Abstract
L-Ascorbic acid (vitamin C, AA) exhibits anti-cancer effects with high-dose treatment through the generation of reactive oxygen species (ROS) and selective damage to cancer cells. The anti-cancer effects of L-ascorbic acid are determined by sodium-dependent vitamin C transporter 2 (SVCT-2), a transporter of L-ascorbic acid. In this study, we demonstrate that L-ascorbic acid treatment showed efficient anti-cancer activity in cell lines with high expression levels of SVCT-2 for a gradient concentration of L-ascorbic acid from 10 μM −2 mM. However, in low SVCT-2 expressing cell lines, high-dose L-ascorbic acid (>1 mM) showed anti-cancer effects but low-dose (<10 μM) treatment induced cell proliferation. Such conflicting results that depend on the concentration are called a hormetic dose response. A hormetic dose response to low-dose L-ascorbic acid was also observed in high SVCT-2 expressing cell lines in the presence of a SVCT family inhibitor. Insufficient uptake of L-ascorbic acid in low SVCT-2 expressing cancer cell lines cannot generate sufficient ROS to kill cancer cells, resulting in the hormetic response. Molecular analysis confirmed the increased expression of cancer proliferation markers in the hormetic dose response. These results suggest that L-ascorbic exhibits a biphasic effect in cancer cells depending on SVCT-2 expression.
Similar content being viewed by others
Introduction
L-Ascorbic acid (Vitamin C, AA), which is known as an antioxidant, acts as a pro-oxidant in cancer cells and selectively kills cancer cells when administered at a high dose1. Through various studies, the anti-cancer effects of L-ascorbic acid were shown to be mediated by inhibition of cellular proliferation and growth through the generation of reactive oxygen species (ROS) and hydrogen peroxide-mediated effects on in vitro systems2,3,4,5,6. ROS induce cellular damage and induce oxidative stress in cancer cells depending on redox status and metabolism7,8. In in vivo systems, a pharmacologic dose of L-ascorbic acid acted as pro-oxidant and showed anti-cancer effects with generation of ascorbate radicals9,10. Many researchers have investigated the mechanism of high-dose L-ascorbic acid therapy through ROS generation, which affects cytochrome c release in mitochondria and finally leads to apoptosis7,11.
Historically, L-ascorbic acid was first recognized as a potential cancer therapeutic agent by Linus Pauling and Ewan Cameron in 197612. High-dose L-ascorbic acid therapy increased the average survival time in previous studies13. However, a study conducted in the Mayo clinic showed that L-ascorbic acid therapy has no benefits in cancer patients14,15. As a possible explanation for this discrepancy, a recent study suggested that sodium-dependent vitamin C transporter family 2 (SVCT-2) is an indicator for high-dose L-ascorbic acid therapy by regulating the uptake of L-ascorbic acid uptake. Moreover, another study suggested that the metabolic state of cancer cells might be linked to the efficacy of high-dose L-ascorbic acid therapy16. However, various questions still remain regarding previous controversial clinical studies12,13,14,15, including (1) the sufficient dose of L-ascorbic acid for various cancer cells, (2) the reason behind the poor survival rate of patients treated with high-dose L-ascorbic acid, which was even lower than that of the placebo group, in the Mayo Clinic study14,15; and (3) whether the anti-cancer activity of L-ascorbic acid changes when the plasma concentration of the delivered L-ascorbic acid decreases and is maintained at a low level for about 4 hours in blood17 due to spontaneous oxidization over a short period of time18.
We hypothesized that these issues can be explained by the hormetic dose response, which is also known as the biphasic dose response in the pharmacological concept and is explained by a U-shaped curve19, that was observed in several cancer research studies20,21,22,23,24,25,26. To address these questions, we proposed the hypothesis that in a gradient concentration of L-ascorbic acid with different expression levels of SVCT-2, the anti-cancer effects of L-ascorbic acid change to hormetic proliferation when insufficient ROS are generated. Since insufficient ROS for cellular apoptosis promotes proliferation of cancer cells through insulin-like growth factor-127 and activation of the Ras gene28, we propose that insufficient uptake of L-ascorbic results in proliferation of cancer cells.
Materials and Methods
Cell culture and reagents
Human colorectal cancer cell lines including Sw620, Sw480, HCT15, HCT116, DLD-1, LoVo, CoLo-205 were purchased from ATCC and human colorectal cancer cell line SNU-C4 and SNU-C5 were purchased from Korea Cell Line Bank (Seoul, Korea). Human colorectal cancer cells were cultured in RPMI1640 media (Gilbco, Cergy Pontoise, France) with 10% fetal bovine serum (Pan Biotech, Aidenbach, Germany) and 1% Penstrep (Pan Biotech) at 37 °C in a humidified incubator with 5% CO2. L-Ascorbic acid was purchased from BCWorld Pharm. Co. (Seoul, Korea) and phloretin was purchased from Sigma Aldrich (St. Louis, MO, USA).
Cell viability assay
Cell viability was measured by Neutral Red assay (Sigma). Cells (1 × 104/well) were seeded and cultured in 96-well plates and incubated for 24 hours. Calls were treated with L-ascorbic acid for 4 hours, washed with phosphate buffered saline (PBS, Pan Biotech), and cultured for an additional 20 hours in RPMI1640 without L-ascorbic acid. Cells were washed two times with PBS, and stained with ref.29.
Heat-map visualization of normalized AUC and hormetic response index
Normalized area under curve (AUC) estimates, measured from the experimental drug response profile and hormetic response index, obtained from statistical consideration of hormetic responses, were used to generate an unbiased clustering of the L-ascorbic acid response profile of cancer cells.
qRT-PCR analysis
Total RNA was extracted from cells using TRI reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. RNA concentrations were determined by absorbance at 260 nm using a spectrophotometer. cDNA was synthesized using MML-V reverse transcriptase (Bioneer Co, Daejeon, Republic of Korea) according to the manufacturer’s protocols. Quantitative real-time PCR was performed using SYBR Premix Ex Taq (TaKaRa, Otsu, Shinga, Japan) and a Rotor-Gene Q system (Qiagen, Chadstone, Victoria, Australia). Data were analyzed using Rotor-Gene Q series software version 2.3.1 (Qiagen). The following genes were amplified with the indicated primers: p53 (forward 5′-AGGCCTTGGACCTCAAGGATG-3′; reverse 5′-TGAGTCAGGCCCTTCTGTCT-3′), cyclin D1 (forward 5′-GCTGCCAAGTGGAAACCARC-3′; reverse 5′-CCTCCTTCTGCACACATTTGAA-3′), E2F1 (forward 5′-ATGTTTTCCTGTGCCCTGAG-3′; reverse 5′-TGGTGGTGGTGACACTATGG-3′) Ki-67 (forward 5′-ACGCCTGGTTACTATCAAAAGG-3′; reverse 5′-CAGACCCATTTACTTGTGTTGGA-3′).
Western blotting
Proteins were extracted from cells with a PRO-PREP protein extraction kit according to the manufacturer’s instructions (iNtRON, Sungnam, Korea). Protein concentrations were measured using the Bradford assay (Bio-Rad, Munich, Germany). A total of 30 μg of protein was denatured in sample buffer for 6 minutes at 95 °C. The samples were loaded on 12% SDS-polyacrylamide gels and transferred to nitrocellulose blotting membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline at room temperature for 30 minutes. After three washes in Tris-buffered saline-0.10% Tween 20, the membranes were incubated with anti-Bax (1:2000; 2774; Cell Signaling Technology, Beverly, MA, USA), anti-SVCT2 (1:2500, NBP2-13319, Novus biologics), cyclin D1 (1:2500; NB600-584; Novus biologics), anti-c-Myc (1:2500; NB200-108; Novus biologics), and anti-beta-actin (1:5000; ab20272; Abcam, Cambridge, MA, USA) antibodies at 4 °C overnight. After four washes in Tris-buffered saline-0.10% Tween 20 for 20 minutes, the membranes were incubated with secondary anti-rabbit, anti-rat, or anti-goat antibodies for 1 hour at room temperature. After additional washing, immune-reactive bands were detected using ECL substrate (Pierce, Rockford, IL, USA) and exposure to X-ray film (Agfa-Gevaert N.V., Septestraat, Mortsel, Belgium).
Detection of ROS generation
Cells were incubated with 20 μM of 2′,7′-Dichlorofluorescin diacetate (Sigma) in culture media for 20 minutes and detached with trypsin and collected in 1 mL of PBS. Cells were washed two times with 500 μL PBS and analyzed on a Guava EasyCyte mini instrument using Cytosoft software version 4.2.1 (Merck Millipore, Billerica, MA, USA).
L-Ascorbic acid uptake
Cells were harvested after 2-hour incubation with 5 mM L-ascorbic acid and washed with PBS. The cells were resuspended in 1 mL of PBS with 10% meta-phosphoric acid (MPA) solution and lysed three times by freeze-thaw cycles in a −80 °C deep freezer. The lysate was centrifuged at 16000 rpm at 4 °C for 5 minutes and the supernatant was harvested. Next, 100 μL of sample was mixed with 100 μL of precipitation reagents of vitamin C diagnostics kits (Chromosystems, Gräfelfing, Germany) and incubated for 10 minutes at 4 °C. The mixture was centrifuged at 13000 rpm for 5 minutes and the supernatant was analyzed using a high-performance liquid chromatography (HPLC) system (Shimadzu Corporation, Tokyo, Japan) equipped with Shim-pack CLC-ODS column (6 mm × 15 cm) connected to a Shim-pack G-ODS guard column (4 mm × 1 cm) (Shimadzu). The mobile phase was provided by Chromsystems and the experiment was performed according to the instruction manual. The concentration of L-ascorbic acid in cells was determined by manual calculation \({C}_{Analyte,Sample}\,(mg/l)=\frac{{A}_{Sample}\times I{S}_{Standard}}{{A}_{Standard}\times I{S}_{Sample}}\times {C}_{Standard}\). The following instrument settings were used: injection volume 20 μL, run time 10 min, flow rate 1 mL/min, column temperature 25 °C, and UV detector wavelength 245 nm.
Results
L-Ascorbic acid exhibited anti-cancer effects according to SVCT-2 expression and L-ascorbic acid uptake
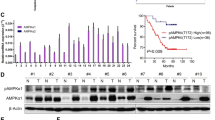
Previous studies demonstrated that SVCT-2 expression acts as an indicator for high-dose L-ascorbic treatment30. Therefore, we investigated SVCT-2 expression, L-ascorbic acid uptake, and cytotoxic effects of L-ascorbic acid in different human colorectal cancer cell lines. The cell lines showed different levels of SVCT-2 expression in western blot analyses (Fig. 1A): Sw620, Sw480, and Lovo expressed high levels of SVCT-2 whereas HCT116, HCT15, and DLD-1 expressed low levels. Results of the cell viability assay showed that the cytotoxicity of L-ascorbic acid was proportional to SVCT-2 expression (Fig. 1B). Moreover, analysis of the uptake of L-ascorbic acid by cells using high performance liquid chromatography (HPLC) showed that uptake of L-ascorbic acid was also proportional to SVCT-2 expression (Fig. 1C).
SVCT-2 expression, cytotoxicity, and uptake of L-ascorbic acid in colorectal cancer cell lines. (A) SVCT-2 expression was analyzed by western blotting. GAPDH was used as a loading control. (B) HPLC analysis of the uptake of L-ascorbic acid. (C) Relative SVCT-2 expression determined with Image J program analysis (black bars) and cell viability with 1 mM L-ascorbic acid treatment (white bars).
Hormetic response to a concentration gradient of L-ascorbic acid in low SVCT-2 expressing cell lines
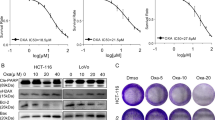
To investigate the cell autonomous impact of L-ascorbic acid on cancer cells, the cell viability assay was performed with a gradient of L-ascorbic acid concentration. The cancer cell lines displayed differential responses to L-ascorbic acid, primarily depending on the expression level of SVCT-2. Some high SVCT-2 expressing cancer cells demonstrated a dramatic cell-autonomous inhibitory effect of L-ascorbic acid (Fig. 2A). In contrast, low SVCT-2 expressing cell lines showed biphasic responses to L-ascorbic acid. This hormetic response to L-ascorbic acid in low SVCT-2 expressing cells was characterized by an anti-cancer effect at a high dose (>1 mM) and a pro-proliferative effect at low doses (<10 μM) (Fig. 2B). High SVCT-2 expressing cell lines showed anti-cancer effects of L-ascorbic acid at all concentrations. These data were further quantitatively assessed with experimental and statistical estimates to generate an unbiased cluster of drug response profile. We particularly focused on the drug sensitivity, presented by the area under curve (AUC), and the hormetic responsiveness index, a statistical estimate of response pattern. Clustering of the data generated three different drug response patterns (Fig. 2C). Cluster 1 included Sw620, Sw480, and LoVo and was largely sensitive to L-ascorbic treatment. Cluster 2 included SNU C4, which showed an intermediate response to L-ascorbic acid. Cluster 3 included DLD-1, Colo-205, HCT116, and HCT15, which were insensitive to L-ascorbic treatment and showed a hormetic response. Cluster 3 co-segregated with the low SVCT-2 expressing group.
Hormetic response with gradient L-ascorbic acid treatment in low SVCT-2 expressing cell lines but not in high SVCT-2 expressing cell lines. (A) Cell viability of high SVCT-2 expressing cell lines with gradient L-ascorbic acid treatment. (B) Cell viability of low SVCT-2 expressing cell lines with gradient L-ascorbic acid treatment. (C) Clustering and heat-map visualization of the response of eight colorectal cancer cell lines to L-ascorbic acid. Drug sensitivity is presented by the area under curve (AUC), and the hormetic responsiveness index, a statistical estimate of the response pattern.
ROS generation, apoptotic response, and gene expression analysis in low SVCT-2 expressing cell lines with hormetic response
Treatment with 1 mM L-ascorbic acid induced anti-cancer effects in low SVCT-2 expressing cell lines whereas 10 μM L-ascorbic acid promoted proliferation of these cells. To investigate ROS generation induced by 1 mM and 10 μM L-ascorbic acid, low SVCT-2 expressing cell lines were stained with 2′,7′-Dichlorofluorescin diacetate (DCF-Da) after L-ascorbic acid treatment (Fig. 3A,B). In the low SVCT-2 expressing cell lines DLD-1 and HCT15, ROS were generated with 1 mM L-ascorbic acid treatment but not with 10 μM L-ascorbic acid. To confirm apoptotic and hormetic proliferation responses with L-ascorbic acid treatment, a quantitative real-time polymerase chain reaction (qRT-PCR) was performed for gene expression analysis. Expression of tumor protein 53 (p53) indicated apoptotic responses after treatment with 10 μM and 1 mM L-ascorbic acid in high SVCT-2 expressing cell lines; however, in low SVCT-2 expressing cell lines, only 1 mM L-ascorbic acid treatment induced p53 expression (Fig. 3C). Cyclin D1 expression was analyzed for determination of proliferation (Fig. 3D). In addition, expression of E2F transcription factor 1 (E2F1) and Antigen KI-67 (Ki-67) was increased in low SVCT-2 expressing cell lines after treatment with 10 μM L-ascorbic acid treatment (Fig. 3E,F). Low-dose L-ascorbic acid treatment induced a hormetic dose response in low SVCT-2 expressing cell lines DLD-1 and HCT15 and also induced cyclin D1, E2F1, and Ki-67 expression. However, in high SVCT-2 expressing cell lines Sw620 and Sw480, the expression of cyclin D1 decreased after treatment with both 10 μM and 1 mM L-ascorbic acid. These results suggest that L-ascorbic acid induced anti-cancer effects at both high and low doses in high SVCT-2 expressing cell lines. In contrast, in low SVCT-2 expressing cell lines, high-dose L-ascorbic acid exhibited anti-cancer effects as evidenced by expression of p53, whereas low-dose L-ascorbic acid treatment induced genes associated with cell proliferation.
ROS generation in low SVCT-2 expressing cell lines. (A) ROS generation was detected by DCF-Da staining in the DLD-1 cell line. (B) ROS generation in the HCT15 cell line was detected with 1 mM L-ascorbic acid but not with 10 μM L-ascorbic acid. (C) Expression of p53 in hormetic response condition and apoptotic response condition with 10 μM and 1 mM L-ascorbic acid treatment. (D–F) Expression of cancer cell proliferation markers E2F1, cyclin D1, and Ki-67 in hormetic response condition and apoptotic response condition with 10 μM and 1 mM L-ascorbic acid treatment. Data are presented as means ± SEMs. *P < 0.05, **P < 0.005.
Molecular analysis of hormetic response of L-ascorbic acid treatment in low SVCT-2 expressing cell lines
To further investigate apoptosis and proliferation of cancer cells in response to L-ascorbic acid treatment at the protein expression level, western blot analysis was performed after treatment with low and high concentrations of L-ascorbic acid. Low SVCT-2 expressing cell lines HCT15 and DLD-1 showed increased c-Myc and cyclin D1 expression as a proliferative response after treatment with 10 μM L-ascorbic acid but decreased expression of these proteins with 1 mM L-ascorbic acid (Fig. 4A,B). After treatment with 10 μM L-ascorbic acid, expression of cyclin D1 was increased and co-localization of cyclin D1 with CDK4 was observed by confocal microscopy (Supplementary Figs 2 and 3).
Hormetic response in high SVCT-2 expressing cell lines with SVCT-2 family inhibitors
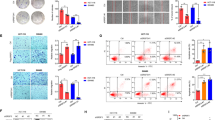
To determine whether the hormetic proliferation response was mediated through L-ascorbic acid uptake via SVCT-2, we treated high SVCT-2 expressing cell lines with 1 mM or 10 μM L-ascorbic acid in combination with phloretin as a SVCT family inhibitor. The concentration of phloretin was confirmed to be non-toxic by a cell viability assay (Supplementary Fig. 1A). L-Ascorbic acid uptake in Sw480 and Sw620 cell lines was inhibited by phloretin in the presence of 1 mM L-ascorbic acid (Supplementary Fig. 1B). With inhibition of the SVCT family, high SVCT-2 expressing cell lines showed a hormetic proliferation response when treated with 10 μM L-ascorbic acid (Fig. 5A,B). These results suggest that the hormetic proliferation response can be triggered when an insufficient amount of L-ascorbic acid is delivered to cancer cells.
Hormetic response in high SVCT-2 expressing cell lines with SVCT-2 inhibition. (A,B) Cell viability assay in Sw620 and Sw480 cell lines treated with 10 μM or 1 mM L-ascorbic acid and phloretin. (C,D) ROS assay by DCF-Da staining in Sw620 and Sw480 cell lines treated with 10 μM or 1 mM L-ascorbic acid and phloretin. Data are presented as means ± SEMs. *P < 0.05, **P < 0.005.
ROS generation in apoptotic response and hormetic response of high SVCT-2 expressing cell lines
In high SVCT-2 expressing cell lines Sw620 and Sw480 both 1 mM and 10 μM L-ascorbic acid induced an apoptotic response, but upon inhibition of SVCT-2 these cells showed a hormetic proliferation response. Since inhibition of SVCT with phloretin induced hormetic proliferation with 10 μM L-ascorbic acid, we investigated ROS generation via DCF-Da staining. ROS generation was induced by treatment with 1 mM and 10 μM L-ascorbic acid. However, inhibition of SVCT-2 by phloretin decreased ROS generation (Fig. 5C,D).
Expression of cancer cell proliferation markers in high SVCT-2 expressing cell lines
To analyze the hormetic proliferation response induced by inhibition of SVCT-2 with phloretin, the expression of cancer proliferation markers and BAX was analyzed by qRT-PCR and western blotting. Expression of Ki-67 and E2F1, which were used as cancer proliferation markers, was investigated by qRT-PCR. The results showed increased expression of Ki-67 and E2F1 in Sw620 and Sw480 cell lines treated with 10 μM L-ascorbic acid and phloretin (Fig. 6A,B). Also, in western blot analysis, c-Myc and cyclin D1 protein expression was significantly decreased by treatment with 1 mM and 10 μM L-ascorbic acid but increased under hormetic proliferation conditions in the presence of phloretin (Fig. 6C,D).
Discussion
The controversy surrounding L-ascorbic acid cancer therapy began with conflicting clinical results from Linus Pauling’s study and the Mayo Clinic study12,13,14,15. To resolve this controversy, numerous studies have focused on the anti-cancer effect of L-ascorbic acid and its mechanism1,2,3,4,8,10. Some studies demonstrated that cytotoxic action of L-ascorbic acid mediated by oxidative DNA breakage in lymphocyte considered with cellular copper ion levels5,6,31. Hadi et al. (2011) demonstrated importance of chromatin-bound copper in the pro-oxidant cellular DNA breakage by ascorbic acid. Similar with other plant derived pro-oxidants such as thymoquinone, epicatechin and eoigallocatechin-3-gallate, L-ascorbic acid generated ROS in cancer cells via SVCT2 and led to cell death32,33,34. Other studies revealed that SVCT-2 expression is a determination factor of L-ascorbic acid cancer treatment30 and that hypoxia-inducible factor–positive cells are susceptible to L-ascorbic acid treatment16. Other studies developed cancer therapy regimens that combined L-ascorbic acid with chemotherapeutic agents or other agents35,36,37. Although these research studies revealed the action mechanism and developed more effective application methods for L-ascorbic acid cancer therapy there was no explanation for why the group of patients who were treated with L-ascorbic acid in the Mayo Clinic study showed a lower survival rate than the placebo group14,15. To interpret these results we investigated the action of L-ascorbic acid on cancer cells using a gradient concentration of 1 μM to 2 mM based on pharmacokinetics results for L-ascorbic acid17.
It was previously reported that SVCT-2 expression is an indicator for L-ascorbic acid treatment30. In this report we confirmed that SVCT-2 functions as a L-ascorbic acid transporter and that the anti-cancer effects of L-ascorbic acid are proportional to SVCT-2 expression of cell lines. To interpret results from the Mayo Clinic and other past clinical studies12,13,14,15 we proposed that L-ascorbic acid has a biphasic role in cancer cells depending on L-ascorbic acid uptake, which is in turn dependent on SVCT-2 expression levels. Our results demonstrated that if there was a sufficient concentration of L-ascorbic acid to generate ROS it functions as an anti-cancer agent regardless of SVCT-2 expression, whereas a hormetic proliferation response was observed with low SVCT-2 expressing cell lines in which there was insufficient uptake of L-ascorbic acid to generate ROS. In high SVCT-2 expressing cell lines, L-ascorbic acid showed cell growth inhibition and apoptotic responses at all concentrations of L-ascorbic acid. Therefore, with sufficient delivery into cancer cells via a high SVCT2 expression level L-ascorbic acid is an effective chemotherapeutic agent but deficient delivery of L-ascorbic acid to cancer cells due to low SVCT2 instead increases the proliferation activity of cancer. Cyclin D1 has prognostic significance through its function in cancer cell proliferation38,39,40, and also plays an essential role in recruiting transcription factors such as E2F1 and regulates gene transcription via inhibition of p30041,42. According to our results, cyclin D1, which was induced by a low concentration of L-ascorbic acid, is a significant factor in the hormetic proliferation response. It remains to be elucidated which proteins or signaling molecules are stimulated by ascorbic acid, but our results suggest that cyclin D1 and cyclin-dependent kinase (CDK4) co-localization could be a critical factor for cell proliferation (Supplementary Figs 2 and 3)43. In addition, increased expression of c-Myc, Ki-67, and E2F1, cyclin D1–related proliferation markers, further supports results of the cell viability assay. Ki-67 and c-Myc increase cell proliferative activity through DNA binding40,44,45. These results are summarized in Fig. 7.
Our findings indicate that L-ascorbic acid has effective chemotherapeutic potential in high SVCT-2 expressing cancer cells. However, an insufficient dose of L-ascorbic acid stimulates cancer cell proliferation, which is mediated by cyclin D1. Therefore, high-dose L-ascorbic acid cancer therapy is an effective treatment for high SVCT-2 expressing cancer with few side effects to patients. However, for low SVCT-2 expressing cancers L-ascorbic acid might not only be less effective, but could also be deleterious to cancer patients. This hypothesis may explain the conflicting clinical results from two different research groups in which the SVCT-2 expression status of patients was unknown12,13,14,15.
In addition, we expect that our results are followed from anti-oxidant property of L-ascorbic acid. Some studies shown anti-oxidants expediting cancer progression46,47. Sayin et al. (2014) showed that N-acetylcysteine (NAC) and vitamin E reduced ROS in cancer cell and increased proliferation of cancer cell. These results demonstrate that when oxidative stress reduced, cancer cells are more proliferative because endogenous anti-oxidant related oncogene nuclear factor erythroid-2–related factor 2 reduced ROS thereby increased cancer proliferation48,49,50. It is possible that other anti-oxidant such as NAC, Vitamin E, glutamine and others could show similar hormetic proliferation response like L-ascorbic acid.
We demonstrated that high-dose L-ascorbic acid cancer therapy requires careful consideration with regard to generating a sufficient concentration of L-ascorbic acid inside cancer cells. Also, determination factors for L-ascorbic acid cancer therapy in addition to SVCT-2 and hypoxia-inducible factor16,30 should be discovered for more effective cancer therapy. Our findings also suggest that the combination of high-dose L-ascorbic acid cancer therapy with SVCT-2 inducible agents or synergistic chemotherapeutic agents could be a more effective cancer therapy and may resolve the current controversies surrounding L-ascorbic acid cancer therapy.
References
Chen, Q. et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 102, 13604–13609 (2005).
Maramag, C., Menon, M., Balaji, K., Reddy, P. G. & Laxmanan, S. Effect of vitamin C on prostate cancer cells in vitro: effect on cell number, viability, and DNA synthesis. Prostate 32, 188–195 (1997).
Park, S. et al. L-Ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int J Biochem Cell Biol. 36, 2180–2195 (2004).
Kim, J.-E. et al. Vitamin C inhibits p53-induced replicative senescence through suppression of ROS production and p38 MAPK activity. Int J Mol Med 22, 651–655 (2008).
Hadi, S., Ullah, M., Shamim, U., Bhatt, S. & Azmi, A. Catalytic therapy of cancer by ascorbic acid involves redox cycling of exogenous/endogenous copper ions and generation of reactive oxygen species. Chemotherapy 56, 280–284 (2010).
Ullah, M., Khan, H., Zubair, H., Shamim, U. & Hadi, S. The antioxidant ascorbic acid mobilizes nuclear copper leading to a prooxidant breakage of cellular DNA: implications for chemotherapeutic action against cancer. Cancer Chemother Pharmacol 67, 103–110 (2011).
Colussi, C. et al. H2O2-induced block of glycolysis as an active ADP-ribosylation reaction protecting cells from apoptosis. FASEB J. 14, 2266–2276 (2000).
Uetaki, M., Tabata, S., Nakasuka, F., Soga, T. & Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci Rep. 5 (2015).
Chen, Q. et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA 105, 11105–11109 (2008).
Chen, Q. et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104, 8749–8754 (2007).
Cai, J. & Jones, D. P. Superoxide in Apoptosis Mitochondrial Generation Triggered by Cytochromec Loss. J Biol Chem. 273, 11401–11404 (1998).
Cameron, E. & Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA 73, 3685–3689 (1976).
Cameron, E. & Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA 75, 4538–4542 (1978).
Creagan, E. T. et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: a controlled trial. N Engl J Med 301, 687–690 (1979).
Moertel, C. G. et al. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: a randomized double-blind comparison. N Engl J Med 312, 137–141 (1985).
Tian, W. et al. The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J Biol Chem 289, 3339–3351 (2014).
Padayatty, S. J. et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 140, 533–537 (2004).
Bode, A., Cunningham, L. & Rose, R. Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin Chem 36, 1807–1809 (1990).
Calabrese, E. J. Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol 35, 463–582 (2005).
Paoletti, P. et al. Characteristics and biological role of steroid hormone receptors in neuroepithelial tumors. J Neurosurg 73, 736–742 (1990).
Love-Schimenti, C. D., Gibson, D. F., Ratnam, A. V. & Bikle, D. D. Antiestrogen potentiation of antiproliferative effects of vitamin D3 analogues in breast cancer cells. Cancer Res 56, 2789–2794 (1996).
A, K., Tamaki, N. & T, K. Effect of dexamethasone on cell proliferation of neuroepithelial tumor cell lines. Neurol Med Chir (Tokyo) 38, 633–640 (1998).
Tamir, S. et al. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res 60, 5704–5709 (2000).
Nakagawa, H. et al. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol 127, 258–264 (2001).
Ying, C. et al. Growth and cell cycle regulation by isoflavones in human breast carcinoma cells. Reprod Nutr Dev 42, 55–64 (2002).
Dings, R. P. et al. Anti-tumor activity of the novel angiogenesis inhibitor anginex. Cancer Lett 194, 55–66 (2003).
Stratton, M. S., Yang, X., Sreejayan, N. & Ren, J. Impact of insulin-like growth factor-I on migration, proliferation and Akt-ERK signaling in early and late-passages of vascular smooth muscle cells. Cardiovasc Toxicol 7, 273–281 (2007).
Finkel, T. Intracellular redox regulation by the family of small GTPases. Antioxid Redox Signal 8, 1857–1863 (2006).
Repetto, G., del Peso, A. & Zurita, J. L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3, 1125–1131 (2008).
Hong, S. et al. SVCT-2 in breast cancer acts as an indicator for L-ascorbate treatment. Oncogene 32, 1508–1517 (2013).
F Ullah, M. et al. Ascorbic acid in cancer chemoprevention: translational perspectives and efficacy. Curr Drug Targets 13, 1757–1771 (2012).
Zubair, H. et al. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis 4, e660 (2013).
Azam, S., Hadi, N., Khan, N. U. & Hadi, S. M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro 18, 555–561 (2004).
Farhan, M., Oves, M., Chibber, S., Hadi, S. M. & Ahmad, A. Mobilization of Nuclear Copper by Green Tea Polyphenol Epicatechin-3-Gallate and Subsequent Prooxidant Breakage of Cellular DNA: Implications for Cancer Chemotherapy. Int J Mol Sci. 18, 34 (2016).
Kurbacher, C. M. et al. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett 103, 183–189 (1996).
Monti, D. A. et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One 7, e29794 (2012).
Vuyyuri, S. B. et al. Ascorbic acid and a cytostatic inhibitor of glycolysis synergistically induce apoptosis in non-small cell lung cancer cells. PLoS One 8, e67081 (2013).
Peurala, E., Koivunen, P., Haapasaari, K.-M., Bloigu, R. & Jukkola-Vuorinen, A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res 15, 1 (2013).
Wang, L. D. et al. Changes in p53 and cyclin D1 protein levels and cell proliferation in different stages of human esophageal and gastric‐cardia carcinogenesis. Int J Cancer 59, 514–519 (1994).
Gordan, J. D., Thompson, C. B. & Simon, M. C. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12, 108–113 (2007).
Fu, M. et al. Cyclin D1 represses p300 transactivation through a cyclin-dependent kinase-independent mechanism. J Biol Chem 280, 29728–29742 (2005).
Fu, M. et al. Cyclin D1 inhibits peroxisome proliferator-activated receptor γ-mediated adipogenesis through histone deacetylase recruitment. J Biol Chem 280, 16934–16941 (2005).
Dong, Y., Sui, L., Sugimoto, K., Tai, Y. & Tokuda, M. Cyclin D1‐CDK4 complex, a possible critical factor for cell proliferation and prognosis in laryngeal squamous cell carcinomas. Int J Cancer 95, 209–215 (2001).
Gordan, J. D., Bertout, J. A., Hu, C.-J., Diehl, J. A. & Simon, M. C. HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11, 335–347 (2007).
Persson, H. & Leder, P. Nuclear localization and DNA binding properties of a protein expressed by human c-myc oncogene. Science 225, 718–721 (1984).
Sayin, V. I. et al. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6, 221ra215–221ra215 (2014).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101 (2013).
Singh, A. et al. RNAi-mediated silencing of nuclear factor erythroid-2–related factor 2 gene expression in non–small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68, 7975–7984 (2008).
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106 (2011).
Bauer, A. K. et al. Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice. PLoS One 6, e26590 (2011).
Author information
Authors and Affiliations
Contributions
S.R.C., C.Y. and S.L. designed the experiment and concepts. S.R.C. performed the experiments and analysis the data with J.C. The manuscript was drafted by S.R.C., H.S. assisted the experiments and Y.S. and H.S. performed HPLC experiments and analysis. Y.K. performed clustering of the cell viability data and AUC curve analysis. B.C.Y. distributed colorectal cancer cells. K.R., S.C.C., E.K., H.B., S.H.C., S.P., analysis and interpretation of data. All the authors discussed about the results and commented on manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, S., Chae, J.S., Shin, H. et al. Hormetic dose response to L-ascorbic acid as an anti-cancer drug in colorectal cancer cell lines according to SVCT-2 expression. Sci Rep 8, 11372 (2018). https://doi.org/10.1038/s41598-018-29386-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29386-7
This article is cited by
-
The level of active DNA demethylation compounds in leukocytes and urine samples as potential epigenetic biomarkers in breast cancer patients
Scientific Reports (2024)
-
Dual-functional lanthanide-MOF probe nanocomposite based on hydroxyapatite nanowires as fluorescent sensor for ascorbic acid
Microchimica Acta (2023)
-
The effects of glucose and ascorbic acid on in vitro development of Echinococcus granulosus metacestodes
Journal of Parasitic Diseases (2022)
-
Evaluation of anticancer activity in vitro of a stable copper(I) complex with phosphine-peptide conjugate
Scientific Reports (2021)
-
Role of p53 in transcriptional repression of SVCT2
Molecular Biology Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.