Abstract
Hormone sensitive lipase (HSL) activation is part of the metabolic adaptations to the negative energy balance common to the mammalian periparturient period. This study determined HSL contribution to adipose tissue (AT) lipolysis and how insulin regulates its activity in periparturient dairy cows. Subcutaneous AT (SCAT) samples were collected at 11 d prepartum (dry) and 11 (fresh) and 24 d (lactation) postpartum. Basal and stimulated lipolysis (ISO) responses were determined using explant cultures. HSL contribution to lipolysis was assessed using an HSL inhibitor (CAY). Basal lipolysis was higher in SCAT at dry compared with fresh. CAY inhibited basal lipolysis negligibly at dry, but at fresh and lactation it reduced basal lipolysis by 36.1 ± 4.51% and 43.1 ± 4.83%, respectively. Insulin inhibited lipolysis more pronouncedly in dry compared to fresh. Results demonstrate that HSL contribution to basal lipolysis is negligible prepartum. However, HSL is a major driver of SCAT lipolytic responses postpartum. Lower basal lipolysis postpartum suggests that reduced lipogenesis is an important contributor to fatty acid release from SCAT. Loss of adipocyte sensitivity to the antilipolytic action of insulin develops in the early lactation period and supports a state of insulin resistance in AT of cows during the first month postpartum.
Similar content being viewed by others
Introduction
Excessive release of fatty acids (FA) from adipose tissue (AT) is linked with metabolic diseases of gestation and early lactation in mammals1,2. Ketosis and fatty liver are prevalent in dairy cows with high concentrations of circulating FA throughout the periparturient period (3 wks before through 3 wks after calving)3,4. In humans and sheep, gestational diabetes mellitus or pregnancy toxemia mainly occur in the third trimester of pregnancy when circulating FA are increased considerably5,6. During the periparturient period, the net release of FA from AT into circulation is a result of reduced lipogenesis (including de novo lipogenesis and re-esterification) and enhanced lipolysis within adipocytes7,8. In humans and sheep, the majority of the FA reserves are mobilized before parturition. In contrast, seals, bears, and dairy cows mobilize these FA after parturition7. Excessive FA release during the periparturient period has been linked to alterations in lipolytic9 and lipogenic10 activity of adipocytes and changes in the response of adipocytes to regulatory hormones11. Consequently, excessive lipolysis is more common in animals with excessive fat accumulation, genetic predisposition, concurrent health disorders, inflammatory conditions, or malnutrition4,12,13,14,15. Endocrine factors regulate the FA release from AT. Catecholamines and growth hormone are the most important hormones increasing FA release while insulin is the most important hormone decreasing FA release7,16. These hormones act by stimulating or inhibiting the activity of hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) through phosphorylation by protein kinase A (PKA) or by changing the sensitivity of adipocytes to catecholamines7,17.
Currently, the exact contribution of lipolytic enzymes, lipogenic activity, and adipocyte insulin resistance to the net release of FA during the immediate periparturient period is not well established. A complete characterization of the lipolytic activity is important in species with a high incidence of gestational and periparturient diseases including humans, dairy cows, and other small ruminants. The present study aimed to determine how the lipolytic enzymes ATGL and HSL contribute to FA mobilization throughout the periparturient period using an adipose explant model in subcutaneous AT samples collected from Holstein Friesian dairy cows before and after calving. Also, the effect of insulin on lipolytic activity of AT was determined by supplementing insulin at physiological concentrations. Protein expression and activity of key enzymes (HSL and protein kinase B or AKT) in the regulation of AT metabolism was determined to identify potential altered pathways. Additionally, changes in the mRNA expression of important lipogenic, lipolytic, and glucose metabolism gene networks were determined.
Materials and Methods
Study design
All animal procedures were approved by the Michigan State University Animal Care and Use Committee. All experiments were performed in accordance with relevant guidelines and regulations. Twenty-two multiparous (3.14 ± 1.28 lactations, mean ± SD) Holstein dairy cows from the Michigan State University Dairy Teaching and Research Center (East Lansing, MI) were enrolled in this study. Cows were non-lactating and pregnant (>235 d of gestation). Body condition score (i.e. adiposity) was assessed at selection by three experienced technicians using a 5-point scale18. Cows were housed in tie-stall barns bedded with sawdust and fed a close-up diet from 3 wk before expected parturition date and a postpartum diet following parturition. All rations were formulated to meet or exceed the predicted requirements for protein, minerals, and vitamins according to NRC19. The ingredient and nutrient composition of the close-up diet and the postpartum diet are described in Supplementary Table A.
Blood samples
Blood samples were collected weekly from 3 wk before expected parturition date until 3 wk after parturition. Blood was drawn before the morning feeding via coccygeal venipuncture using uncoated serum collection tubes, centrifuged for 20 min at 3,000 × g (15 °C) for serum fraction collection and then stored at −80 °C until further analysis. Serum concentrations of insulin, free fatty acids (FFA), and β-hydroxybutyrate (BHB) were determined using an Olympus AU640e chemistry analyzer (Olympus America, Center Valley, PA, USA) at the Diagnostic Center for Population and Animal Health of Michigan State University (Lansing, MI, USA).
Adipose tissue samples
Subcutaneous AT (SCAT) samples were obtained from the right flank at 11 ± 1 d before expected parturition date (dry) and 11 ± 0.2 d (fresh) and 24 ± 0.4 d (lactation) after calving, using a surgical procedure described in detail by Mann, et al.20. Briefly, after local anesthesia (15 mL of 2% lidocaine hydrochloride 2%, VetTek, Middleburg, VA, USA) and aseptic preparation of the surgical field using iodine scrub, iodine prep, and alcohol, a vertical skin incision of 5 cm was made. Five grams of SCAT were collected. A part of the adipose sample was snap frozen in liquid nitrogen and stored at −80 °C until further analysis, a part was fixed in formaldehyde for 72 h, and a part was used for the in vitro lipolysis assay. The skin was closed using a continuous interlocking suture with Braunamid (USP1, Aesculap, Center Valley, PA, USA). Sutures were removed after 14 d.
In vitro lipolysis assay
AT lipolysis was determined using a short-term in vitro explant culture as described by De Koster, et al.9. Krebs Ringer Bicarbonate HEPES buffer (KRBH, pH 7.4) containing 3% FA-free bovine serum albumin (BSA, Millipore-Sigma, Burlington, MA, USA) was prepared fresh on the day of the experiment. Immediately after sampling, AT was placed in 20 mL KRBH supplemented with 3% BSA and minced using scissors. Samples were stored and transported to the lab at 38 °C. Adipose explants (approximately 100 mg per culture dish) were incubated in 6 well plates containing 3 mL KRBH + 3% BSA on a shaker at 38 °C. After 20 min pre-incubation, reagents were added to the culture plates as described below. All reagents were prepared fresh on the day of the experiment. Basal lipolysis was determined without addition of any reagent. Stimulated lipolysis was determined by adding (-)-isoproterenol (ISO, I6504, Millipore-Sigma) at a concentration of 10−6 M. The inhibitory effect of insulin on the stimulated lipolytic activity was determined by adding insulin and (-)-isoproterenol (10−6 M) simultaneously to the culture dishes. Insulin was added at two different concentrations: 1 µg/L and 0.2 µg/L. The effect of CAY10499 (CAY, Cayman chemical, Ann Arbor, MI, USA, #10007875) on lipolytic activity was determined after adding CAY (dissolved in DMSO) to culture plates at a concentration of 2 µM. After 1 hour of pre-incubation with CAY, 2 different conditions were tested. To determine the effect of CAY on basal lipolytic activity, no reagents were added. To assess the effect of CAY on stimulated lipolytic activity, ISO was added at a concentration of 10−6 M. After 3 h of incubation, samples of medium were taken, snap-frozen in liquid N2 and stored at −80 °C until further analysis. The weight of the explants was determined precisely on an analytical scale (Mettler Toledo, Columbus, OH, USA, MS303TS/00) and the explants were snap frozen and stored at −80 °C for further analysis.
Lipolysis responses were assessed by glycerol concentrations released in the culture medium during the 3 h assay using free glycerol reagent (Millipore-Sigma, F6428). Intra-assay CV was 5.70%; inter-assay CV was 6.95%. Results of the lipolysis assays are expressed as glycerol release per million adipocytes per 3 hours. The number of adipocytes was determined as described by DiGirolamo and Fine21 using an Olympus BX-40 microscope (Olympus America). The diameter of the adipocytes (n = 100 per sample) was determined after digestion of 1 g of AT in 3 ml KRBH + 3% BSA containing 2 mg/mL collagenase type 2 (Worthington, Lakewood, NJ, USA) using ImageJ. Mean, and standard deviation of the diameter were used to calculate the volume of the adipocytes using the formula of Goldrick22. All conditions were performed in duplicate. Statistical analysis was performed using the average glycerol concentration of the duplicates. The intra-assay CV for the in vitro lipolysis assay was 10.4%. The effect of CAY on basal and ISO stimulated glycerol release was expressed as a percentage of the basal and ISO stimulated glycerol release, respectively. The effect of ISO on glycerol release was expressed as a percentage of the basal glycerol release, to correct for differences in basal glycerol release. The effect of insulin (1 µg/L and 0.2 µg/L) was expressed as a percentage of ISO stimulated glycerol release, to correct for differences in ISO stimulated glycerol release.
RNA extraction from adipose samples
Snap-frozen SCAT samples weighing less than 200 mg were transferred into screwcap tubes containing 1 mL of TRizol reagent (Thermofisher Scientific, Waltham, MA, USA) and 2.3 mm zirconia/silica beads (Biospec, Bartlesville, OK, USA). The tubes containing tissues were placed in liquid nitrogen and then homogenized at 6,000 rpm for 3 times 30 sec using a bead mill homogenizer (Precellys, Bertin Instruments, Montigny-le-Bretonneu, France). Following homogenization, the TRizol supernatant was collected carefully, leaving the debris and beads at the bottom and avoiding the lipid layer on the top. After performing the chloroform phase separation, a commercially available kit (QIAGEN, Hilden, Germany) was used to extract total RNA according to the manufacturer’s protocol. Purity, concentration, and integrity of total RNA were evaluated using a NanoDrop 1000 spectrophotometer (Thermofisher Scientific) and an Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). All samples had a 260:280 nm ratio between 1.96 and 2.01 and RNA integrity number >6. Reverse transcription was performed using the qScript cDNA SuperMix (Quantabio, Beverly, MA, USA).
qRT-PCR analysis
Transcriptional studies were performed using the Wafergen SmartChip Real-time PCR system (Takara Bio, Mountain View, CA, USA) as described in23. Each run of SmartChip Real-time PCR system can perform 5,184 real-time PCR reactions with a volume of 100 nL each, and they were filled using the SmartChip Multisample Nanodispenser. Quality assurance and quality control checks were performed according to a standard protocol provided by Wafergen Biosystems. Each 100 nL PCR reaction contains 1X Applied Biosystems TaqMan Universal PCR Master mix (Thermofisher Scientific), 1X TaqMan gene expression assays (Supplementary Table B) and 1.5 ng/μL sample cDNA. The following cycling conditions were used on Wafergen SmartChip Real-time PCR system, initial enzyme activation at 95 °C for 10 min, 45 cycles of denaturation at 95 °C for 10 sec and annealing at 60 °C for 53 sec. Finally, qPCR results were analyzed using SmartChip qPCR software (v 2.8.6.1), an amplification efficiency beyond the range (1.5–2.2) and a threshold cycle (Ct) above 40 were discarded. All qPCR reactions were performed in duplicates, and no template controls (NTC) were included on each chip/plate for each TaqMan gene expression assay/ custom designed primers.
Gene expression data of 7 endogenous control genes (ACTB, B2M, EIF3K, GAPDH, PPIA, RPLP0, and RPS9) were analyzed using qBase+analysis software, which calculates the stability of endogenous control genes (M-value). Following qBase+analysis of gene expression data, endogenous control genes RPS9, RPLP0 and EIF3K were ranked best. The Cq (quantification cycle) values of the target genes (PNPLA2, ABDH5, LIPE, LPL, ACACA, FASN, ELOVL6, SCD1, AGPAT2, DGAT1, DGAT2, GLUT4 and, PGK1) were converted to normalized relative gene expression as described by Hellemans, et al.24. Information on the endogenous control genes and target genes can be found in Supplementary Table B.
Western blot
A bead mill homogenizer was used to extract proteins from snap frozen SCAT samples weighing less than 100 mg using RIPA buffer (Teknova, Hollister, CA, USA) supplemented with cocktails of protease (Roche, San Francisco, CA, USA) and phosphatase (Thermofisher Scientific) inhibitors. A concentration of 0.2 mg/mL was found optimal to be used on all antibodies tested on 12–230 kDa Wes Separation Module capillary cartridges of Simple Protein Wes system (ProteinSimple, Santa Clara, CA, USA). Rabbit monoclonal antibody specific for total HSL (#4107, Cell Signaling, Danvers, MA, USA) and Akt (#9272 s, Cell Signaling) were used at a dilution of 1:50, while phosphorylated HSL (pHSL(Ser563), #4139t, Cell Signaling) was used at a dilution of 1:25 and phosphorylated Akt (pAkt (Ser473), #9271t, Cell Signaling) at a dilution of 1:10. A rabbit polyclonal antibody specific for vinculin (#4650, Cell signaling), a cytoskeletal protein, was used as a loading control (1:100 dilution). Anti-rabbit detection modules for Wes (ProteinSimple) kits include Luminol-S, Peroxide, antibody Diluent 2, Streptavidin-HRP and anti-rabbit secondary antibody. Sample proteins were allowed to separate by a capillary technology and were analyzed based on the chemiluminescence signal peaks generated, which were transformed into digital images depicting bands as observed in western blot analysis. Using Compass software (ProteinSimple), the peak areas of Akt, pAkt, HSL and pHSL proteins were estimated and normalized against vinculin. The peak areas are directly proportional to the amount of target protein. Protein abundance of total HSL and pHSL(Ser563) were determined in adipose explants from basal and CAY culture conditions. Protein abundance of total AKT and AKT phosphorylation were determined in adipose explants from basal and insulin stimulated (insulin 1 µg/L) culture conditions. Raw WES data were normalized to the area of vinculin. The normalized data are expressed as a ratio of phosphorylated protein over total protein (ratio pHSL(Ser563):HSL, ratio pAKT:AKT). The change in the ratio of phosphorylated protein by CAY and insulin (1 µg/L) was calculated as the difference in the ratio of phosphorylated protein between the basal sample and the CAY and insulin (1 µg/L) sample, respectively.
Statistical analyses
Statistical analyses were performed using R25. Normality of the variables was checked using the Kolmogorov-Smirnov test (P < 0.05). Non-normally distributed variables (serum BHB and FFA concentrations, number of adipocytes per gram AT, gene expression data, ratio pHSL(Ser563):HSL, ratio pAKT:AKT) were ln-transformed. The effect of independent variables on the dependent variables was checked using a linear mixed effect model. Parity of the animals was included as a covariate in all models. Period or wk relative to the calving date was included as a repeated observation within the random factor cow. Two-way interactions were removed from the model if non-significant (P > 0.05). Pairwise comparisons were done using the Tukey’s post hoc test. Residuals of the models were checked and found to be normally distributed. Significance and tendency were declared at P < 0.05 and 0.05 < P < 0.10, respectively. Results are presented as LSMEANS ± SEM unless otherwise stated.
Results
Lipolysis during the periparturient period
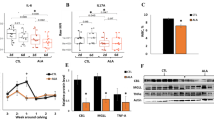
Reflecting the intense FA mobilization from AT during the periparturient period, serum FFA and BHB concentrations increased and body weight and body condition score were reduced in fresh and lactation compared with dry (Fig. 1 and Supplementary Table C). Insulin concentration decreased after parturition (fresh and lactation) compared to values observed at dry (Fig. 1). As expected, the diameter and volume of adipocytes decreased continuously throughout the experimental period. The volume of adipocytes was decreased by 40% at lactation compared with dry (Fig. 2). Due to the interrelationship between the size and the number of the adipocytes, the number of adipocytes per gram of AT increased at fresh and lactation compared with dry (Fig. 2).
Serum free fatty acid (FFA), β-hydroxybutyrate (BHB) and insulin concentrations. Least squares means of FFA concentration (mEq/L) (A), BHB concentration (mmol/L) (B) and insulin concentration (µg/L) (C) during the periparturient period. Error bars represent the SEM. Time-points with different letters differ significantly (abcd, P < 0.05). P-values for the effect of wk relative to calving on the different metabolites are derived from the linear mixed effect model.
Adipocyte volume and diameter and number of adipocytes per gram adipose tissue. Least squares means of adipocyte diameter (µm) (A), adipocyte volume (pL) (B) and number of adipocytes per gram AT (×106 adipocytes per g AT) (C) in the SCAT samples at dry (−11 ± 1 d), fresh (+11 ± 0.2 d) and lactation (+24 ± 0.4 d relative to calving) from Holstein dairy cows. Error bars represent the SEM. Time-points with different letters differ significantly (abc, P < 0.05). P-values for the effect of period on the different variables are derived from the linear mixed effect model.
Basal lipolysis
Basal lipolytic activity was higher in adipose explants from dry compared with fresh (Fig. 3A). In adipose samples taken at lactation, basal lipolytic activity was numerically increased compared with those taken at fresh (Fig. 3A, P = 0.13). Independent of the period, explants with larger adipocytes demonstrated higher basal lipolytic activity (Fig. 3G). Inhibition of basal lipolytic activity by CAY was minimal at dry (Fig. 3B). In fresh and lactation, CAY inhibited basal lipolysis by 36.05 ± 4.51% and 43.05 ± 4.83%, respectively (Fig. 3B).
Glycerol release and the effect of adipocyte volume during inhibition of HSL activity. Least squares means of basal glycerol release (nmol/106 adipocytes per 3 h) (A), CAY inhibited basal glycerol release (% decrease of basal glycerol release) (B), isoproterenol (ISO) stimulated glycerol release (% increase of basal glycerol release) (C), CAY inhibited ISO stimulated glycerol release (% decrease of ISO stimulated glycerol release) (D) and insulin (0.2 and 1 µg/L) inhibited ISO stimulated glycerol release (% decrease of ISO stimulated glycerol release) (E,F). Effect of adipocyte volume on basal glycerol release (G) and CAY inhibited ISO stimulated glycerol release (H) in SCAT explants from Holstein dairy cows at dry (−11 ± 1 d), fresh (+11 ± 0.2 d) and lactation (+24 ± 0.4 d relative to calving). Error bars represent the SEM. Time-points with different letters differ significantly (ab, P < 0.05). P-values for the effect of period and adipocyte size (volume in pL) on the different variables are derived from the linear mixed effect model.
Stimulated lipolysis is affected by adipocyte size and time relative to parturition
The beta-adrenergic agonist ISO stimulated the lipolytic activity of adipose explants in all periods in a similar way (Fig. 3C). Compared with basal lipolysis, ISO increased lipolysis 5- to 10-fold (Fig. 3A,C). Inhibition of HSL activity by CAY during ISO stimulation was more pronounced in the fresh and lactation compared with dry (Fig. 3D). The inhibition of stimulated lipolytic activity by CAY was more pronounced in larger adipocytes (Fig. 3H, P < 0.001).
Insulin inhibition of lipolytic activity is reduced after calving
Insulin inhibited ISO stimulated lipolytic activity in SCAT explants, and the inhibition was more pronounced for the 1 µg/L compared with 0.2 µg/L insulin dose (Fig. 3E,F). Inhibition of stimulated lipolysis by insulin was more marked in dry compared with fresh for both insulin doses (Fig. 3E,F). Adipocyte size did not influence the inhibitory effect of insulin.
Lipolytic and lipogenic gene networks after parturition
Expression of the lipolytic gene network was paradoxically reduced during the peak of FA mobilization postpartum. AT expression of ABDH5, LIPE, LPL, and PNPLA2 (encoding ATGL) decreased in fresh and lactation compared with samples taken at dry (Table 1). The expression of LPL was decreased pronouncedly in fresh and lactation compared to dry (Table 1).
Similarly, the lipogenic gene network was downregulated after parturition (fresh and lactation). The expression of genes involved in the FA synthesis process of the de-novo lipogenic pathway such as ACACA and FASN decreased dramatically in AT samples taken after calving (fresh and lactation) compared with dry (Table 1). Genes of the glycerol-3-phosphate pathway that are part of the triacylglycerol synthesis process including AGPAT2, DGAT2 and those encoding enzymes related to the elongation and desaturation of FA such as ELOVL6 and SCD1 decreased profoundly after calving (fresh and lactation) (Table 1). Remarkably, the expression of DGAT1 was not affected by period (Table 1). Higher expression of DGAT2 was associated with larger adipocytes in adipose samples at fresh and lactation samples but not in the dry samples (Fig. 4). The expression of two genes encoding important proteins from the gene network involving the metabolism of glucose, SLC2A4 (formerly known as GLUT4 or glucose transporter 4) and PGK1, were decreased in fresh and lactation compared with the dry sample (Table 1).
HSL and AKT activity in periparturient cows
HSL content in AT did not change over time in the basal samples: the ratio of HSL:vinculin was 0.50 ± 0.23, 0.24 ± 0.11, 0.50 ± 0.24 in the dry, fresh and, lactation periods, respectively (P value for the period effect = 0.39). The ratio of pHSL(Ser563):HSL reflected the HSL activation of the explants and was numerically increased in the fresh period compared with the dry and lactation period (Fig. 5A). CAY decreased the ratio of pHSL(Ser563):HSL especially in the fresh period, when the ratio of phosphorylation of HSL was maximal (Fig. 5B).
HSL and PKA activity in periparturient cows. Least squares means of the ratio of pHSL(Ser563):HSL (A) and pAKT:AKT (C) in explants cultured in basal incubation condition from SCAT samples taken during the dry (−11 ± 1 d), fresh (+11 ± 0.2 d) and lactation (+24 ± 0.4 d relative to calving) period from Holstein dairy cows. Change in ratio of pHSL(Ser563):HSL in explants incubated with CAY compared with basal incubation conditions (B) and change in ratio of pAKT:AKT in explants incubated with insulin (1 µg/L) compared with basal incubation conditions (D). Error bars represent the SEM. Time-points with different letters differ significantly (ab, P < 0.05). P-values for the effect of period on the ratio of protein phosphorylation and the change in ratio of protein phosphorylation are derived from the linear mixed effect model.
The total content of AKT in AT decreased over time: the ratio of AKT:vinculin was 1.49 ± 0.57, 0.36 ± 0.14, 0.55 ± 0.21 in the dry, fresh and, lactation period, respectively (P value for the period effect < 0.01). The ratio of pAKT:AKT reflects the activation of the insulin signaling cascade of the explants. There was an increase in the ratio of pAKT:AKT in the basal samples taken during the fresh and lactation period (Fig. 5C). Addition of insulin (1 µg/L) to the explants increased the ratio of pAKT:AKT. The effect of insulin on the activation of AKT was more pronounced in the dry and lactation period compared with the fresh period (Fig. 5D).
Discussion
Mobilization of FA reserves from AT supports energy deficits generated by rapid fetal growth and lactation during the periparturient period. In the present study, net FA release from adipocytes into circulation was reflected by an increase in plasma BHB and FFA concentrations, a reduction in the diameter and volume of adipocytes, and an increase in adipocyte number per g of tissue26. The 40% decrease in volume of the adipocytes between the dry and lactation periods observed in our experiment is in agreement with previous studies26,27.
Our results demonstrate that the level of basal lipolytic activity in SCAT is dynamic throughout the periparturient period and decreases in the fresh compared with the dry period. Similarly, Kokkonen, et al.28 and Kenez, et al.29 observed a decrease in basal lipolysis in the immediate postpartum period. Other studies reported that basal lipolysis did not increase substantially after calving30,31. Although our observation of lower basal lipolysis postpartum is in contrast with the general perception that lipolysis increases after parturition, during this time higher concentrations of BHB exert an inhibitory effect on lipolysis via the G protein-coupled receptor 109 A (GRP109A)32. As lactation progresses, this inhibitory effect declines due to reductions in blood BHB concentrations. Additionally, results from the present study indicate that the postpartum peak in circulating FFA may be related to the drastic reduction in the lipogenesis process, that includes re-esterification of FFA, rather than an increase in lipolysis33. Although AT explant lipogenic activity was not measured in the present study, the mRNA expression of the lipogenic network decreased after parturition supporting our hypothesis. It is important to note that the present study did not evaluate basal lipolysis in visceral AT. Thus it is possible that, given the substantial differences in lipolytic activity between adipocytes from different depots9,34, circulating FFA could be derived from internal adipose depots rather than SCAT.
CAY is a known inhibitor of HSL activity in murine adipocytes35. In humans, CAY also appears to have an additional inhibitory effect on ATGL and monoacylglycerol lipase36. As far as the authors are aware, this is the first study using pharmacological inhibition of HSL in explants from dairy cattle. The observed effect of CAY on ISO stimulated lipolysis suggests an important inhibitory effect of CAY on HSL activity in bovine adipocytes that is supported by decreased phosphorylation of HSL on serine 563 in the explants treated with CAY. The absence of any effect of CAY on basal lipolysis in the dry period may suggest that CAY does not affect ATGL activity in bovine adipocytes or that the activity of ATGL and HSL is so low that it cannot be further reduced. Based on our results we cannot exclude any effect of CAY on MGL. However, MGL activity accounts for only 5% of lipolysis in murine AT37.
Based on the effects of CAY on basal and stimulated lipolysis, and pHSL(Ser563):HSL, we hypothesize that HSL contribution to basal lipolysis is dynamic during the transition period. In the dry period, we observed that HSL activity is of minor importance and most of the glycerol release was due to ATGL activity. In the fresh and lactation periods, nearly half of the basal lipolysis was attributable to HSL activity. These observations are in agreement with the increased rate of HSL phosphorylation in the postpartum period despite a decrease in total HSL protein content20,29. Interestingly, basal lipolysis (combined ATGL and HSL activity) was positively related with adipocyte size while the effect of CAY (only HSL activity) on basal lipolysis was not. It is known that basal lipolysis is related to adipocyte size9,38 and regulated by ATGL activity39,40. Overfed cows, which are expected to have larger adipocytes, had higher expression of ATGL but not of HSL11,41. In most studies, ATGL protein content or gene expression remain unchanged or downregulated in early lactation and are independent of energy balance status40,42,43. In our study, this was reflected by the decrease in ATGL dependent basal lipolytic activity (i.e. residual basal lipolytic activity in the presence of CAY). In agreement with Koltes and Spurlock40, this finding reflects a shift from ATGL dependent basal lipolysis during positive energy balance (dry period) to HSL dependent basal lipolysis during negative energy balance (fresh and lactation period). The increase in HSL activity postpartum is caused by different mechanisms including decreased insulin concentrations, potential decreased insulin signaling in adipocytes, increased growth hormone concentrations, and increased catecholamine sensitivity of the adipocytes7,44,45. Based on these observations, we suggest that ATGL determines a certain level of basal lipolytic activity that is directly related to adipocyte size. HSL modulates the level of lipolysis based on the individual requirements of each animal44. Future studies inhibiting ATGL activity may elucidate the role of this lipase in modulating basal lipolysis in dairy cows.
In adipocytes, stimulated lipolysis is triggered by catecholamines signaling through β adrenergic receptors that in turn activate HSL and ATGL37,40,46. HSL is more responsive to catecholamines compared with ATGL44. In our study, ISO, a non-selective β agonist, increased lipolysis independently of the period when biopsies were collected. Around 80% of the ISO stimulated lipolytic activity was due to HSL as indicated by the inhibitory effect of CAY on ISO stimulated glycerol release. This finding is in contrast with the observation that lipolytic responses of adipocytes to catecholamines is increased in the postpartum period47. Based on our calculations of the contribution of HSL to both basal and demand lipolysis, we propose that higher basal HSL activity (i.e., the inhibitory effect of CAY on basal glycerol release) in the fresh and lactation period explains in part the higher inhibitory effect of CAY on ISO stimulated lipolysis postpartum.
Insulin is the most potent physiological inhibitor of lipolysis in AT48. The effect of insulin on stimulated lipolysis was determined at insulin doses reflecting physiological insulin concentrations of dry (1 µg/L)49 and fresh (0.2 µg/L)11,50 periods of dairy cows. Since insulin exerts an inhibitory effect on stimulated lipolysis, by decreasing HSL phosphorylation, it was necessary to determine the effect of insulin after ISO treatment51. Inhibition of ISO stumulated lipolysis by both doses of insulin was less pronounced in the fresh period compared with the dry period. Also, protein phosphorylation of AKT, measured as pAKT:AKT, was reduced in the fresh period compared with the dry and lactation period. These observations indicate that during the fresh period adipocytes exhibit limited sensitivity to the anti-lipolytic actions of insulin. This insulin resistant state appears to be limited in time since the anti-lipolytic effect of insulin increased during the lactation period. Similar observations were reported by Ji, et al.11 in AT collected from cows at 1 and 3 weeks after parturition using insulin at supra-physiological concentrations (1 µM). Insulin signaling in adipose samples taken during intravenous glucose tolerance tests was lower in the postpartum period compared with the prepartum period20,52. However, it is important to clarify that insulin concentrations during intravenous glucose tolerance tests are usually supra-physiological and typically a reduction in insulin secretion is observed in the postpartum period.
This is the first study to demonstrate an insulin resistant state in adipocytes from cows during the first week of lactation (fresh) using physiologically relevant insulin concentrations. In the immediate postpartum period, minimal lipolysis inhibition and reduced lipogenesis stimulation driven by low insulin concentrations and reduced sensitivity to the anti-lipolytic effects of insulin increase the net release of FA into circulation. Insulin resistance during the first 2 weeks after parturition might support a sufficient FA release from AT while adipocytes adapt their metabolism to the intense energy requirements of lactation. However, AT insulin resistance in the immediate postpartum period may have a detrimental impact on health and productivity of dairy cows because it promotes excessive lipolysis52. Further research is needed to determine the physiological and pathological consequences of periparturient adipocyte insulin resistance.
Insulin inhibits lipolytic activity by decreasing the phosphorylation and thus activity of HSL. After insulin binds to its receptor, intracellular signaling is activated via phosphorylation of insulin receptor substrate 1 (IRS1), PI 3-kinase (PI3K), AKT, and the activity of phosphodiesterase 3b (PDE3b)53,54. In vitro studies using adipocyte cell lines demonstrated that, depending on the metabolic environment, the anti-lipolytic effect of insulin occurs via an AKT-dependent or an AKT-independent pathway. At high concentrations of ISO, maximal HSL activity, insulin inhibits lipolysis by an AKT-dependent pathway. At lower ISO concentrations, lipolysis is inhibited in an AKT-independent pathway55. In the present study, ISO dose (1 µM) was chosen to maximize adipocyte lipolytic activity before and after parturition56. We conclude that since ISO stimulated lipolysis was not affected by time relative to parturition, the anti-lipolytic effect of insulin is directly related to a state of insulin resistance in the adipocyte. Given that insulin only inhibits lipolysis through the reduction of HSL phosphorylation, it would not be possible to determine the inhibitory effect of insulin on basal lipolysis at dry when HSL activity was minimal (i.e., low ratio of pHSL(Ser563):HSL). Hence it is necessary to standardize the lipolytic stimulus before insulin responses can be assessed in vitro explants.
In the present study, expression of lipolytic genes (PNPLA2, ABDH5, LIPE, and LPL) decreased after calving. In line with these results, other groups reported lower protein expression of ATGL, HSL and ABHD5 (encoded by PNPLA2, LIPE, and ABDH5 respectively) during early lactation compared to the dry period30,40,57. Accordingly, in the present study, total HSL protein expression was lower in the fresh period compared with the dry and lactation periods. In a study of Koltes and Spurlock40, protein expression of ATGL decreased in early lactation. And other studies have demonstrated that AT lipolysis is mainly regulated by posttranslational control mechanisms, i.e., phosphorylation of key enzymes, and related to milk production30,31,58. The decreased protein expression of HSL is accompanied by an increase in the ratio of phosphorylated HSL in the fresh period, indicating an increased lipolytic activity of HSL57.
Basal glycerol release was influenced by adipocyte size. Larger adipocytes had higher basal lipolytic activity compared with smaller adipocytes independent of the period when the samples were collected. Contrary, mRNA expression of lipogenic genes (except DGAT2) was not affected by adipocyte size. The imbalance between the lipolysis and lipogenesis in larger adipocytes favoring lipolytic activity in the basal state may provide an explanation why over-conditioned cows, which have larger adipocytes9, are at increased risk of having higher concentrations of circulating FFA and BHB during the periparturient period59,60. Remarkably, the anti-lipolytic effect of insulin was not influenced by adipocyte size as previously shown in over-conditioned cows9 which indicates that stimulated lipolysis in adipocytes from over-conditioned cows is not insulin resistant. More research is needed to substantiate the effect of lipogenic enzymes in the postpartum period and to determine the potential effect of modulating lipogenic activity on FA mobilization from AT.
Despite being lipolytic, LPL is responsible for the provision of FA to the adipocytes by breaking down circulating triacylglycerol in the capillaries of AT37. Decreased expression of LPL is thus an indication of decreased availability of FA for lipogenesis in AT11. The activity of lipogenic enzymes is controlled by transcriptional mechanisms and affected by the availability of energy11,58. Negative energy balance at the beginning of lactation leads to a pronounced decrease in the expression and activity of genes encoding proteins of the de-novo lipogenic, and glycerol-3-phosphate pathways7,11,29. Reduced lipogenic activity in AT may contribute to the increase in circulating FFA levels in the immediate postpartum period33. More research is warranted to determine the impact of modulating lipogenic enzyme activity on FA release from AT in the periparturient period of dairy cows.
The decreased expression of genes for glucose metabolism, GLUT4, and PGK1, in the postpartum period, is in agreement with the homeorhetic mechanisms that preserve glucose for milk production. In the postpartum period, lower PGK1 will lead to lower glycolysis within adipocytes. And at the same time, downregulation of GLUT4 expression leads to lower insulin-dependent glucose uptake in the adipocytes11,61. GLUT4 expression was specially reduced in large adipocytes during the dry period. This may indicate that the glucose metabolism of large adipocytes is less sensitive to insulin compared with small adipocytes prior to calving. The abundance of GLUT4 protein was lower in SCAT samples of over-conditioned cows in the prepartum period but not in the postpartum period61. Similarly, insulin sensitivity of glucose metabolism was negatively associated with fat accumulation in dairy cows at the end of the dry period62. These results provide further evidence of an insulin resistant state of glucose metabolism of adipocytes by excessive accumulation of triacylglycerol through a decreased expression of GLUT4.
Conclusion
AT lipolytic responses during the periparturient period are characterized by a shift from ATGL dependent basal lipolysis during positive energy balance (dry period) to HSL dependent basal lipolysis during negative energy balance (fresh and lactation period). The decrease in basal lipolytic activity and a broadly decreased transcriptional regulation of the lipogenic gene network suggests that decreased lipogenesis is an important contributor to FA release from SCAT postpartum. In addition, insulin resistance of lipolytic activity develops during the early lactation period and is characterized by a decreased activation of AKT.
References
Herdt, T. H. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Veterinary Clinics of North America: Food Animal Practice 16, 215–230 (2000).
Boden, G. Fuel metabolism in pregnancy and in gestational diabetes mellitus. Obstetrics and Gynecology Clinics of North America 23, 1–10 (1996).
McArt, J. A. A., Nydam, D. V. & Oetzel, G. R. Epidemiology of subclinical ketosis in early lactation dairy cattle. Journal of Dairy Science 95, 5056–5066 (2012).
Bobe, G., Young, J. W. & Beitz, D. C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. Journal of Dairy Science 87, 3105–3124 (2004).
Butte, N. F. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. American Journal of Clinical Nutrition 71, 1256S–1261S (2000).
Rook, J. S. Pregnancy toxemia of ewes, does, and beef cows. Veterinary Clinics of North America. Food Animal Practice 16, 293–317, vi-vii (2000).
Vernon, R. G. & Pond, C. M. Adaptations of maternal adipose tissue to lactation. Journal of Mammary Gland Biology and Neoplasia 2, 231–241 (1997).
Contreras, G. A., Strieder-Barboza, C. & De Koster, J. Modulating adipose tissue lipolysis and remodeling to improve immune function during the transition period and early lactation of dairy cows. Journal of Dairy Science (2017).
De Koster, J. et al. Influence of adipocyte size and adipose depot on the in vitro lipolytic activity and insulin sensitivity of adipose tissue in dairy cows at the end of the dry period. Journal of Dairy Science 99, 2319–2328 (2016).
Urrutia, N., Ying, Y. & Harvatine, K. The effect of conjugated linoleic acid, acetate, and their interaction on adipose tissue lipid metabolism in nonlactating cows. Journal of Dairy Science 100, 5058–5067 (2017).
Ji, P., Osorio, J. S., Drackley, J. K. & Loor, J. J. Overfeeding a moderate energy diet prepartum does not impair bovine subcutaneous adipose tissue insulin signal transduction and induces marked changes in peripartal gene network expression. Journal of Dairy Science 95, 4333–4351 (2012).
Drackley, J. K. et al. Physiological and pathological adaptations in dairy cows that may increase susceptibility to periparturient diseases and disorders. Italian Journal of Animal Science 4, 323–344 (2005).
Garro, C. J., Mian, L. & Cobos Roldan, M. Subclinical ketosis in dairy cows: prevalence and risk factors in grazing production system. J Anim Physiol Anim Nutr (Berl) 98, 838–844 (2014).
Pons, R. S., Rockett, F. C., de Almeida Rubin, B., Oppermann, M. L. R. & Bosa, V. L. In Diabetology and Metabolic Syndrome. A80 (Springer).
Bertoni, G., Trevisi, E., Han, X. & Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J Dairy Sci 91, 3300–3310 (2008).
McNamara, J. P. & Murray, C. E. Sympathetic nervous system activity in adipose tissues during pregnancy and lactation of the rat. Journal of Dairy Science 84, 1382–1389 (2001).
Roche, J. R. et al. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. Journal of Dairy Science 92, 5769–5801 (2009).
Edmonson, A. J., Lean, I. J., Weaver, L. D., Farver, T. & Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. Journal of Dairy Science 72, 68–78 (1989).
NRC. Vol. 1 (National Academies Press, Washington, District of Columbia, 2001).
Mann, S. et al. Insulin signaling, inflammation, and lipolysis in subcutaneous adipose tissue of transition dairy cows either overfed energy during the prepartum period or fed a controlled-energy diet. Journal of Dairy Science 99, 6737–6752 (2016).
DiGirolamo, M. & Fine, J. B. Cellularity measurements. Methods in Molecular Biology 155, 65–75 (2001).
Goldrick, R. B. Morphological changes in the adipocyte during fat deposition and mobilization. American Journal of Physiology 212, 777–782 (1967).
Contreras, G. A. et al. Periparturient lipolysis and oxylipid biosynthesis in bovine adipose tissues. PLOS ONE 12, e0188621 (2017).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology 8, R19 (2007).
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org/ (2013).
McNamara, J. P. Regulation of Adipose Tissue Metabolism in Support of Lactation. Journal of Dairy Science 74, 706–719 (1991).
Akter, S. H. et al. Physiological and conjugated linoleic acid-induced changes of adipocyte size in different fat depots of dairy cows during early lactation. Journal of Dairy Science 94, 2871–2882 (2011).
Kokkonen, T. et al. Effect of body fatness and glucogenic supplement on lipid and protein mobilization and plasma leptin in dairy cows. Journal of Dairy Science 88, 1127–1141 (2005).
Kenez, A. et al. Changes in lipid metabolism and beta-adrenergic response of adipose tissues of periparturient dairy cows affected by an energy-dense diet and nicotinic acid supplementation. Journal of Animal Science 93, 4012–4022 (2015).
Khan, M. J. et al. Change in subcutaneous adipose tissue metabolism and gene network expression during the transition period in dairy cows, including differences due to sire genetic merit. Journal of Dairy Science 96, 2171–2182 (2013).
Rocco, S. M. & McNamara, J. P. Regulation of bovine adipose tissue metabolism during lactation. 7. Metabolism and gene expression as a function of genetic merit and dietary energy intake. Journal of Dairy Science 96 (2013).
Kenez, A., Locher, L., Rehage, J., Danicke, S. & Huber, K. Agonists of the G protein-coupled receptor 109A-mediated pathway promote antilipolysis by reducing serine residue 563 phosphorylation of hormone-sensitive lipase in bovine adipose tissue explants. Journal of Dairy Science 97, 3626–3634 (2014).
Bell, A. W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. Journal of Animal Science 73, 2804–2819 (1995).
Hostens, M. et al. The fatty acid profile of subcutaneous and abdominal fat in dairy cows with left displacement of the abomasum. Journal of Dairy Science 95, 3756–3765 (2012).
Marcelin, G., Liu, S. M., Li, X., Schwartz, G. J. & Chua, S. Genetic control of ATGL-mediated lipolysis modulates adipose triglyceride stores in leptin-deficient mice. Journal of Lipid Research 53, 964–972 (2012).
Iglesias, J. et al. Simplified assays of lipolysis enzymes for drug discovery and specificity assessment of known inhibitors. Journal of Lipid Research 57, 131–141 (2016).
Lafontan, M. & Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Progress in Lipid Research 48, 275–297 (2009).
Gagliostro, G. & Chilliard, Y. Duodenal Rapeseed Oil Infusion in Early and Midlactation Cows .4. In vivo and In vitro Adipose Tissue Lipolytic Responses. Journal of Dairy Science 74, 1830–1843 (1991).
Elkins, D. A. & Spurlock, D. M. Phosphorylation of Perilipin is Associated with Indicators of Lipolysis in Holstein Cows. Hormone and Metabolic Research 41, 736–740 (2009).
Koltes, D. A. & Spurlock, D. M. Coordination of lipid droplet-associated proteins during the transition period of Holstein dairy cows. Journal of Dairy Science 94, 1839–1848 (2011).
Ji, P., Drackley, J. K., Khan, M. J. & Loor, J. J. Overfeeding energy upregulates peroxisome proliferator-activated receptor (PPAR)-γ controlled adipogenic and lipolytic gene networks but does not affect proinflammatory markers in visceral and subcutaneous adipose depots of Holstein cows. Journal of Dairy Science 97, 3431–3440 (2014).
Elkins, D. A. The involvement of the novel proteins, perilipin, ATGL, and CGI-58, in lipolysis of early and mid lactation Holstein cows. (Iowa State University, 2008).
Deiuliis, J. A. The metabolic and molecular regulation of adipose triglyceride lipase, The Ohio State University (2007).
McNamara, J. P., Huber, K. & Kenez, A. A dynamic, mechanistic model of metabolism in adipose tissue of lactating dairy cattle. Journal of Dairy Science 99, 5649–5661 (2016).
Chilliard, Y. et al. Adipose tissue metabolism and its role in adaptations to undernutrition in ruminants. Proceedings of the Nutrition Society 59, 127–134 (2000).
Koltes, D. A., Spurlock, M. E. & Spurlock, D. M. Adipose triglyceride lipase protein abundance and translocation to the lipid droplet increase during leptin-induced lipolysis in bovine adipocytes. Domestic Animal Endocrinology 61, 62–76 (2017).
Vernon, R. G. in Proceedings of the 12th World Buiatrics Congress, 18–23 August 2002. 210–223.
Zechner, R., Kienesberger, P. C., Haemmerle, G., Zimmermann, R. & Lass, A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. Journal of Lipid Research 50, 3–21 (2009).
Abuelo, A., Hernandez, J., Benedito, J. L. & Castillo, C. Association of oxidative status and insulin sensitivity in periparturient dairy cattle: an observational study. J Anim Physiol Anim Nutr (Berl) 100, 279–286 (2016).
Strieder-Barboza, C., de Souza, J., Raphael, W., Lock, A. L. & Contreras, G. A. Fetuin-A: A negative acute-phase protein linked to adipose tissue function in periparturient dairy cows. Journal of Dairy Science (2017).
Morimoto, C., Tsujita, T. & Okuda, H. Antilipolytic actions of insulin on basal and hormone-induced lipolysis in rat adipocytes. Journal of Lipid Research 39, 957–962 (1998).
Zachut, M. et al. Periparturient dairy cows do not exhibit hepatic insulin resistance, yet adipose-specific insulin resistance occurs in cows prone to high weight loss. Journal of Dairy Science 96, 5656–5669 (2013).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Sasaki, S. Mechanism of insulin action on glucose metabolism in ruminants. Animal Science Journal 73, 423–433 (2002).
Choi, S. M. et al. Insulin regulates adipocyte lipolysis via an akt-independent signaling pathway. Molecular and Cellular Biology 30 (2010).
Carpene, C. Assays of adrenergic receptors. Including lipolysis and binding measurements. Methods in Molecular Biology 155, 129–140 (2001).
Locher, L. F. et al. Hormone-sensitive lipase protein expression and extent of phosphorylation in subcutaneous and retroperitoneal adipose tissues in the periparturient dairy cow. Journal of Dairy Science 94, 4514–4523 (2011).
McNamara, J. P. Ruminant Nutrition Symposium: a systems approach to integrating genetics, nutrition, and metabolic efficiency in dairy cattle. Journal of Animal Science 90, 1846–1854 (2012).
Roche, J. R., Kay, J. K., Friggens, N. C., Loor, J. J. & Berry, D. P. Assessing and Managing Body Condition Score for the Prevention of Metabolic Disease in Dairy Cows. Veterinary Clinics of North America: Food Animal Practice 29, 323–336 (2013).
Lacetera, N. et al. Lymphocyte functions in overconditioned cows around parturition. Journal of Dairy Science 88, 2010–2016 (2005).
Jaakson, H. et al. Adipose tissue insulin receptor and glucose transporter 4 expression, and blood glucose and insulin responses during glucose tolerance tests in transition Holstein cows with different body condition. Journal of Dairy Science 101, 752–766 (2018).
De Koster, J. et al. Insulin response of the glucose and fatty acid metabolism in dry dairy cows across a range of body condition scores. Journal of Dairy Science 98, 4580–4592 (2015).
Acknowledgements
The authors acknowledge the technical assistance of Lynn Worden at the Department of Animal Science, Libby Greenberg, and the staff at the Michigan State University Dairy Teaching and Research Center (East Lansing). This project was supported in part by USDA-National Institute of Food and Agriculture (Washington, DC) grant 2015-67015-23207, Department of Large Animal Clinical Sciences (East Lansing, MI) and Global Agri-Trade Corporation (Gardena, CA).
Author information
Authors and Affiliations
Contributions
J.D.K., J.D.S., A.L.L., and G.A.C. conception and design of research; J.D.K., C.S.B., and R.K.N. performed experiments; J.D.K. analyzed data; J.D.K., R.K.N., and G.A.C. interpreted results of experiments; J.D.K. prepared figures; J.D.K. drafted manuscript; all authors edited and revised manuscript drafts and approved final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Koster, J., Nelli, R.K., Strieder-Barboza, C. et al. The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows. Sci Rep 8, 13378 (2018). https://doi.org/10.1038/s41598-018-31582-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31582-4
This article is cited by
-
Endotoxin-induced alterations of adipose tissue function: a pathway to bovine metabolic stress
Journal of Animal Science and Biotechnology (2024)
-
Mechanisms and Treatment of Dyslipidemia in Diabetes
Current Cardiology Reports (2021)
-
The Impact of Fasting on Major Metabolic Pathways of Macronutrients and Pharmacokinetics Steps of Drugs
European Journal of Drug Metabolism and Pharmacokinetics (2021)
-
Transcriptomic profiling of adipose tissue inflammation, remodeling, and lipid metabolism in periparturient dairy cows (Bos taurus)
BMC Genomics (2020)
-
Effect of anti-inflammatory treatment on systemic inflammation, immune function, and endometrial health in postpartum dairy cows
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.