Abstract
2-Cl-C.OXT-A (COA-Cl) is a novel nucleic acid analogue that promotes tube-forming activity of human umbilical vein endothelial cells (HUVEC) through vascular endothelial growth factor (VEGF). The development of coronary collateral circulation is critical to rescue the ischemic myocardium and to prevent subsequent irreversible ischemic injury. We evaluated whether COA-Cl can promote angiogenesis in ischemic tissue, reduce infarct size and preserve cardiac contractility in vivo. Mice received COA-Cl or placebo daily for three days after myocardial infarction (MI) by coronary ligation. The degree of angiogenesis in ischemic myocardium was assessed by staining endothelial cells and vascular smooth muscle cells, and measuring infarct size/area-at-risk. In mice treated with COA-Cl, enhanced angiogenesis and smaller infarct size were recognized, even given a similar area at risk. We observed increases in the protein expression levels of VEGF and in the protein phosphorylation level of eNOS. In addition, the heart weight to body weight ratio and myocardial fibrosis in COA-Cl mice were decreased on Day 7. Administration of COA-Cl after MI promotes angiogenesis, which is associated with reduced infarct size and attenuated cardiac remodeling. This may help to prevent heart failure due to cardiac dysfunction after MI.
Similar content being viewed by others
Introduction
It has been recognized that collateral arteries tend to develop in ischemic vascular disease. Blood flow is increased either by angiogenesis, defined as the sprouting of new capillaries, or by the recruitment of pre-existing coronary collateral vessels. The development of collateral coronary circulation in the ischemic myocardium can salvage it from irreversible myocardial injury1. Therefore, it is known that coronary angiogenesis in the ischemic heart provides myocardial protective effects, potentially by reducing infarct size, subsequent myocardial dysfunction and incidence of arrhythmias. Angiogenesis is regulated by mechanical, chemical, and molecular factors2. Various peptide growth factors, including vascular endothelial growth factor (VEGF)3, hepatocyte growth factor4, and fibroblast growth factor (FGF)5 have been identified in cardiac angiogenesis due to myocardial ischemia2,6.
2-Cl-C.OXT-A (COA-Cl) was developed as a novel nucleic acid analogue that may possess the property of angiogenesis. Results of a previous study showed that the angiogenic activity of COA-Cl might confer clinical therapeutic value to COA-Cl as a novel angiogenic drug for ischemic stroke7. Our present study aimed to evaluate the angiogenic effect of COA-Cl after myocardial infarction (MI) in vivo, because expansion of collateral artery circulation in the ischemic myocardium leads to increased myocardial perfusion and eventual improvements in ventricular function.
Results
Reduced myocardial infarct size and remodeling in mice treated with COA-Cl
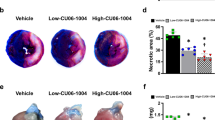
After MI, COA-Cl or saline was administered for three days. On Day 3 after MI, infarct size (IS) was reduced significantly in the group treated with COA-Cl (COA-Cl group) compared with the group treated with saline (saline group) (6.6 ± 0.6% versus 13.7 ± 1.6%, respectively; P < 0.01), and the area at risk (AAR), the ischemic area by LAD ligation, also tended to decrease in the COA-Cl group (38.6 ± 3.8% versus 48.4 ± 4.9%, respectively; P = 0.15). However, the IS/AAR ratio was smaller in the COA-Cl group than in the saline group (17.6 ± 1.6% versus 28.4 ± 2.2%, respectively; P < 0.01; Fig. 1a). We evaluated the effect of COA-Cl on cardiac dysfunction 7 days after MI with transthoracic echocardiography. The reduction of % fractional shortening (FS) and the dilation of the left ventricular dimension in the COA-Cl group were not as remarkable as those of the saline group. COA-Cl suppressed the dilation of the left ventricular dimension and decreased systolic function. Moreover, the heart weight (HW) to body weight (BW) ratio decreased significantly in the COA-Cl group (Table 1).
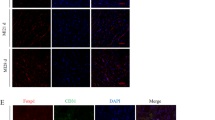
(a) Myocardial infarct size and area at risk on Day 3. The blue area is perfused tissue, the red and white area is the area at risk (AAR), and the white area is infarcted tissue (IS). The graphs show the IS as a percentage of left ventricular (LV) area (IS/LV), AAR as a percentage of LV area (AAR/LV), and IS as a percentage of AAR (IS/AAR). Saline group (n = 5) versus COA-Cl group (n = 5) *Significant difference by Student’s t-test at P < 0.05; (b) Immunofluorescent staining of α-smooth muscle actin (α-SMA) and lectin. Sections of hearts from the saline group (n = 5) versus the COA-Cl group (n = 5) 3 days after MI. Scale bar corresponds to 50 μm; (c) Vascular density in the border area was measured by quantitation of lectin staining. *Significant difference by Student’s t-test at P < 0.05. (d) The numbers of capillary vessels and arterioles (n = 4). *p < 0.05, **p < 0.01 vs saline group. (e) Immunofluorescent staining of CD31(upper panel) and VEGF (lower panel). Section of hearts from each group (n = 4) on day 7 after MI. Scale bar corresponds to 100 μm.
Preserved capillary density after myocardial infarction
We visualized the intramyocardial arteries and microvasculature 3 days after MI. Vasculardensity including capillary and arterioles in the heart was estimated with lectin specialized to bind to specific oligosaccharide side chains of vascular endothelial cells and α-smooth muscle actin antibody to bind to vascular smooth muscle cells. We quantified the blood vessel area stained by lectin in both the ischemic area and the border zone (Fig. 1b). The number of myocardial vessels including capillaries and arterioles in the COA-Cl group was significantly greater compared with that in the saline group (Fig. 1c–e), which proved to promote angiogenesis by COA-Cl in the ischemic area.
Protein expression after treatment with COA-Cl
VEGF expression in the COA-Cl group was increased significantly compared to that in the saline group, as documented by Western blot analysis (P = 0.04; Fig. 2a). Furthermore, eNOS expression was not different between the saline group and the COA-Cl group. In contrast, the expression of phosphor-eNOS and phosphor-eNOS/eNOS was increased in the COA-Cl group (Fig. 2d–f; P = 0.78, 0.03, and 0.03, respectively). The degree of eNOS phosphorylation was higher in the COA-Cl group than in the saline group, although the amount of total eNOS protein expression was not. MMP-9 tended to be expressed more in the COA-Cl group (P = 0.07; Fig. 2b). Previous studies indicated that these factors also have a role in the progression of angiogenesis8,9. iNOS was induced by the inflammatory response after myocardial damage10, which was less in the COA-Cl group (P < 0.01; Fig. 2c). This finding suggests that collateral arteries in the ischemic region increased blood flow, which suppressed necrosis of myocardial tissue and the subsequent inflammatory response. Moreover, there was no significant difference in the expression of cleaved caspase-3 (CCP3) between the groups (P = 0.392; Fig. 2g), however, COA-Cl tended to reduce the expression of nitrotyrosine (NTS) by 28% (P = 0.302; Fig. 2h) and the level of TBARS was significantly reduced (P < 0.05; Fig. 2i). On Day 7 after MI, myocardial interstitial fibrosis was significantly less in the COA-Cl group than in the saline group. (6.8 ± 1.3 vs. 11.1 ± 0.7, respectively; P = 0.02; Fig. 3) These results indicated that COA-Cl reduced oxidative stress, and infarct area by enhancing blood vessel formation after myocardial infarction (MI), and prevented subsequent cardiac dysfunction.
(a) Vascular endothelial growth factor (VEGF); (b) Matrix metalloproteinase (MMP)−9; (c) Inducible nitric oxide synthase (iNOS); (d) Endothelial nitric oxide synthase (eNOS); (e) phosphor-eNOS; (f) phosoho-eNOS/eNOS; (g) Cleaved caspase-3 (CCP3); (h) Nitrotyrosine (NTS) in heart measured by Western blot analysis. (I) Myocardial TBARS assay. Saline group (n = 8) versus COA-Cl group (n = 8). β-actin was used as a control (45 kDa: lower band above the graph). *Significant difference by Student’s t-test at P < 0.05.
(a) Immunohistochemical staining of hearts in the saline group (n = 5) and the COA-Cl group (n = 5) on day 7. Both sections were stained by Sirius red stains. Scale bar corresponds to 300 μm; (b) fibrosis was measured by quantitation with Sirius red staining. *Significant difference by Student’s t-test at P < 0.05.
Discussion
In this study, we demonstrated that COA-Cl reduced infarct area and developed myocardial collateral vessels with activating angiogenic molecules such as VEGF, eNOS, and MMP-9 in murine MI model which leads to suppressing inappropriate cardiac remodeling. These results suggested that the administration of COA-Cl resulted in suppression of cardiac damage and subsequent cardiac remodeling due to strong angiogenic activation.
Clinically the development of collaterals in the ischemic myocardium is critical to reduce jeopatized ischemic tissue and prevent LV dysfunction resulting in heart failure., which is caused by angiogenic factors including hypoxia, inflammation, hemodynamics, and shear stress2. Therefore, along with the immediate implementation of coronary angioplasty to salvage the ischemic myocardium, it is desirable to establish a method of treatment aimed at protecting the ischemic myocardium by forming sufficient collateral circulation and attenuating the ischemic damage.
Previously we showed that COA-Cl induced a strong angiogenic response in cultured human umbilical vein endothelial cells (HUVEC) that are co-cultured with fibroblasts, as well as in chicken chorioallantoic membrane and rabbit corneal matrigel implant models8, and COA-Cl promoted the phosphorylation and activation of the mitogen-activated protein kinase (MAPK) cascade, which caused angiogenesis involving the G-protein coupled sphingosine 1-phosphate receptor 1 (S1P1), which can modulate angiogenesis, in the tube-forming activity of HUVECs8,9. As serum-borne lysophospholipid sphingosine 1-phosphate (S1P), endogenous ligand for S1P1, is produced in various cell types including vascular endothelial cells10,11, S1P-S1P1 pathway acts to regulate vascular maturation as a vascular intrinsic stabilization mechanism12, which converts extracellular stimulation by COA-Cl into intracellular signaling and then leads to the development of angiogenesis9.
COA-Cl also induces the expression and secretion of VEGF in the presence of normal human dermal fibroblasts, which is caused by the expression of a transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator (PGC)−1α in concert with ERRα13. VEGF has a high angiogenic potential that regulates angiogenesis and vasculogenesis, as well as vascular maintenance. In addition, it is the strongest inducer of vascular permeability due to efficient simultaneous nitric oxide (NO) and prostacyclin production14,15, which is the necessary step for angiogenesis by providing endothelial cell proliferation and migration16. Therefore, COA-Cl may not only prompt angiogenesis in the process of sprouting vascular endothelial cells initially by the induction of VEGF, but also stimulate mature endothelial tube formation gradually via the S1P-S1P1 pathway. We also confirmed that COA-Cl induces VEGF expression and secretion in fibroblasts from adjacent fibroblasts, which is promoted by expression not of HIF1α but of PGC-1α17. Moreover, COA-Cl may also cause a stronger angiogenic response by activating the S1P-S1P1 system in endothelial cells.
In this study, VEGF and eNOS were activated by COA-Cl after MI, but iNOS expression induced by inflammatory cytokines was decreased, despite the fact that VEGF induces eNOS and iNOS expression in vascular endothelial cells18. This might be because COA-Cl promoted angiogenesis due to the effect of VEGF and eNOS, and then enhanced blood perfusion that attenuated inflammation after MI, leading to declining iNOS activity. In post-infarct myocardium, angiogenic growth factors and inflammatory cytokines induce matrix metalloproteinase (MMP)19, which has a beneficial role in proper wound healing in early stages of cardiac remodeling, thus contributing to tissue replacement and scar formation20. In addition, it was shown that MMP-9 has a significant role in neovascularization through the proteolytic degradation of the basal lamina in the blood vessels and release of activated VEGF21. The results of the present study showed that MMP-9 expression was greater with COA-Cl, which might be caused by the degradation of the extracellular matrix with subsequent activation of major proangiogenic factors, such as VEGF in vasculogenesis.
Certainly, VEGF is one of the major angiogenic factors and is produced early in the angiogenic cascade and is associated significantly with the initial activation of endothelial cells22 to proliferate and result in sprouting in angiogenesis. However, the administration of VEGF for ischemic heart disease was not sufficient to induce angiogenesis in a clinical study23. Despite the fact that angiogenic factors, including VEGF, would have potential clinical therapeutic value, they are unstable and difficult to synthesize chemically and biologically, in addition to being expensive. On the other hand, COA-Cl is a Xeno-free novel adenosine-like nucleic acid analogue, and it is stable and easy to synthesize. Therefore, COA-Cl may have potential benefits as a novel treatment for increasing collateral circulation for ischemic diseases including coronary artery disease, stroke, and peripheral artery disease. However, there is a possibility to increase nutrient blood vessels for tumor etc. and to advance cancer extension, so further research including safety is required. Moreover, clinical studies of therapeutic angiogenesis with COA-Cl will be required to demonstrate long-term clinical benefits on the morbidity and mortality of ischemic disease as well as pharmaceutical toxicity. In addition, it is hard to deny other cardioprotective possibilities of COA-Cl through unknow mechanisms beyond neovascularization. The results of future basic and clinical researches on COA-Cl will be awaited.
In summary, COA-Cl promotes the development of collateral artery circulation after MI, which leads to reduced infarct size and cardiac remodeling. COA-Cl has potential as a novel therapeutic agent for ischemic heart disease; therefore, we expect further studies of the pharmacological effects of COA-Cl to improve clinical outcomes.
Materials and Experimental Procedures
Experimental animals and experimental design
C57 BL/6 mice purchased from Kyudo Co., Ltd. (Tosu, Japan) were housed in a specific pathogen-free environment. Healthy adult males were aged 12 to 16 weeks and weighed 20 to 25 grams. All experiments were performed in accordance with the “Position of the American Heart Association on Research Animal Use” and approved by the Institutional Animal Care and Use Committee at Saga University.
Surgery to induce myocardial infarction
Mice were anesthetized with ketamine (100 ml/kg) + xylazine (10 mg/kg) and intubated for mechanical ventilation. After left thoracotomy to expose the heart, MI was achieved by ligation of the left anterior descending coronary artery with an 8-0 silk suture. After closure of the chest wall and extubation, the hearts were harvested 3 or 7 days later. Mice were randomly assigned to two groups: 12 mg/kg COA-Cl (COA-Cl group)24 or saline (saline group) were administered intraperitoneally right after MI for 3 days. We confirmed that there are no significant differences in heart weight and data about hemodynamics using COA-Cl among sham-operated mice.
Measurement of infarct size
After internal carotid artery cannulation, 1% Evans blue dye was infused into the aorta and coronary artery to assess the area at risk. The hearts were sliced transversely into 2-mm thick sections and incubated with 1% triphenyltetrazolium chloride solution (TTC) (Sigma Aldrich Chemical, Buchs, Switzerland) at 37 °C for 15 minutes as previously described25. Each section was stained in a different color for the infarct area (IS), area-at-risk (AAR), and viable myocardium, and each color was measured with Image J software.
Echocardiographic analysis
Echocardiographic analysis was performed with an echocardiographic machine with a 15-MHz transducer (Toshiba, Japan) at 7 days after MI, as described previously26. From 2-dimensional short-axis imaging with the M-mode, left ventricular end-diastolic diameter (LVEDD) and end-systolic diameters (LVESD) were measured, and fractional shortening was calculated as {(LVEDD-LVESD)/LVEDD × 100 (%FS) as described previously26. The left ventricular ejection fraction (LVEF) was calculated using the formula EF% = [(LVEDV − LVESV)/LVEDV] × 100; where LVEDV and LVESV are left ventricular end-diastolic volume and left ventricular end-systolic volume, respectively.
Histological analysis
Samples were taken from the cardiac tissue around the infarct regions and fixed in 4% formalin for 24 hours, dehydrated in 70% ethyl alcohol, and embedded in paraffin before cryopexy. Left ventricular sections (5 µm) were cut and stained with hematoxylin-eosin. Immunofluorescence staining for neovascularization was visualized on a Zeiss confocal microscopy system, and then blood vessel densities were compared. New capillary blood vessels, including vascular endothelium and smooth muscle, were stained with α-smooth muscle actin (α-SMA) (A2547, 1:1000) (Sigma Chemical Co. Lt. Louis, MO) and Griffonia simplicifolia Lectin I- ISOLECTIN B4 (LECTIN) (FL-1201, 1:3000) (Vector Laboratories Ltd., Peterborough, UK). In addition, collagen density due to fibrosis was assessed with Sirius red staining. For each myocardial specimen, 10 independent fields from of 5 stained sections were photographed at 200x magnification. All areas were analyzed by quantitation with Image J software.
Assay for protein expression
Heart sections were homogenized in cytoplasmic and nuclear extraction regents (Thermo scientific, Hudson, NH) containing protease inhibitors. Samples of myocardium lysate were resolved on SDS-PAGE according to a standard protocol. After protein transfer to membranes, the membranes were probed with primary antibodies followed by secondary antibodies conjugated to horseradish peroxidase, and immunoreactive bands were developed with ECL Plus Western Blotting Detection Regents (GE Healthcare, Buckinghamshire, UK). Enhanced chemiluminescence was detected with an LAS-3000 image analyzer (Fujifilm, Tokyo, Japan), and band density was quantified with Image J software. We used the following primary antibodies: inducible nitric oxide synthase (iNOS) (#6 10432, 1:1000) (BD Transduction Laboratories, Lexington, KY), endothelial nitric oxide synthase (eNOS) (#9586, 1:1000), Phospho-eNOS (Ser1177) (#9570, 1:1000), MMP-9 (#3852, 1:1000) (Cell Signaling, Danvers, MA), β-actin I-19 (sc-1616, 1:1000), VEGF (A-20) (sc-152, 1: 200) (Santa Cruz Biotechnology, INC., Santa Cruz, CA), cleaved caspase-3 (CCP3) and nitrotyrosine (N0409, 1:1000) (Sigma-Aldrich, St. Louis, MO). Myocardial TBARS was measured as previously described (Cayman Chemical, Ann Arbor, MI)26.
Statistical analysis
Data are expressed as mean ± SEM. Multiple group comparison were analyzed using one-way ANOVA with Tukey post-hoc multiple comparison. Comparisons between groups were made with a two-sample t-test assuming equal variances. A P-value less than 0.05 was deemed statistically significant. All statistical analysis was performed with the SPSS software statistics package (version. 22; IBM SPSS, Chicago, IL).
References
Murry, C. E., Jennings, R. B. & Reimer, K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74, 1124–1136 (1986).
Tabibiazar, R. & Rockson, S. G. Angiogenesis and the ischaemic heart. Eur Heart J 22, 903–918, https://doi.org/10.1053/euhj.2000.2372 (2001).
Crafts, T. D., Jensen, A. R., Blocher-Smith, E. C. & Markel, T. A. Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine 71, 385–393, https://doi.org/10.1016/j.cyto.2014.08.005 (2015).
Miyagawa, S. et al. Myocardial regeneration therapy for heart failure: hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation 105, 2556–2561 (2002).
Engelmann, G. L., Dionne, C. A. & Jaye, M. C. Acidic fibroblast growth factor and heart development. Role in myocyte proliferation and capillary angiogenesis. Circ Res 72, 7–19 (1993).
Vandervelde, S., van Luyn, M. J., Tio, R. A. & Harmsen, M. C. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol 39, 363–376, https://doi.org/10.1016/j.yjmcc.2005.05.012 (2005).
Okabe, N. et al. Delayed administration of the nucleic acid analog 2Cl-C.OXT-A attenuates brain damage and enhances functional recovery after ischemic stroke. Brain Res 1506, 115–131, https://doi.org/10.1016/j.brainres.2013.02.009 (2013).
Tsukamoto, I. et al. A novel nucleic acid analogue shows strong angiogenic activity. Biochem Biophys Res Commun 399, 699–704, https://doi.org/10.1016/j.bbrc.2010.08.003 (2010).
Igarashi, J. et al. Involvement of S1P1 receptor pathway in angiogenic effects of a novel adenosine-like nucleic acid analog COA-Cl in cultured human vascular endothelial cells. Pharmacol Res Perspect 2, e00068, https://doi.org/10.1002/prp2.68 (2014).
Allende, M. L., Yamashita, T. & Proia, R. L. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102, 3665–3667, https://doi.org/10.1182/blood-2003-02-0460 (2003).
Venkataraman, K. et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102, 669–676, https://doi.org/10.1161/CIRCRESAHA.107.165845 (2008).
Gaengel, K. et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 23, 587–599, https://doi.org/10.1016/j.devcel.2012.08.005 (2012).
Jain, R. K. Molecular regulation of vessel maturation. Nat Med 9, 685–693, https://doi.org/10.1038/nm0603-685 (2003).
Parenti, A. et al. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem 273, 4220–4226 (1998).
Ziche, M. et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99, 2625–2634, https://doi.org/10.1172/JCI119451 (1997).
Fukumura, D. et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98, 2604–2609, https://doi.org/10.1073/pnas.041359198 (2001).
Igarashi, J. et al. A key role of PGC-1alpha transcriptional coactivator in production of VEGF by a novel angiogenic agent COA-Cl in cultured human fibroblasts. Physiol Rep 4, https://doi.org/10.14814/phy2.12742 (2016).
Hood, J. D., Meininger, C. J., Ziche, M. & Granger, H. J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol 274, H1054–1058 (1998).
van Hinsbergh, V. W. & Koolwijk, P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res 78, 203–212, https://doi.org/10.1093/cvr/cvm102 (2008).
van den Borne, S. W. et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 7, 30–37, https://doi.org/10.1038/nrcardio.2009.199 (2010).
Yabluchanskiy, A., Ma, Y., Iyer, R. P., Hall, M. E. & Lindsey, M. L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda) 28, 391–403, https://doi.org/10.1152/physiol.00029.2013 (2013).
Semenza, G. L. V. Angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem 102, 840–847, https://doi.org/10.1002/jcb.21523 (2007).
Henry, T. D. et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 107, 1359–1365 (2003).
Sakakibara, N. et al. Synthesis and Evaluation of Novel Carbocyclic Oxetanocin A (COA-Cl) Derivatives as Potential Tube Formation Agents. Chem Pharm Bull (Tokyo) 63, 701–709, https://doi.org/10.1248/cpb.c15-00386 (2015).
Oyama, J. et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109, 784–789 (2004).
Oyama, J. et al. Repetitive hyperthermia attenuates progression of left ventricular hypertrophy and increases telomerase activity in hypertensive rats. Am J Physiol Heart Circ Physiol. 302, H2092–2101, https://doi.org/10.1152/ajpheart.00225.2011 (2012).
Acknowledgements
We thank for Masami Haraguchi, Ruriko Imaizumi and Tomoko Yajima for technical assistance.
Author information
Authors and Affiliations
Contributions
Toshiyuki Nishikido, Aya Shiraki, and Jun-ichi Oyama performed the experiments and wrote the manuscript. Ikuko Tsukamoto and Junsuke Igarashi provided guidance on the research direction. Jun-ichi Oyama and Koichi Node supervised overall activities.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishikido, T., Oyama, Ji., Shiraki, A. et al. COA-Cl (2-Cl-C.OXT-A) can promote coronary collateral development following acute myocardial infarction in mice. Sci Rep 9, 2533 (2019). https://doi.org/10.1038/s41598-019-39222-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39222-1
This article is cited by
-
A novel soluble epoxide hydrolase vaccine protects murine cardiac muscle against myocardial infarction
Scientific Reports (2022)
-
COA-Cl Evokes Protective Responses Against H2O2-and 6-OHDA-Induced Toxic Injury in PC12 Cells
Neurotoxicity Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.