients beginning the next reproductive cycle in poorer body condition, which could in turn lower pup weaning mass58 and diminish post-weaning survival and recruitment59. However, higher quality individuals may be able to make up for these additional costs by foraging more over the intervening winter and spring60,61.
Inter-annual variation in ice dynamics affects moult phenology
We found a significant effect of year on moult initiation dates, with moult beginning earliest in 2013 and latest in 2016 (see Table 1, Fig. 4) in most reproductive categories. The marked inter-annual differences in moulting phenology across 2013 and 2016 was reflective of sea ice break-out phenology: in 2016, the McMurdo Sound ice break-out occurred 21 days later62 and the moult occurred 10–15 days later than in 2013. Limited pack ice retreat has been found to stunt and delay the annual phytoplankton bloom63 which would impact the food resources of Weddell seals64. Low resource availability and consequently poor body condition may delay moult via increased cortisol levels and suppressed thyroid hormones28,65 as has been found in birds8.
Cross-year carryover effects between moulting and pupping
While there were links between Year 1 moult and Year 2 pupping, our data suggest Year 2 reproductive success is driven primarily by Year 1 pupping success and phenology rather than Year 1 moulting phenology (Tables S2, S3). In general, parous seals were likely to remain in the same pupping categories across Years 1 and 2 (Table S3); however, we found that Attendant Late-Parturients were more likely than expected to become Non-Attendant the following year. It is common for sexually mature birds and mammals to intermittently skip reproduction66 because it takes individuals more than one calendar year to acquire the capital needed for future reproduction67. In support of this mechanism, non-breeding individuals are often in poorer quality due to stress, starvation, diseases, or parasites68. We suggest that energetic constraints may be responsible for the increased probability of Attendant Late-Parturients becoming Non-Attendant: individuals with lower energy reserves are commensurately less likely to attend breeding colonies69. Similar effects have been seen in other species. In red voles, for example, females that successfully reproduce and consequently moult later have lower overwinter survival due to delayed winter preparation53,70.
We found no effect of sex, reproductive category, or year on moult duration, which we estimated to be 29.2 ± 7.7 (mean ± SD) days in Weddell seals. This moult duration is similar to non-catastrophic moult durations in other phocid seals and notably shorter than those of fur seals and sea lions (Family Otariidae; Table S5). Recent evidence suggests that Weddell seal life history events fill nearly an entire year, with embryonic diapause being very short or non-existent71, gestation lasting 10 months72, visible moult lasting 29 days (this study), and lactation lasting 45 days73; however, some Weddell seals produce pups in many sequential years74 so a >365 day life history cycle is unlikely, at least for the best performing individuals.
In our study, Non-Attendant individuals were 10% more likely than expected to remain Non-Attendant (Table S3), although 76% of individuals returned to breeding colonies the subsequent year. Temporary emigration can reduce conspecific conflict and food competition but precludes breeding opportunities and may increase predation risk because there is more open water access for predators75. We found that the probability of colony attendance fluctuated across years, with more Non-Attendants and less Attendant Parturients following years of early (2015) or late (2016) ice break-out relative to years with more typical ice break-out phenology (Table 1). In juvenile Weddell seals, increased sea ice extent has been found to result in more frequent emigration, probably because higher sea ice extent corresponds to lower primary production and presumed lower foraging success69, which in turn lowers the number of individuals able to reach the body condition threshold necessary for attending colonies76. Another explanation is a shift in age structure following highly productive years77 to a higher frequency of older females, which are more likely to become Non-Parturient78 and Non-Attendant39. In our study, inter-annual variation in colony attendance is likely an interaction between shifts in population age structure and fluctuating environmental conditions.
Implications of phenology disruptions
Phenological disruptions are increasingly likely under predicted global change scenarios79 and have already been documented in several species. For instance, breeding phenology advancement has been associated with spring temperature increases80,81,82,83. Phenology disruptions may carry-over to other life history events or other years84, and have larger impacts on population health than predicted if treated in isolation. These carry-over effects are particularly concerning in high-latitude environments that have stronger selection pressures85,86. Furthermore, species may differ in their phenological plasticity, which can lead to mismatches between interacting species such as predators and prey.
To fully understand the ecological impacts of changing environments, researchers must first characterize the full annual cycle of life history events and how they interact with each other physiologically. Unlike Weddell seal birth phenology that is consistent across years and individuals (range = 37 days, SD = 7 days,74,87), we found that moult timing is much more flexible (range = 75 days, SD = 14 days) without compromising reproductive success or altering future reproductive phenology in subsequent years. Thus, our data provide encouraging evidence that Weddell seals have some inherent phenological flexibility within the annual cycle with which to respond to fluctuating environmental conditions.
Data Availability
Data are available at: http://www.usap-dc.org/view/dataset/601131.
References
Barbraud, C. & Weimerskirch, H. Antarctic birds breed later in response to climate change. Proceedings of the National Academy of Sciences 103, 6248–6251 (2006).
Gordo, O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Climate Research 35, 37–58 (2007).
Morrison, C. A. et al. Flexibility in the timing of post‐breeding moult in passerines in the UK. Ibis 157, 340–350 (2015).
Harrison, X. A., Blount, J. D., Inger, R., Norris, D. R. & Bearhop, S. Carry‐over effects as drivers of fitness differences in animals. Journal of Animal Ecology 80, 4–18 (2011).
Watanuki, Y., Ito, M., Deguchi, T. & Minobe, S. Climate-forced seasonal mismatch between the hatching of rhinoceros auklets and the availability of anchovy. Marine Ecology Progress Series 393, 259–271 (2009).
Miller-Rushing, A. J., Høye, T. T., Inouye, D. W. & Post, E. The effects of phenological mismatches on demography. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365, 3177–3186 (2010).
Hipfner, J. M. Matches and mismatches: ocean climate, prey phenology and breeding success in a zooplanktivorous seabird. Marine Ecology Progress Series 368, 295–304 (2008).
Danner, R. M., Greenberg, R. S., Danner, J. E. & Walters, J. R. Winter food limits timing of pre‐alternate moult in a short‐distance migratory bird. Functional Ecology 29, 259–267 (2015).
Hemborg, C. & Lundberg, A. Costs of overlapping reproduction and moult in passerine birds: an experiment with the pied flycatcher. Behavioral Ecology and Sociobiology 43, 19–23 (1998).
Payne, R. B., Farner, D. & King, J. Mechanisms and control of molt. Avian Biology 2, 103–155 (1972).
Carey, C. The impacts of climate change on the annual cycles of birds. Philosophical Transactions of the Royal Society of London B: Biological Sciences 364, 3321–3330 (2009).
Gaston, A. J., Gilchrist, H. G., Mallory, M. L. & Smith, P. A. Changes in seasonal events, peak food availability, and consequent breeding adjustment in a marine bird: a case of progressive mismatching. The Condor 111, 111–119 (2009).
Pitelka, F. A. Timing of molt in Steller Jays of the Queen Charlotte Islands, British Columbia. The Condor 60, 38–49 (1958).
Dietz, M. W., Rogers, K. G. & Piersma, T. When the seasons don’t fit: speedy molt as a routine carry-over cost of reproduction. PLoS One 8, e53890 (2013).
Helm, B. & Gwinner, E. Timing of molt as a buffer in the avian annual cycle. Current Zoology 52, 703–706 (2006).
Ling, J. Adaptive functions of vertebrate molting cycles. American Zoologist, 77–93 (1972).
McNamara, J. M. & Houston, A. I. Optimal annual routines: behaviour in the context of physiology and ecology. Philosophical Transactions of the Royal Society of London B: Biological Sciences 363, 301–319 (2008).
Dawson, A., Hinsley, S., Ferns, P., Bonser, R. C. & Eccleston, L. Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. Proceedings of the Royal Society of London B: Biological Sciences 267, 2093–2098 (2000).
Pistorius, P. A., Bester, M. N., Hofmeyr, G. J., Kirkman, S. P. & Taylor, F. E. Seasonal survival and the relative cost of first reproduction in adult female southern elephant seals. Journal of Mammalogy 89, 567–574 (2008).
Forcada, J., Trathan, P. N. & Murphy, E. J. Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Global Change Biology, https://doi.org/10.1111/j.1365-2486.2008.01678.x (2008).
Fraser, W. R. & Hofmann, E. E. A predator’s perspective on causal links between climate change, physical forcing and ecosystem. Marine Ecology Progress Series 265, 1–15 (2003).
Marra, P. P., Cohen, E. B., Loss, S. R., Rutter, J. E. & Tonra, C. M. A call for full annual cycle research in animal ecology. Biology Letters 11, 20150552 (2015).
Newton, I. & Rothery, P. The timing, duration and pattern of moult and its relationship to breeding in a population of the European greenfinch Carduelis chloris. Ibis 147, 667–679 (2005).
Newton, I. Moult and plumage. Ringing & Migration 24, 220–226 (2009).
Morton, G. A. & Morton, M. L. Dynamics of postnuptial molt in free-living mountain white-crowned sparrows. Condor, 813–828 (1990).
Newton, I. The moult of the Bullfinch Pyrrhula pyrrhula. Ibis 108, 41–67 (1966).
Flinks, H., Helm, B. & Rothery, P. Plasticity of moult and breeding schedules in migratory European Stonechats Saxicola rubicola. Ibis 150, 687–697 (2008).
Romero, L. M., Strochlic, D. & Wingfield, J. C. Corticosterone inhibits feather growth: potential mechanism explaining seasonal down regulation of corticosterone during molt. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 142, 65–73 (2005).
Stutz, S. S. Pelage patterns and population distributions in the Pacific harbour seal (Phoca vitulina richardi). Journal of the Fisheries Board of Canada 24, 451–455 (1967).
Ling, J. K. Pelage and molting in wild mammals with special reference to aquatic forms. Quarterly Review of Biology, 16–54 (1970).
Green, K., Burton, H. R. & Watts, D. J. Studies of the Weddell seal in the Vestfold Hills, east Antarctica (Australian Antarctic Division 1995).
Daniel, R. G., Jemison, L. A., Pendleton, G. W. & Crowley, S. M. Molting phenology of harbor seals on Tugidak Island, Alaska. Marine Mammal Science 19, 128–140 (2003).
Boily, P. Metabolic and hormonal changes during the molt of captive gray seals (Halichoerus grypus). American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 270, 1051–1058 (1996).
Kirkman, S. et al. Variation in the timing of moult in southern elephant seals at Marion Island. South African Journal of Wildlife Research 33, 79–84 (2003).
Badosa, E., Pastor, T., Gazo, M. & Aguilar, A. Moult in the Mediterranean monk seal from Cap Blanc, western Sahara. African Zoology 41, 183–192 (2006).
Johanos, T. C., Becker, B. L. & Ragen, T. J. Annual reproductive cycle of the female Hawaiian monk seal (Monachus schauinslandi). Marine Mammal Science 10, 13–30 (1994).
Moss, J. Environmental and biological factors that influence harbor seal (Phoca vitulina richardsi) haulout behavior in Washington and their consequences for the design of population surveys, University of Washington (1992).
Rotella, J. J., Link, W. A., Chambert, T., Stauffer, G. E. & Garrott, R. A. Evaluating the demographic buffering hypothesis with vital rates estimated for Weddell seals from 30 years of mark-recapture data. Journal of Animal Ecology 81, 162–173, https://doi.org/10.1111/j.1365-2656.2011.01902.x (2012).
Cameron, M. & Siniff, D. Age-specific survival, abundance, and immigration rates of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica. Canadian Journal of Zoology 82, 601–615, https://doi.org/10.1139/z04-025 (2004).
Chambert, T., Rotella, J. J., Higgs, M. D. & Garrott, R. A. Individual heterogeneity in reproductive rates and cost of reproduction in a long-lived vertebrate. Ecology and Evolution 3, 2047–2060, https://doi.org/10.1002/ece3.615 (2013).
Ainley, D. et al. Antarctic mesopelagic micronekton: evidence from seabirds that pack ice affects community structure. Science 232, 847–850 (1986).
Beltran, R. S. Bridging the gap between pupping and molting phenology: behavioral and ecological drivers in Weddell seals PhD thesis, University of Alaska Fairbanks (2018).
Erni, B., Bonnevie, B. T., Oschadleus, H.-D., Altwegg, R. & Underhill, L. G. Moult: an r package to analyse moult in birds. Journal of Statistical Software 52, 1–23 (2013).
Akaike, H. In Selected Papers of Hirotugu Akaike 199–213 (Springer 1998).
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical information-theoretic approach (Springer Science & Business Media 2003).
Hochberg, Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75, 800–802 (1988).
Thompson, P. & Rothery, P. Age and sex differences in the timing of moult in the common seal, Phoca vitulina. Journal of Zoology 212, 597–603 (1987).
Raeside, J. & Ronald, K. Plasma concentrations of oestrone, progesterone and corticosteroids during late pregnancy and after parturition in the harbour seal, Phoca vitulina. Journal of Reproduction and Fertility 61, 135–139 (1981).
Boyd, I. Changes in plasma progesterone and prolactin concentrations during the annual cycle and the role of prolactin in the maintenance of lactation and luteal development in the Antarctic fur seal (Arctocephalus gazella). Journal of Reproduction and Fertility 91, 637–647 (1991).
Bartsh, S. S., Johnston, S. D. & Siniff, D. B. Territorial behavior and breeding frequency of male Weddell seals (Leptonychotes weddelli) in relation to age, size, and concentrations of serum testosterone and cortisol. Canadian Journal of Zoology 70, 680–692 (1992).
Postma, M., Bester, M. N. & De Bruyn, P. N. Spatial variation in female southern elephant seal mass change assessed by an accurate non-invasive photogrammetry method. Antarctic Science 25, 731–740 (2013).
Nowak, K. et al. Molt phenology in the mountain goat (U.S. National Park Service, Y2Y Conservation Initiative, Wildlife Conservation Society, and The Safina Center 2018).
Ylönen, H. Vole cycles and antipredatory behaviour. Trends in Ecology & Evolution 9, 426–430 (1994).
Déry, F., Hamel, S. & Côté, S. D. Getting ready for the winter: Timing and determinants of molt in an alpine ungulate. Ecology and Evolution (2019).
Feltz, E. T. & Fay, F. H. Thermal requirements in vitro of epidermal cells from seals. Cryobiology 3, 261–264 (1966).
Kanamitsu, M. et al. NCEP–DOE AMIP-II Reanalysis (R-2). Bulletin of the American Meteorological Society 83, 1631–1643, https://doi.org/10.1175/bams-83-11-1631 (2002).
Wheatley, K. E., Bradshaw, C. J., Davis, L. S., Harcourt, R. G. & Hindell, M. A. Influence of maternal mass and condition on energy transfer in Weddell seals. Journal of Animal Ecology 75, 724–733, https://doi.org/10.1111/j.1365-2656.2006.01093.x (2006).
Oosthuizen, W. C., Bester, M. N., Altwegg, R., McIntyre, T. & de Bruyn, P. N. Decomposing the variance in southern elephant seal weaning mass: partitioning environmental signals and maternal effects. Ecosphere 6, 1–22 (2015).
Oosthuizen, W. C., Altwegg, R., Nevoux, M., Bester, M. & de Bruyn, P. N. Phenotypic selection and covariation in the life‐history traits of elephant seals: heavier offspring gain a double selective advantage. Oikos 127, 875–889 (2018).
Johns, M. E. et al. Increased reproductive investment associated with greater survival and longevity in Cassin’s auklets. Proceedings of the Royal Society of London B: Biological Sciences 285, 20181464 (2018).
Shero, M. R. et al. Temporal changes in Weddell seal dive behavior over winter: Are females increasing foraging effort to support gestation? Ecology and Evolution 8(23), 11857–11874 (2018).
Comiso, J. (NASA National Snow and Ice Data Center Distributed Active Archive Center, Boulder, Colorado USA 2017).
Arrigo, K. R., Worthen, D., Schnell, A. & Lizotte, M. P. Primary production in Southern Ocean waters. Journal of Geophysical Research: Oceans 103, 15587–15600 (1998).
Seibel, B. A. & Dierssen, H. M. Cascading trophic impacts of reduced biomass in the Ross Sea, Antarctica: Just the tip of the iceberg? The Biological Bulletin 205, 93–97 (2003).
St. Aubin, D. & Geraci, J. Capture and handling stress suppresses circulating levels of thyroxine (T4) and triiodothyronine (T3) in beluga whales Delphinapterus leucas. Physiological Zoology 61, 170–175 (1988).
Baron, J. P., Galliard, J. F., Ferrière, R. & Tully, T. Intermittent breeding and the dynamics of resource allocation to reproduction, growth and survival. Functional Ecology 27, 173–183 (2013).
Shaw, A. K. & Levin, S. A. The evolution of intermittent breeding. Journal of Mathematical Biology 66, 685–703 (2013).
Bradley, J., Wooller, R. & Skira, I. Intermittent breeding in the short‐tailed shearwater Puffinus tenuirostris. Journal of Animal Ecology 69, 639–650 (2000).
Stauffer, G. E., Rotella, J. J. & Garrott, R. A. Variability in temporary emigration rates of individually marked female Weddell seals prior to first reproduction. Oecologia 172, 129–140, https://doi.org/10.1007/s00442-012-2472-z (2013).
Viitala, J. The red vole, Clethrionomys rutilus (Pall.), as a subordinate member of the rodent community at Kilpisjaervi, Finnish Lapland. Acta Zoologica Fennica (1984).
Shero, M. R., Adams, G. P. & Burns, J. M. Field use of ultrasonography to characterize the reproductive tract and early pregnancy in a phocid, the weddell seal (Leptonychotes weddellii). The Anatomical Record 298, 1970–1977 (2015).
Shero, M., Adams, G., McCorkell, R., Kirkham, A. & Burns, J. In Society for Marine Mammalogy (Canada) 2017).
Tedman, R. & Green, B. Water and sodium fluxes and lactational energetics in suckling pups of Weddell seals (Leptonychotes weddellii). Journal of Zoology 212, 29–42 (1987).
Rotella, J. J., Paterson, J. T. & Garrott, R. A. Birth dates vary with fixed and dynamic maternal features, offspring sex, and extreme climatic events in a high‐latitude marine mammal. Ecology and Evolution 6, 1930–1941 (2016).
Siniff, D., DeMaster, D., Hofman, R. & Eberhardt, L. An analysis of the dynamics of a Weddell seal population. Ecological Monographs 47, 319–335 (1977).
Laws, R. M. Growth and sexual maturity in aquatic mammals. Nature 178, 193–194 (1956).
Chambert, T., Rotella, J. J. & Garrott, R. A. Environmental extremes versus ecological extremes: impact of a massive iceberg on the population dynamics of a high-level Antarctic marine predator. Proceedings of the Royal Society of London B: Biological Sciences 279, 4532–4541 (2012).
Proffitt, K. M., Garrott, R. A., Rotella, J. J. & Wheatley, K. E. Environmental and senescent related variations in Weddell seal body mass: implications for age‐specific reproductive performance. Oikos 116, 1683–1690 (2007).
Vegvari, Z., Bokony, V., Barta, Z. & Kovacs, G. Life history predicts advancement of avian spring migration in response to climate change. Global Change Biology 16, 1–11 (2010).
Crick, H. Q., Dudley, C., Glue, D. E. & Thomson, D. L. UK birds are laying eggs earlier. Nature 388, 526–526 (1997).
Both, C., Bouwhuis, S., Lessells, C. & Visser, M. E. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (2006).
Both, C. & Visser, M. E. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (2001).
Lane, J. E. et al. Phenological shifts in North American red squirrels: disentangling the roles of phenotypic plasticity and microevolution. Journal of evolutionary biology (2018).
Fayet, A. L. et al. Carry‐over effects on the annual cycle of a migratory seabird: an experimental study. Journal of Animal Ecology 85, 1516–1527 (2016).
Fretwell, S. D. Populations in a seasonal environment (Princeton University Press 1972).
Reed, T. E., Schindler, D. E. & Waples, R. S. Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conservation Biology 25, 56–63 (2011).
Proffitt, K. M., Rotella, J. J. & Garrott, R. A. Effects of pup age, maternal age, and birth date on pre‐weaning survival rates of Weddell seals in Erebus Bay, Antarctica. Oikos 119, 1255–1264 (2010).
Chambert, T., Rotella, J. J. & Garrott, R. A. Female Weddell seals show flexible strategies of colony attendance related to varying environmental conditions. Ecology 96, 479–488 (2015).
Acknowledgements
We thank the B-009 team members, especially Robert Garrott, Terrill Paterson and Jay Rotella for contributing information on many birth dates and on female ages and reproductive states, as it was crucial to our paper. We also thank B-292 field members Michelle Shero, Rachel Berngartt, Linnea Pearson, Patrick Robinson, Skyla Walcott, Gregg Adams, and Rob McCorkell for data collection; Marm Kilpatrick and Tim Tinker for statistical advice; and Matt Cameron, Rachel Holser, and Joe Eisaguirre for helpful comments on previous drafts. This project was made possible by logistical support from the United States National Science Foundation (NSF) through the U.S. Antarctic Program, Lockheed Martin Antarctic Support Contract, and support staff in Christchurch, New Zealand and McMurdo Station. Research was conducted with financial support from NSF grant ANT-1246463 to JMB and JWT and an NSF graduate research fellowship to RSB (Grant No. 2015174455). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program (RSB) and while serving at the National Science Foundation (JMB). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
R.S.B., J.W.T., and J.M.B. conceived the ideas and designed methodology; R.S.B., A.L.K., and J.M.B. collected the data; R.S.B., J.W.T., A.L.K., G.A.B., and J.M.B. analysed the data; R.S.B. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beltran, R.S., Kirkham, A.L., Breed, G.A. et al. Reproductive success delays moult phenology in a polar mammal. Sci Rep 9, 5221 (2019). https://doi.org/10.1038/s41598-019-41635-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41635-x
This article is cited by
-
Temporal variation in the summer diet of the weddell seal, leptonychotes weddellii, at hope bay, antarctic peninsula
Polar Biology (2023)
-
A mowing strategy for urban parks to support spring flowers in a mediterranean climate city in South Africa
Urban Ecosystems (2023)
-
Iron mobilization during lactation reduces oxygen stores in a diving mammal
Nature Communications (2022)
-
Determinants of moult haulout phenology and duration in southern elephant seals
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.