Abstract
Given the potentially distinctive histological variations in northwest of China, the aim of current study was to compare the efficacy of induction chemotherapy plus concurrent chemoradiotherapy (IC + CCRT) with concurrent chemoradiotherapy (CCRT) in nasopharyngeal carcinoma (NPC) patients with different histological types. A total of 301 patients were included in this study. Patients were classified in two cohorts according to the 2005 WHO World Health Organization histological classification: WHO type IIa group and WHO type IIb group. The Kaplan-Meier method was used to detect the efficacy between IC + CCRT and CCRT in two WHO types cohorts. Propensity score matching method was adopted to balance the baseline covariate and eliminate potential selection bias. On propensity matched analyses, IC + CCRT was found to produce better 3-year DMFS and OS than CCRT in WHO type IIa cohort (DMFS, 76.2% vs. 42.2%, p = 0.029; OS, 78.3% vs. 65.5%, p = 0.027). For WHO type IIb cohort, IC + CCRT was associated with a better 3-year OS (87.4% vs. 77.9%, p = 0.029) and a trend of better 3-year DMFS (85.9% vs. 76%, p = 0.162) compared with CCRT. IC + CCRT was benefit for advanced stage nasopharyngeal carcinoma with different nonkeratinizing carcinoma subtypes.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is an unbalanced geographic distribution disease. The incidence of NPC in endemic area of China is approximately 15–30 for every 100,000 people per year1. In China, the highest incidence rate occurs in Southern China, whereas in northwest China, the incidence rate is low. WHO histological type has been identified as a significantly prognostic factor to impact the survival of NPC patients by several epidemiology studies2,3. According to the 2005 WHO classification, NPC histological types were classified as keratinizing carcinoma (type I) and nonkeratinizing squamous cell carcinoma (type II). Type II was further classified to two subtypes: differentiation subtype (type IIa) and undifferentiation subtype (type IIb)4. Our previous studies reported that NPC in northwest China had higher proportion of WHO type IIa (approximate 30%) than endemic area of China (<5%)5,6,7. Compared with the most common WHO type IIb, several studies showed WHO type IIa contributed to poor overall survival (OS) and distant metastasis-free survival (DMFS) for patients with NPC5,8,9. These results implied more intensity treatment modality should be delivered to patients with WHO type IIa.
Concurrent chemoradiotherapy (CCRT) plus adjuvant chemotherapy has become a standard treatment modalities for advanced NPC for many years since it was established by the intergroup 009910. However, the treatment modalities were changing along with the combinations of new radiotherapy technique and drugs. In the era of intensity modulated radiotherapy (IMRT), 5-year local recurrence-free survival (LRFS) of locoregionally advanced stage NPC has achieved more than 90%, whereas 5-year DMFS and OS are limited to70–80%11. MAC-NPC meta-analysis suggested induction or adjuvant chemotherapy might be a promising treatment modalities to further improve the DMFS and OS for patients with advanced stage disease12. Recently, a phase III randomized study showed that adjuvant cisplatin and fluorouracil (PF) chemotherapy did not improve the treatment outcomes13. However, compared with CCRT, induction chemotherapy followed by concurrent chemoradiotherapy (IC + CCRT) was identified to be benefit to improve the 3-year DMFS and OS by another phase III randomized study from endemic region of China14. At present, IC + CCRT has been considered as a dominating treatment modality for endemic NPC with clinical advanced stage. However, two important issue remain unsettled for the clinicians from northwest region of China: (1) which is the better choice between IC + CCRT and CCRT for the locoregionally advanced NPC in non-endemic region of China? (2) especially for patients with WHO type IIa, whether IC + CCRT is a better option to improve survival outcomes compared with CCRT alone.
The aim of current study was to compare the efficacy of IC + CCRT with CCRT in patients with different nonkeratinizing carcinoma subtypes from northwest of China. Propensity score matching method was adopted to balance the baseline covariate and eliminate potential selection bias.
Materials and Methods
Patients selection
We reviewed 524 cases of histologically proven NPC patients, who received initial treatment at our institute between January 2006 to December 2014. The inclusion criteria were as follow: histologically confirmed non-keratinizing carcinoma of nasopharynx by biopsy; locoregionally advanced stage III-IVB without metastasis; receiving IC + CCRT or CCRT as initial treatment modality; receiving IMRT as definitive radiotherapy; patients’ primary residences limited to the northwest of China. We excluded patients who did not complete the prescribed course of radiotherapy, who developed non-cancer specific death. Ultimately, a total of 301 patients were included for analysis. All methods were carried out in accordance with the guidelines and regulations of ethics committee of Xijing hospital. This study was approved by the ethics committee of XiJing Hospital, Xi’an, China. The ethics committee of our hospital confirmed it was not necessary to obtain informed consent in this study because there were no participants involved during the research process. All research materials were obtained on the base of the computerized patient record system of xijing hospital.
Clinical staging
The routine staging workup included a complete history and physical examinations, blood work, direct fibreoptic nasopharyngoscopy, imaged by computed tomography (CT) and magnetic resonance imaging (MRI) of head and neck, and chest images, abdominal sonography, and whole body bone scan, as well as positron emission tomography (PET)-CT, if necessary. All patients MRI detail were evaluated by two experienced radiologists. Consensus meetings were conducted to resolve the disagreements. All patients were restaged according to the 7th editions of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) system.
Histological type
The haematoxylin and eosin stained sections of biopsy material obtained for first diagnosis were retrieved and reviewed by two senior pathologists. The histological type was identified according to the 2005 WHO World Health Organization classification based on the microscopy morphology4. The non-keratinizing undifferentiated type (WHO type IIb) was characterized by syncytial-appearing large tumor cells with indistinct cell borders, round to oval vesicular nuclei, and large central nucleoli. The non-keratinizing differentiated type (WHO type II) usually showed cellular stratification and pavementing, often with a plexiform growth, reminiscent of transitional cell carcinoma of the bladder. In the present study, when both types were seen in a specimen, the differentiated component had to constitute more than 50% of the tumor tissue to be qualified as differentiated type.
Clinical treatment
The treatment planning approaches were described by our previous studies5,9,15. The prescribe dose were 72.6 Gy in 33 fractions to the planning target volume (PTV) of gross tumor volume of nasopharynx (GTVnx), 66 to 72.6 Gy to PTV of gross tumor volume of positive lymph nodes (GTVnd), 66 Gy to the entire nasopharynx mucosa, 60 to 63 Gy to PTV of high risk clinical target volume (CTV1), and 50.4 to 56 Gy to PTV of low risk clinical target volume (CTV2). The dose received by each organ at risk (OAR) should be no more than its tolerance16.
The neoadjuvant chemotherapy included TP regimen (docetaxel 75 mg/m2, cisplatin 75 mg/m2), PF regimen (cisplatin 80 mg/m2, 5-FU 800–1000 mg/m2 days 1 to 5), GP regimen (gemcitabine 1000 mg/m2, cisplatin 75 mg/m2) and TPF regimen (docetaxel 75 mg/m2, cisplatin 75 mg/m2, 5-FU 750 mg/m2 days1 to 5) every 3 weeks for 2–3 cycles at a 2–3 weeks’ interval before the initial radiotherapy. Concurrent chemotherapy was only consisted of cisplatin (100 mg/m2 every 3 weeks or 40 mg/m2 weekly).
Statistical analysis
The endpoints included LRFS, DMFS, progression-free survival (PFS) and OS which were defined as time to first local recurrence and/or distant metastasis. Propensity scores were computed by logistic regression for each patient using the following covariates: age, gender, smoking, drinking, race, blood EBV DNA copies, T category, N category, clinical stage, histological WHO type, tumor volume. Initial propensity matching was conducted with a 1:1 match of IC + CCRT to CCRT. Because the sample of IC + CCRT was almost two times as large as CCRT, another propensity matching was undertaken with two IC + CCRT patients matched to one CCRT patients through a Greedy algorithm with caliper being 0.2 times of standard deviation of logit propensity score. Numerical variable was transformed to categorical variable using interquartile range method if it was not Gaussian distribution, such as tumor volume. Means were compared by the Student’s t test. Categorical variables were compared by the χ2 test. The Kaplan-Meier method was used to calculate the accurate rate of endpoints. Because of high distant failure rate in advanced NPC, the prognostic analysis only focused on DMFS and OS. Only the factors which were found to be associated with the endpoints by univariate analyses entered into multivariate Cox proportional hazards regression analysis. The hazard ratio (HR) and its 95% confidence interval (95% CI) were used to indicate the prognostic value of risk factors. A 2-sided p value of less than 0.05 was considered significant. SAS statistical package 9.1.3 (SAS institute USA) and GraphPad Prsim 5.0 (GraphPad Software Inc, USA) were used for all analyses.
Results
Patient characteristics
Patient characteristics are presented in Table 1. The patients’ overall median age was 47 years old (range, 18–78 years). Clinical stage IV was the most common stage in this study (72.1%). Besides T stage and N stage, the baseline demographic and clinical characteristics of the treatment groups were well balanced before matching. But the distribution biases were eliminated after matching.
Survival outcomes according to treatment modality
With a median follow-up of 41 months (range, 7–90 months), the survival outcomes of IC + CCRT were superior to those of CCRT. The 3-year LRFS, DMFS, PFS and OS were 96.6%, 81%, 80.7% and 84.7% for IC + CCRT and 90.1%, 71.1%, 62% and 75.6% for CCRT, respectively (LRFS, p < 0.001; DFMS, p = 0.056; PFS, p < 0.001; OS, p = 0.007; Table 2). On propensity matched survival analysis, there was no significant difference between two treatment groups for 3-year LRFS (p = 0.65), however, IC + CCRT showed significantly better outcomes than CCRT in 3-year DMFS, PFS and OS (DFMS, p = 0.045; PFS, p < 0.001; OS, p = 0.011 Table 2).
Prognostic analysis
On univariate analysis, WHO histological type, N stage, treatment modality were associated with DMFS and OS (Table 3). Multivariate analysis showed that WHO histological type, N stage, treatment modality were independently prognostic factors for DMFS (WHO histological type, hazard ratio (HR) 1.845, 95% confidence interval (95% CI) 1.18–3.047, p = 0.017; N stage, HR 1.916, 95% CI 1.35–2.717, p < 0001; treatment modality, HR 2.525, 95% CI 1.469–4.34, p = 0.001) and OS (WHO histological type, HR 1.982, 95% CI 1.204–3.262, p = 0.007; N stage, HR 1.824, 95% CI 1.317–2.526, p < 0001; treatment modality, HR 2.852, 95% CI 1.68–4.844, p < 0.001) (Table 4).
Efficacy of IC + CCRT for patients stratified as WHO type IIa and WHO type IIb
The stratified analysis was conducted to further evaluate the efficacy of IC + CCRT in different WHO types. The clinical characteristics of treatment groups were well balanced in different nonkeratinizing carcinoma subtypes (Tables A1 and 2).
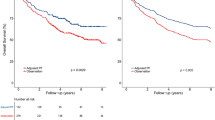
For the WHO type IIa cohort, a propensity matched analysis was conducted with 1:1 matching (18 IC + CCRT patients with 18 CCRT patients). IC + CCRT showed a better DMFS and OS than CCRT (DMFS, HR: 0.221, 95% CI: 0.072–0677, p = 0.0082; OS, HR: 0.147, 95% CI: 0.046–0.476, p = 0.0014) (Supplementary Figure). To improve the statistical power, another propensity matched analysis was conducted with 2:1 matching (34 IC + CCRT patients with 17 CCRT patients). IC + CCRT was also found to produce better 3-year DMFS and OS than CCRT (DMFS, 76.2% vs. 42.2%, p = 0.029; OS, 78.3% vs. 65.5%, p = 0.027) (Fig. 1). For the WHO type IIb cohort, IC + CCRT showed a better trend for DMFS (HR: 0.397, 95% CI: 0.147–1.067, p = 0.067) and a statistically significant improvement for OS (HR: 0.241, 95% CI: 0.089–0.647, p = 0.0047) compared with CCRT based on the 1:1 propensity matching (54 IC + CCRT patients with 54 CCRT patients) (Supplementary Figure). On propensity matched with 2:1 matching (88 IC + CCRT patients, 44 CCRT patients), there was a trend of better 3-year DMFS for IC + CCRT compared with CCRT (85.9% vs. 76%, p = 0.162), but a statistically significant difference was showed between IC + CCRT and CCRT for 3-year OS (87.4% vs. 77.9%, p = 0.029) (Fig. 2).
Before and after propensity matching, Kaplan-Meier DMFS and OS curves for the two treatment group in patients with WHO histological type IIa. (A,B) Distant metastasis-free survival; (C,D) overall survival; IC + CCRT, induction chemotherapy plus concurrent chemoradiotherapy; CCRT, concurrent chemoradiotherapy.
Before and after propensity matching, Kaplan-Meier DMFS and OS curves for the two treatment group in patients with WHO histological type IIb. (A,B) Distant metastasis-free survival; (C,D) overall survival; IC + CCRT, induction chemotherapy plus concurrent chemoradiotherapy; CCRT, concurrent chemoradiotherapy.
Discussion
To the best of our knowledge, this is a first study to detect the efficacy of IC + CCRT in patients of NPC with different non-keratinizing subtypes. According to the results of our study, IC + CCRT had better efficacy to increase the OS compared with CCRT in patients with WHO type IIb. Moreover, IC + CCRT had a superior efficacy to improve OS and DMFS for patients with WHO type IIa compared with CCRT.
Recently, a randomized trial from endemic region of China indicated IC + CCRT produced a excellent 3-year DMFS (90%) and OS (92%) for locoregionally advanced NPC compared with CCRT (83% for DMFS, 86% for OS)14. Our previous experience of using IC + CCRT in locaregionaly advanced NPC in non-endemic of China confirmed the promising efficacy of IC + CCRT. The 3-year local control, DMFS and OS achieved 94.9%, 78.6% and 84.5%, respectively7. However, our previous study could not answer this question that whether IC + CCRT could provide any additional survival benefit compared with standard CCRT for non-endemic patients. In this propensity matched study, we reported IC + CCRT produced superior survival outcomes than CCRT for patients from northwest of China. Although the results of this study had similar outcomes to reports from endemic data of China, the survival data of IC + CCRT were lower than the endemic data14,17,18. It may be explained by three reasons: (1) this study included higher proportion of stage IV patients (approximately 70%) than ever reports from endemic data; (2) high proportion of WHO type IIa contributed the slightly worse outcomes; (3) a few of patients received PF (4.7%, data was not shown) as induction chemotherapy regimens, which was indicated as a worse option than other regimens contained docetaxel and gemcitabine in NPC7,14,19.
A few of studies have revealed worse prognosis in keratinizing squamous carcinoma of the nasopharynx compared with the non-keratinizing category in non-endemic and endemic areas3,20,21,22. Similarly, for patients with non-keratinizing categories of NPC, differentiated subtype generated worse prognosis than undifferentiated subtype. Cheng et al. reported that WHO type IIa contribute a worse locoregional control due to radioresistance2. Another study also found patients with WHO type IIa contributed to a worse DMFS and OS than patients with WHO type IIb8. Our previous study also found that WHO type IIa was an independently poor prognostic factor for DMFS and OS in patients from northwest of China5. These studies above mentioned implied that patients with WHO type IIa might be received more intensity treatment modalities. In the subgroup propensity matched analysis of this study, IC + CCRT demonstrated better survival outcomes than CCRT in patients with WHO types IIb. The study could offer a reference for clinicians that locoregionally advanced NPC patients with WHO type IIa could obtained survival benefit from IC + CCRT but CCRT. However, the results should also be understood carefully because the samples size of WHO type IIa was too small to have enough power to obtain a reliable conclusion. Prospective studies with large cohort should be designed to investigate the role of IC + CCRT in patients with WHO type IIa of NPC.
The mechanism of WHO type IIa leading to the poor prognosis remains unclear. Some studies reported high expression of IKK-αcould induce NPC cell differentiation via regulating the NF-κB signaling pathway23,24,25. Others reported expression of ERCC1, which was relative to cisplatin-based chemotherapy resistance in cancers, was higher in the WHO type IIa compared with WHO type IIb8,26. These studies implied that WHO type IIa had distinct characteristics of molecular biology, and the treatment for this subtype should focus on inducing the differentiation of NPC cells via regulating the NF-κB signaling pathway or decreasing the internal chemotherapy-resistance molecular to increase the treatment sensitivity in the future.
According to the subgroup analysis for patients with WHO type IIc, IC + CCRT could significantly improve OS compared with CCRT (3-year OS, IC + CCRT 87.4% vs. CCRT 77.9%). This result was similar to endemic data. However, only better trend but significant improvement was detected for DMFS when IC + CCRT compared with CCRT after propensity matching. Maybe a positive result would be possible to get by a longer follow-up time.
EBV DNA copies were well established as an independently prognostic factor for NPC outcomes. In endemic region of China, EBV DNA was detected in nearly 90% patients, which was significantly higher than the detection rate (only approximate 10%) in this study and our previous studies7,15. Due to the small number of patients with EBV DNA positivity, we could not analyze any correlation between EBV and prognosis in this study. Maybe it was not reasonable to define 5000 copies as cut-off value in this study because of lack of verification by the receiver operating characteristic curves. However, the reported cut-off value of EBV DNA load was variable in many studies, even variable EBV cut-off value were reported in same center17,27. Overall, the proportions of more than 5000 copies in our center were lower than the reports of endemic region17,27,28. The potential reasons for this situation might be explained as follows: (1) lack of unified methods for the detection of EBV DNA; (2) low copies of EBV DNA in non-endemic NPC; (3) specific infection status of EBV in non-endemic region of China; (4) presence of specific EBV strain infection in non-endemic region of China.
Conclusion
Compared with CCRT, IC + CCRT could improve the distant metastasis-free survival and overall survival in advanced stage NPC patients with different nonkeratinizing carcinoma subtypes. However, prospective studies with large cohort are needed to further assess the eventually efficacy of IC + CCRT for patients with WHO type IIa.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108, https://doi.org/10.3322/caac.21262 (2015).
Cheng, S. H. et al. Examining prognostic factors and patterns of failure in nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy: impact on future clinical trials. International journal of radiation oncology, biology, physics 50, 717–726 (2001).
Ou, S. H., Zell, J. A., Ziogas, A. & Anton-Culver, H. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Annals of oncology: official journal of the European Society for Medical Oncology 18, 29–35, https://doi.org/10.1093/annonc/mdl320 (2007).
Barnes, L. Pathology and genetics of head and neck tumours. (IARC Press, 2005).
Zang, J. et al. Prognostic Model of Death and Distant Metastasis for Nasopharyngeal Carcinoma Patients Receiving 3DCRT/IMRT in Nonendemic Area of China. Medicine 95, e3794, https://doi.org/10.1097/MD.0000000000003794 (2016).
Guo, R. et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 104, 294–299, https://doi.org/10.1016/j.radonc.2012.09.001 (2012).
Zhao, L. et al. Induction chemotherapy for the treatment of non-endemic locally advanced nasopharyngeal carcinoma. Oncotarget, https://doi.org/10.18632/oncotarget.14279 (2016).
Cheung, F. et al. The prognostic value of histological typing in nasopharyngeal carcinoma. Oral Oncol 48, 429–433, https://doi.org/10.1016/j.oraloncology.2011.11.017 (2012).
Zhao, L. N. et al. Clinical outcome for nasopharyngeal carcinoma with predominantly WHO II histology treated with intensity-modulated radiation therapy in non-endemic region of China. Oral Oncol 48, 864–869, https://doi.org/10.1016/j.oraloncology.2012.03.001 (2012).
Al-Sarraf, M. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 16, 1310–1317, https://doi.org/10.1200/jco.1998.16.4.1310 (1998).
Sun, X. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 110, 398–403, https://doi.org/10.1016/j.radonc.2013.10.020 (2014).
Blanchard, P. et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. The Lancet. Oncology 16, 645–655, https://doi.org/10.1016/S1470-2045(15)70126-9 (2015).
Chen, L. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. The Lancet. Oncology 13, 163–171, https://doi.org/10.1016/S1470-2045(11)70320-5 (2012).
Sun, Y. et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. The Lancet. Oncology 17, 1509–1520, https://doi.org/10.1016/S1470-2045(16)30410-7 (2016).
Wang, J. et al. Failure patterns and survival in patients with nasopharyngeal carcinoma treated with intensity modulated radiation in Northwest China: a pilot study. Radiat Oncol 7, 2, https://doi.org/10.1186/1748-717X-7-2 (2012).
Lee, N. et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 27, 3684–3690, https://doi.org/10.1200/jco.2008.19.9109 (2009).
Guo, S. S. et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in stage III-IVb nasopharyngeal carcinoma patients with Epstein-Barr virus DNA >/= 4000 copies/ml: a matched study. Oncotarget 7, 29739–29748, https://doi.org/10.18632/oncotarget.8828 (2016).
Hui, E. P. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 27, 242–249, https://doi.org/10.1200/jco.2008.18.1545 (2009).
Zhang, L. et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet (London, England) 388, 1883–1892, https://doi.org/10.1016/s0140-6736(16)31388-5 (2016).
Reddy, S. P., Raslan, W. F., Gooneratne, S., Kathuria, S. & Marks, J. E. Prognostic significance of keratinization in nasopharyngeal carcinoma. American journal of otolaryngology 16, 103–108 (1995).
Shanmugaratnam, K. et al. Histopathology of nasopharyngeal carcinoma: correlations with epidemiology, survival rates and other biological characteristics. Cancer 44, 1029–1044 (1979).
Liu, Q., Chen, J. O., Huang, Q. H. & Li, Y. H. Trends in the survival of patients with nasopharyngeal carcinoma between 1976 and 2005 in Sihui, China: a population-based study. Chinese journal of cancer 32, 325–333, https://doi.org/10.5732/cjc.012.10189 (2013).
Xiao, D. et al. Opposed expression of IKKalpha: loss in keratinizing carcinomas and gain in non-keratinizing carcinomas. Oncotarget 6, 25499–25505, https://doi.org/10.18632/oncotarget.4548 (2015).
Yan, M. et al. IKKalpha restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiation. Nature communications 5, 3661, https://doi.org/10.1038/ncomms4661 (2014).
Deng, L. et al. Increase in IkappaB kinase alpha expression suppresses the tumor progression and improves the prognosis for nasopharyngeal carcinoma. Molecular carcinogenesis 54, 156–165, https://doi.org/10.1002/mc.22087 (2015).
Lee, H. W. et al. High expression of excision repair cross-complementation group 1 protein predicts poor outcome in patients with nasopharyngeal cancer. Oral Oncol 46, 209–213, https://doi.org/10.1016/j.oraloncology.2009.12.007 (2010).
Peng, H. et al. Prognostic Value of Neoadjuvant Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma with Low Pre-treatment Epstein-Barr Virus DNA: a Propensity-matchedAnalysis. Journal of Cancer 7, 1465–1471, https://doi.org/10.7150/jca.15736 (2016).
Peng, H. et al. Optimize the cycle of neoadjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: A propensity score matching analysis. Oral Oncol 62, 78–84, https://doi.org/10.1016/j.oraloncology.2016.10.014 (2016).
Acknowledgements
This work was supported by the national natural science foundation of ShannXi, China (No. 2016JQ8038).
Author information
Authors and Affiliations
Contributions
Jian zang and mei shi contributed the design of study. Chen li contributed the statistical method. Jian zang and chen li wrote the manuscript and prepared for all the tables and figures. Man xu mainly collected the data. Wanni xu contributed the pathological review and xiaowei kang contributed the image review for all the patients data. Jianhua wang and shanquan luo contributed the data collection.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zang, J., Li, C., Xu, M. et al. Induction chemotherapy followed by concurrent chemoradiotherapy is benefit for advanced stage nasopharyngeal carcinoma with different nonkeratinizing carcinoma subtypes. Sci Rep 8, 13318 (2018). https://doi.org/10.1038/s41598-018-31050-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31050-z
This article is cited by
-
Detailed analysis of recovery process of cranial nerve palsy after IMRT-based comprehensive treatment in nasopharyngeal carcinoma
Radiation Oncology (2021)
-
Gemcitabine and cisplatin versus docetaxel and cisplatin as induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma from non-endemic area of China
Journal of Cancer Research and Clinical Oncology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.