Abstract
Kinase-family with sequence similarity 20, member C (Fam20C) is a protein kinase, which can phosphorylate biomineralization related proteins in vertebrate animals. However, the function of Fam20C in invertebrate animals especially the role in biomineralization is still unknown. Herein, we cloned the cDNA of fam20C from the pearl oyster, Pinctada fucata. It is showed that the expression of fam20C in the mantle edge was much higher than other tissues. In situ hybridization showed that fam20C was expressed mostly in the outer epithelial cells of the middle fold, indicating it may play important roles in the shell formation. Besides, fam20C expression increased greatly in the D-shape stage of pearl oyster development, when the shell was first formed. During the shell repair process, the expression level of fam20C increased 1.5 times at 6 h after shell notching. Knockdown of fam20C in vivo by RNA interference resulted in abnormally stacking of calcium carbonate crystals at the edges of nacre tablets, showing direct evidence that fam20C participates in the shell formation. This study provides an insight into the role of kinase protein in the shell formation in mollusk and broaden our understanding of biomineralization mechanism.
Similar content being viewed by others
Introduction
Biomineralization is a process that minerals are formed by organisms1. Pearl oyster, Pinctada fucata is one of the most important cultured pearl species in China, and is also a good model species to study biomineralization2. The shell of P. fucata includes two layers, an inner nacreous aragonite layer, and an outer prismatic calcite layer. Both layers are composed of more than 95% calcium carbonate and <5% of organic macromolecules especially proteins, which are important for crystal nucleation, polymorphism, orientation and organization during shell formation3,4. Previous studies have reported many matrix proteins from shells. For instance, acidic matrix proteins with cation binding properties are known as important proteins in calcium carbonate crystallization and shell formation5,6,7. Several matrix protein domains have been identified including carbonic anhydrase domain in nacrein8 and N669, lectin domain in perlucin10 and pontin protein domain in dermatopontin11.
It is noted that some matrix proteins have post-translational modification such as glycosylation, phosphorylation and sulfation, which are crucial for their functions12. Phosphorylation is one of the most widespread post-translational modifications of proteins and also occurs in the organic matrix of biominerals13,14. Kinase is a series of evolutionary conserved enzyme, playing important roles in regulating cellular events by phosphorylating substrates15. Fam20C, also called dentin matrix protein 4, is a kind of kinase encoded by fam20C gene in homo sapiens16,17,18. Fam20C family was first identified in the process of hematopoietic stem cell differentiation into bone marrow cell16. In recent years, Fam20C is found to be a serine protein kinase located in Golgi and is able to phosphorylate casein and SIBLING (Small integrin-binding ligand, the N-linked glycoproteins) family by phosphorylating S-x-E motif19. The finding of Fam20C locating in Golgi proves that the ATP-dependent protein phosphorylation can occur in secreted pathways20,21. It is also revealed that Fam20C can modulate the differentiation of mesenchymal stem cells into odontoblast and regulate enamel mineralization17. In addition, Fam20C was highly expressed in dental tissues in mice and was highly expressed in odontoblast during the developing process of mice teeth, indicating that the Fam20C was closely related to mineral formation17. These studies confirmed that Fam20C was closely involved in the biomineralization process of vertebrate animals. However, the function of Fam20C in mollusk and the role of kinase in mollusk shell formation is poorly understood.
In pacific oyster C. gigas, tissue-specific microarray analysis showed that fam20C was highly expressed in the mantle22. The Lottia gigantea shell matrix phosphoproteome revealed that one third of phosphorylation sites were at the serine site of S-x-E motif, compared with 24% in human secreted phosphoproteins23. Recently, a dentin-matrix protein-like (DMP-like), exhibiting a remarkable Fam20C domain was detected in two freshwater mussels unionoid proteomes24. cfMSP-1, an extremely acidic matrix protein involved in shell formation of the scallop Chlamys farreri, had eight S-D-E motifs, which were possibly phosphorylated by Fam20C25. Despite these studies, no direct evidence has been given on the role of Fam20C in shell formation. Therefore, in this study, we cloned the cDNA of fam20C from the pearl oyster P. fucata and studied the tissue-specific distribution as well as the expression profiles during different development stages. In addition, shell notching experiment and RNA interference were performed to investigate the role of Fam20C in biomineralization in vivo.
Methods
Ethics statement
This study was approved by the Animal Ethics Committee of Tsinghua University, Beijing, China.
Experimental animals
All pearl oysters P. fucata used in this study were collected from Zhanjiang, Guangdong province of China and were cultured at 20 degrees centigrade in artificial seawater (3% salinity).
Tissue collection and preparation
Different tissues were extracted from the control or treated oysters. Then the tissues were immediately flash-frozen and were powdered in liquid nitrogen for further experiments. Especially, the samples of different developmental stages including oosperm stage, trochophore stage, D-shape stage, umbonal stage and juvenile stage were stored in RNAlater RNA stabilization reagent (Qiagen) and were collected from Zhanjiang, Guangdong province of China.
Total RNA extraction
Total RNA was extracted using Trizol reagent (Life technologies) following the manufacturer’s instruction. RNA integrity and purity were checked by 1.2% agarose gel electrophoresis and an UV/visible spectrophotometer (Ultrospec 3000, Amersham). RNA concentration was determined by NanoDrop 2000 (Thermo Scientific).
cDNA library construction
cDNA library was prepared by reverse transcription-PCR of the total RNA with GoScriptTM Reverse Transcription System (Promega) following the manufacturer’s instructions.
Full-length cDNA cloning by RACE
A conserved DNA sequence of fam20C gene was acquired by alignment between transcriptome of P. fucata and human fam20C gene sequence by Tagliabracci et al.19. The full-length cDNA of fam20C was cloned from the mantle total RNA using SMARTTM RACE cDNA Amplification kit (Clontech) following the instructions. RACE PCR was performed using primers named as Fam20C-3′RACE and Fam20C-5′RACE. Confirmation PCR ‘rimers were named as Fam20C-confirm-F and Fam20C-confirm-R. All primers used in this study were listed in Table S1.
Nucleotide and amino acid sequences analysis
Blast of sequences was attempted using Blastp and Blastn searches against NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The theoretical molecular mass, isoelectric point and amino acid composition of the proteins were computed using ProtParam from the EXPASY online server. Conserved domains were predicted using SMART (http://smart.embl-heidelberg.de/) and InterproScan (http://www.ebi.ac.uk/interpro/search/sequence-search). The alignment of sequences from different species was performed by online software Clustal Ω (http://www.ebi.ac.uk/Tools/msa/clustalo/). Signal peptide was predicted by online tool signal P4.1 (http://www.cbs.dtu.dk/services/SignalP/).
Gene expression analysis by real-time PCR
Real-time PCR was performed using StepOnePlusTM Real-time PCR systems (Applied Biosystems) with SYBR® Premix Ex TaqTM II Kit (Takara), with GAPDH as an internal reference due to its relatively stable expression in different tissues. Templates were cDNA libraries of different tissues. Primers were designed according to fam20C sequence named as qFam20C-F and qFam20C-R. The control primers were named as qGAPDH-F and qGAPDH-R. Different gene expression level was calculated using 2−ΔΔCt method by Livak and Schmittgen26.
In situ hybridization experiment
The mantle of pearl oyster was removed and was immediately fixed overnight in 4% paraformaldehyde containing 0.1% diethyl pyrocarbonate (Sigma) and was then washed in 0.1 M PBS. Washed sample was soaked in 20% sucrose solution at 4 degrees centigrade. Then frozen mantle section was prepared for in situ hybridization. The DNA fragments were amplified with the primer pair Fam20C-F and Fam20C-R and were inserted in multiple cloning sites of vector pEASY-T3 (Promega). Synthesized RNA probe was produced using DIG RNA Labeling Kit (Roche) with T7 and SP6 RNA polymerase for the sense and anti-sense probe respectively. In situ hybridization was carried out using Enhanced Sensitive ISH Detection Kit II (BOSTER). To avoid false positive signals, the hybridization temperature was increased to 58 degrees centigrade.
Shell notching experiment
The shell notching of pearl oysters was performed as described by Mount et al.27. Pearl oysters were randomly divided into eight groups with five animals each and were cultured in seawater tanks. At 0, 6, 12, 24, 36, 48, 72, and 96 h after shell notching, oysters were killed and mantle tissues were collected. The methods of RNA extraction, reverse transcription and real-time PCR were mentioned above.
Gene silencing in vivo by RNA interference (RNAi)
RNAi experiment was conducted according to the method by Suzuki et al.28 with some modifications. Firstly, DNA segments were produced by PCR. The primers were dsFam20C-F and dsFam20C-R; dsGFP-F and dsGFP-R. fam20C gene and gfp segment were produced from the mantle cDNA and pEGFP-C1(Clontech) respectively. Next, dsRNA was transcribed from DNA segment by RiboMaXTM Large Scale RNA Production System (T7) Kit (Promega) following manufacturer’s instructions. Then the synthesized dsRNA products were diluted to 80 μg/100 μL and 160 μg/100 μL by 0.1 M PBS. 100 μL of dsRNA was injected into four oysters for RNAi and 0.1 M PBS was injected as the control. All oysters were killed after six days and mantle tissues were collected. The shells were sampled after 6 days after RNAi experiment because it usually took about 6 days for the growth of new shells. We want to check the effect of fam20C knockdown on the new generated shells. Therefore, the time point of 6 days were chosen and the method has been used for RNAi experiment of matrix proteins28. The methods of RNA extraction, reverse transcription and real-time PCR were mentioned above. The shells were collected for further scanning electron microscope (SEM) observation.
SEM observation of shells
Collected shells were rinsed in MiliQ water 3 times before air drying in an incubator. Cleaned shell was sprayed gold nanoparticles for 60 s before observation. The morphologies of the inner surface of shells were examined by SEM (FEI Quanta 200, 15 keV).
Data accessibility
Nucleotide sequence of fam20C of P. fucata is available in the GenBank database under the accession numbers MF785096.
Statistical analysis
Multigroup comparisons of the means were carried out by one-way analysis of variance (ANOVA) test with post hoc contrasts by Bonferroni test (IBM SPSS Statistics 22 software). The statistical significance for all tests was set at P < 0.05. (*P < 0.05, **P < 0.01).
Results
Cloning and bioinformatics analysis of fam20C
Based on the homology of fam20C gene in different species, we found a highly conserved DNA sequence in the mantle transcriptome of P. fucata. Then, we cloned fam20C full-length sequence from the mantle cDNA library and obtained a 2739-bp transcript including a 5′-untranslated region of 28 bp, an open reading frame of 1512 bp encoding a protein containing 503 amino acids, and a 3′-untranslated region of 1199 bp (Fig. 1). The deduced mature protein had a molecular mass of 58.9 kDa and the theoretical isoelectric point was 8.36. After retrieving sequences of several important species including Danio rerio, Mus musculus, Homo sapiens, Lottia gigantean, Villosa lienosa and Crassostrea gigas from NCBI data base, a sequence alignment analysis was performed by Clustal Ω. It is showed that fam20C had more similarity at C-ends among different species, but sequences at N-ends were species-specific (Fig. 2A). The human and mouse fam20C gene were more complex than other species. fam20C of P. fucata, Lottia gigantean and Crassostrea gigas lacked transmembrane peptide, while all three vertebrates species and Villosa lienosa had transmembrane peptides. Like human and mouse, fam20C of P. fucata had a low complexity region from amino acid 125 to 140 (Fig. 1), which may be involved in flexible binding29. Fam20C among different species had a conserved function domain with their own features (Figs 1, 2B). SignalP 4.1 showed that Fam20C of P. fucata had no signal peptide (Fig. S1), which conformed to the result of domain prediction, indicating that Fam20C of mollusks may have a different function compared with well-studied Fam20C of vertebrates.
The distribution of fam20C expression in different tissues
To determine the distribution of fam20C mRNA in different tissues, we conducted real-time PCR. fam20C mRNA was expressed in all tissues except hemocytes (Fig. 3A). However, the expression level was much higher in mantle edge than in gonad, mantle pallial and adductor muscle. Moreover, in situ hybridization was carried out to study the spatial distribution of fam20C in the mantle. Strong hybridization signals were detected at the outer epithelial cells of the middle fold, while a faint signal were detected at the inner epithelial cells of the inner fold of the mantle (Fig. 3C left). Meantime, no obvious signal was found using the control probe for hybridization (Fig. 3C right).
Expression and distribution of fam20C mRNA. (A) Relative gene expression levels of fam20C in different tissues (From left to right: mantle edge, mantle pallial, adductor muscle, gonad, gill, foot, visceral mass and hemocytes). (B) fam20C expression levels varies during five development stages. (C) In situ hybridization of fam20C mRNA in the mantle of pearl oyster. Among the three folds of the mantle, hybridization signals (arrows) were observed at the outer epithelial cells of the middle fold and the inner epithelial cells of the inner fold of the mantle (left). No hybridization signal is apparent in the control section stained with the antisense probe (right). OF, outer fold; MF, middle fold; IF, inner fold. The error bars are the standard deviation of four independent samples.
fam20C expression level varies during development stages
In order to investigate the function of fam20C gene in the larval development, we conducted real-time PCR to study the gene expression during the development process. fam20C expression increased by 3 times in D-shape stage than in oosperm and the expression level dropped in umbonal stage and juvenile stage (Fig. 3B). The D-shape stage is the time when the shell is first formed, suggesting that fam20C is closely related to shell formation.
The role of fam20C in biomineralization in vivo: shell notching and RNA interference experiment
To investigate the fam20C functions in shell formation, we conducted shell notching assays to induce shell repairing. The expression of fam20C increased 1.5 times 6 h after shell notching and maintained a high level until 36 h (Fig. 4A). At 36 h after shell notching, fam20C expression decreased to the initial level. Regenerated shell was firstly found 3–6 days after shell notching (Fig. S2). During the shell repair process, the gene expression was not greatly changed, but it had a tendency of increasing and then decreasing to the normal state. These results may be related to the phosphorylation of proteins during the shell repair process, which needed further study.
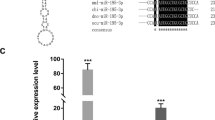
Shell notching and RNAi of fam20C. (A) Response of the fam20C gene during shell repairing after shell notching. 0 h after shell notching groups had a relative value of 1.0. (B) Relative fam20C gene expression level after RNAi. PBS-injected groups had a relative value of 1.0. The non-target control was gfp dsRNA injected groups. Two experimental groups were injected with fam20C dsRNA with the dose of 80 μg and 160 μg. The error bars are the standard deviation of four independent samples. The stars represent a significant (**p < 0.01) difference compared with the PBS-injected groups (p = 0.006 and p = 0.002 compared to the PBS-injected group for 80 μg and 160 μg group, respectively). In contrast, there is no significant difference between PBS-injected group and gfp-injected group. The method used is One-way ANOVA performed by SPSS Statistics 22 software.
The function of fam20C during the shell formation was further analyzed by RNAi. fam20C dsRNA were injected into adductor muscles. For the control, gfp dsRNA and PBS were injected. fam20C expression in the PBS-injected and gfp dsRNA-injected groups were similar. Compared to the PBS-injected group, fam20C expression level decreased by approximately 30% in the group injected with 80 μg fam20C dsRNA and 50% in the group injected with 160 μg fam20C dsRNA (Fig. 4B). The surfaces of the shells were observed by SEM. In the gfp dsRNA-injected group, the prismatic layer showed normal prism structures (Fig. 5A) and the nacreous layer showed a normal pattern like “brick wall” accumulated by small nacreous tablets (Fig. 5B). After injecting 80 μg fam20C dsRNA, cavities started to appear in the center of the nacre tablets and they were covered with randomly accumulated crystals (Fig. 5D). When the concentration increased to 160 μg, randomly accumulated crystals became more interconnected and thick (Fig. 5F). The prismatic layer was not severely disturbed as the nacreous layer, while the edges of prisms became slightly unclear when injected 80 (Fig. 5C) or 160 μg fam20C dsRNA (Fig. 5E). Moreover, more images of shell phenotypes from the control animals and treatment animals (both 80 and 160 treatments) have been provided in the Fig. S3 (SEM images of prismatic layers) and Fig. S4 (SEM images of nacreous layers). In our experiment, we mixed the samples so three images for each group were shown and we believed that they should represent the typical results of each experiment based on our groups’ experience in similar experiment30. As we can see in Fig. S3, no significant difference of prismatic layers have been observed in the gfp-injected group and fam20C dsRNA-injected group. In contrast, significant difference of nacreous layers have been observed in the gfp-injected group and fam20C dsRNA-injected group (Fig. S4). Therefore, to statistically analyze the effects after RNAi injection, we have quantified the diameter of overgrowth crystals on nacre (red arrows in Fig. S4B and C1). The statistics showed that the average diameters of overgrowth crystals on nacre tablets of 80 μg fam20C dsRNA-injected group and 160 μg fam20C dsRNA-injected group were 0.44 μm and 1.72 μm, respectively, both significantly larger than that of gfp-injected group (Fig. S4D). Moreover, we estimated the normal hexagon nacre tablets in different groups and found that the percent of normal hexagon nacre tablets were decreasing with the increase of fam20C dsRNA injection (Fig. S4E).
SEM observation of shell surfaces after RNAi. (A) The prismatic layer and the nacreous layer (B) of gfp dsRNA-injected group. (C) The prismatic layer and the nacreous layer (D) of 80 μg fam20C dsRNA-injected group. (E) The prismatic layer and the nacreous layer (F) of 160 μg fam20C dsRNA-injected group.
Discussion
Recent studies were mainly focused on the functions of matrix proteins in shell formation. Although the roles of matrix proteins in crystal growth have been gradually revealed, more upstream regulating proteins should be studied. Fam20C could be one of the candidates due to its kinase property. We showed that Fam20C was a highly conserved protein among different species. Interestingly, Fam20C lacked signal peptides and transmembrane peptides in most invertebrates, which has implications for understanding the different function of Fam20C.
RT-PCR results indicated that Fam20C may have diverse functions besides mineralization function. The distribution in gonads suggested that Fam20C may be related to development and cell differentiation. Its abundant distribution in mantle edge indicated that it plays important roles in shell formation. As it is known, the mantle is the most important tissue involved in biomineralization and different regions of mantle are responsible for periostracum, prismatic layer, nacreous layer according to their expression and secretion of matrix proteins or regulating proteins. Previous studies revealed that the proteins such as KRMP family31 expressed in mantle edge were responsible for prismatic layer formation, and proteins expressed in mantle pallial such as MSI6032 and nacrein8 constructed the nacreous layer. The proteins from the outer epithelial cells of the middle fold, such as tyrosinase31 were responsible for periostracum formation. The proteins from the inner epithelial cells of the inner fold, such as MSI733, participated in the formation of nacreous layer. Based on that, Fam20C may be related to the formation of all shell layers. However, recent studies showed that protein secreted from specific regions cannot determine its function in biomineralization from the studies of PfN2334 and PfN4435. The relation between protein locating regions and shell layer formation needs to be studied further. Previous studies revealed that Prodissoconch I, composed of amorphous calcium carbonate, forms at the early D-shaped stage; whereas Prodissoconch II, which contains aragonite and calcite, appears in the late D-shaped and umbonal stages36. Dissoconch shell, the original shell with an inner nacreous layer and an outer prismatic layer, forms at the juvenile stage and grows throughout life30. The distribution of gene expression in different development stages showed that Fam20C had a close connection with Prodissoconchi I formation and may be involved in larval development due to its high expression in gonad.
Shell notching experiments were carried out to detect the in vivo effect of fam20C during shell repair process. fam20C expression responded positively during the shell regeneration process after shell notching and the expression level of fam20C then decreased gradually to a relatively stable value. The RNAi experiment is an effective way to detect functions of matrix proteins in vivo, such as Pif, PfN23, and PfN4428,34,35. Knockdown of fam20C affected the shell formation especially the nacre formation.
In conclusion, we provided evidences that fam20C participated in shell formation. Regulation of shell biomineralization is a sophisticated biological process, which requires participation of many cells and proteins. Shell formation is not regulated by one protein. We assume that Fam20C is a regulator in biomineralization and it acts as a controller rather than an inhibitor or enhancer. However, whether Fam20C phosphorylates matrix proteins in the shell formation process still remain unexplored and warrants further study. Nevertheless, this study provides a novel understanding of the function of fam20C in mollusk, which has implications for understanding the regulation mechanisms of shell formation as well as for the development of nacre-like materials. Moreover, it lays foundation for future study about the relationship between regulating proteins and matrix proteins, which can give us more information about biomineralization from a new perspective.
References
Mann, S. Biomineralization: principles and concepts in bioinorganic materials chemistry, (Oxford University Press, 2001).
Addadi, L., Joester, D., Nudelman, F. & Weiner, S. Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem. Eur. J. 12, 980–987 (2006).
Belcher, A. M. et al. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 381, 56–58 (1996).
Marin, F., Luquet, G., Marie, B. & Medakovic, D. Molluscan shell proteins: primary structure, origin, and evolution. Curr.Top. Dev. Biol. 80, 209–276 (2007).
Maruyama, K., mikawa, T. & Ebashi, S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J. Biochem. 95, 511–519 (1984).
Ueno, M. Calcium transport in crayfish gastrolith disc: morphology of gastrolith disc and ultrahistochemical demonstration of calcium. J. Exp. Zoo. Part A 213, 161–171 (1980).
Weiner, S. Aspartic acid-rich proteins: major components of the soluble organic matrix of mollusk shells. Calcified Tissue Int. 29, 163–167 (1979).
Miyamoto, H. et al. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc. Natl. Acad. Sci. USA 93, 9657–9660 (1996).
Kono, M., Hayashi, N. & Samata, T. Molecular mechanism of the nacreous layer formation in Pinctada maxima. Biochem. Bioph. Res. Co. 269, 213–218 (2000).
Mann, K., Weiss, I. M., Andre, S., Gabius, H. J. & Fritz, M. The amino-acid sequence of the abalone (Haliotis laevigata) nacre protein perlucin. European J.Biochem. 267, 5257–5264 (2000).
Marxen, J. C., Nimtz, M., Becker, W. & Mann, K. The major soluble 19.6 kDa protein of the organic shell matrix of the freshwater snail Biomphalaria glabrata is an N-glycosylated dermatopontin. BBA-Proteins Proteom. 1650, 92–98 (2003).
Nudelman, F., Gotliv, B. A., Addadi, L. & Weiner, S. Mollusk shell formation: mapping the distribution of organic matrix components underlying a single aragonitic tablet in nacre. J. Struct.Biol. 153, 176–187 (2006).
Veis, A., Sfeir, C. & Wu, C. B. Phosphorylation of the proteins of the extracellular matrix of mineralized tissues by casein kinase-like activity. Crit. Rev. Oral Biol. & Med. 8, 360–379 (1997).
George, A. & Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 108, 4670–4693 (2008).
Cohen, P. The origins of protein phosphorylation. Nat.Cell Biol. 4, E127–E130 (2002).
Nalbant, D. et al. FAM20: an evolutionarily conserved family of secreted proteins expressed in hematopoietic cells. BMC Genomics 6, 11 (2005).
Hao, J., Narayanan, K., Muni, T., Ramachandran, A. & George, A. Dentin matrix protein 4, a novel secretory calcium-binding protein that modulates odontoblast differentiation. J. Biol. Chem. 282, 15357–15365 (2007).
Simpson, M. A. et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am. J. Hum. Genet. 81, 906–912 (2007).
Tagliabracci, V. S. et al. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 336, 1150–1153 (2012).
Yalak, G. & Vogel, V. Extracellular phosphorylation and phosphorylated proteins: not just curiosities but physiologically important. Sci. Signal. 5, re7–re7 (2012).
Tagliabracci, V. S., Pinna, L. A. & Dixon, J. E. Secreted protein kinases. Trends Biochem. Sci. 38, 121–130 (2013).
Dheilly, N. M., Lelong, C., Huvet, A. & Favrel, P. Development of a Pacific oyster (Crassostrea gigas) 31,918-feature microarray: identification of reference genes and tissue-enriched expression patterns. BMC Genomics 12, 468 (2011).
Mann, K. & Edsinger, E. The Lottia gigantea shell matrix proteome: re-analysis including MaxQuant iBAQ quantitation and phosphoproteome analysis. Proteome Sci. 12, 28 (2014).
Marie, B. et al. Deep conservation of bivalve nacre proteins highlighted by shell matrix proteomics of the Unionoida Elliptio complanata and Villosa lienosa. J. R. Soc. Interface 14, 20160846 (2017).
Jia, G. et al. cfMSP-1, an extremely acidic matrix protein involved in shell formation of the scallop Chlamys farreri. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 185, 34–41 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25, 402–408 (2001).
Mount, A. S., Wheeler, A. P., Paradkar, R. P. & Snider, D. Hemocyte-mediated shell mineralization in the eastern oyster. Science 304, 297–300 (2004).
Suzuki, M. et al. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 325, 1388–1390 (2009).
Coletta, A. et al. Low-complexity regions within protein sequences have position-dependent roles. BMC Syst. Biol. 4, 43 (2010).
Fang, D. et al. Identification of Genes Directly Involved in Shell Formation and Their Functions in Pearl Oyster, Pinctada fucata. Plos One 6, e21860 (2011).
Zhang, C., Xie, L., Huang, J., Chen, L. & Zhang, R. A novel putative tyrosinase involved in periostracum formation from the pearl oyster (Pinctada fucata). Biochem. Bioph. Res. Co. 342, 632–639 (2006).
Sudo, S., Fujikawa, T., Nagakura, T. & Ohkubo, T. Structures of mollusc shell framework proteins. Nature 387, 563 (1997).
Zhang, Y. et al. A novel matrix protein participating in the nacre framework formation of pearl oyster, Pinctada fucata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 135, 565–573 (2003).
Fang, D. et al. Novel basic protein, PfN23, functions as key macromolecule during nacre formation. J. Biol. Chem. 287, 15776–15785 (2012).
Pan, C. et al. A novel acidic matrix protein, PfN44, stabilizes magnesium calcite to inhibit the crystallization of aragonite. J. Biol. Chem. 289, 2776–2787 (2014).
Miyazaki, Y., Nishida, T., Aoki, H. & Samata, T. Expression of genes responsible for biomineralization of Pinctada fucata during development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 155, 241–248 (2010).
Acknowledgements
This study was supported by the National Natural Science Foundation of China, Grants 31372502 and 31572594.
Author information
Authors and Affiliations
Contributions
J.Z.D., C.L., L.P.X. and R.Q.Z. designed the experiments. J.Z.D. and C.L. wrote the main manuscript text. J.Z.D., C.L., G.R.X. and J.X. performed experiment. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, J., Liu, C., Xu, G. et al. fam20C participates in the shell formation in the pearl oyster, Pinctada fucata. Sci Rep 8, 3563 (2018). https://doi.org/10.1038/s41598-018-21797-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21797-w
This article is cited by
-
A shell matrix protein of Pinctada mazatlanica produces nacre platelets in vitro
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.