Abstract
The mortality of acute-on-chronic liver failure (ACLF) patients complicated with invasive pulmonary aspergillosis (IPA) was extremely high. We aimed to explore prognostic value of the Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) lung score and to establish an optimal voriconazole regimen for ACLF patients complicated with IPA. We retrospectively screened hospitalized ACLF patients in our hospital from July 2011 to April 2016, from which 20 probable IPA cases were diagnosed. Along with onsets of IPA, deteriorated diseases severity, especially lung conditions were found in those 20 ACLF patients. It was found that IPA patients with CLIF-SOFA lung score <2 had better 28-day survival than those with lung score >1 (11/13 vs 0/7, p < 0.001). Based on plasma voriconazole concentration measurement, an optimal voriconazole regimen (loading doses: 0.2 g twice daily; maintenance doses, 0.1 g once daily) was established, which resulted in rational trough plasma drug concentrations (1–5 μg/mL), good clinical outcomes (90-day survival rate of 6/8) and no observed adverse events. In conclusion, CLIF-SOFA lung score >1 was able to identify ACLF patients complicated with IPA encountering much higher 28-day mortality. An optimal voriconazole regimen was safe and effective in our ACLF patients complicated with IPA.
Similar content being viewed by others
Introduction
Acute-on-chronic liver failure (ACLF) is a distinct clinical entity characterized by acute deterioration of liver function, multi-organ failure and high mortality1,2. Infections, including spontaneous bacterial peritonitis and pulmonary infection, are consistently attributed to the development and progression of ACLF1,3.
Moreover, some fungal infections, especially invasive pulmonary aspergillosis (IPA), are observed in patients with decompensated cirrhosis, liver failure or severe alcoholic hepatitis4,5,6. Patients with critical liver disorders are now considered as additional risk factors7, along with the classic factors such as allogeneic bone marrow transplantation8.
The prevalence of IPA in HBV-related ACLF patients has been reported to be 5.0–8.3%9,10,11, reaching 13.8% (13/94) in patients with severe alcoholic hepatitis6. The short-term mortality observed in these patients ranged from 73.5% to 100%6,9,10. However, currently no criteria are available to identify patients with poor prognosis.
Voriconazole, liposomal amphotericin B and echinocandins are three frequently prescribed drugs for IPA patients, among which voriconazole is recommended as the first-line option for primary treatment of IPA12. However, use of oral or intravenous voriconazole in ACLF patients has been limited due to its potential hepatotoxicity, and also the lack of pharmacokinetics or pharmaco-dynamics data in such critical situations13. Additionally, acute kidney injuries related to voriconazole treatment is believed to be related with sulfobutylether-β-cyclodextrin in the intravenous formulation of voriconazole14, and treatment with oral instead of intravenous voriconazole should eliminate any risk of acute kidney injury related to sulfobutylether-β-cyclodextrin.
Management strategies for IPA in ACLF patients have evolved significantly in our Hepatology Unit since 2012, from which an intensive IPA screening strategy was implemented following physicians’ consistent awareness of IPA in liver failure patients. And since plasma voriconazole monitoring methods became available in our hospital in 2014, we explored an optimal voriconazole regimen for ACLF patients complicated with IPA. Our retrospective study aimed to prove the association between CLIF-SOFA lung score1 and short-term outcomes, and to introduce an optimal voriconazole regimen for ACLF patients complicated with IPA.
Results
IPA cohort derived from ACLF patients
In total, 790 adult patients who had been diagnosed with liver failure in the Hepatology Unit of Nanfang Hospital between July 2011 and April 2016 were screened and 565 ACLF cases were included, among which 101 ACLF patients who underwent pulmonary CT scanning and were diagnosed with lung infection were re-evaluated (Fig. 1).
Thirty-nine patients were identified as potential IPA cases with corresponding radiological features, among which 18 plasma samples from 16 patients were available for galactomannan (GM) tests. Positive GM were obtained in 7/10 plasma samples collected on the second day of voriconazole treatment, in 4/6 plasma samples collected 1–2 days prior to anti-fungal treatment, and in 0/2 samples collected 5–6 days prior to anti-fungal treatment. Overall 11 patients had positive GM tests (median GM index: 1.89, ranging from 1.07 to 3.21).
Appropriate sputum cultures were performed in 27 cases, and 51.9% (14/27) cases were identified as positive for Aspergillus spp. (13 with A. fumigatus and 1 with A. Flavus). Overall, 20 probable IPA were established by positive culture of Aspergillus from respiratory secretions (n = 9), positive plasma GM tests (n = 6) or both (n = 5).
In order to avoid potential bias from the possible diagnosed IPA, the following analysis was focused on the 20 probable IPA patients. The main underlying causes of the ACLF patients with probable IPA were HBV-related liver disease (19/20). The median time from ACLF diagnosis to IPA development was 13 days, ranging from 0–44 days. Supplementary Table 1 showed the detailed radiologic features of 20 probable IPA patients. Concomitant bacterial lung infections occurred in three IPA patients (1 Staphylococcus haemolyticus, 1 Enterobacter cloacae, 1 Klebsiella pneumoniae). One IPA patients complicated with bacterimia, and three IPA cases complicated with spontaneous bacterial peritonitis.
We enrolled 62 ACLF patients diagnosed with non-aspergillus lung infection and compared their characteristics with those 20 probable IPA patients (Table 1). Corticosteroids were prescribed more frequently in ACLF patients who developed IPA (60% vs 11.3%, p < 0.001), and the cumulative prednisone-equivalent dosages were also higher (240 mg vs 70 mg, p = 0.045) than those with non-aspergillus lung infection. IPA developments were not attributed to severity of liver failures, as indicated by comparable MELD, MELD-Na, CLIF-C ACLFs and CLIF-SOFA scores15 between these two groups (Table 1).
IPA worsened overall conditions of ACLF patients
To figure out the clinical feature of ACLF patients complicated with IPA, we analyzed the changes in clinical and laboratory index in ACLF patients as IPA developed, compared with those at enrolment (Table 1).
The temperature elevated as IPA developed (median: 37°C vs 38.6°C, p = 0.001). Some infection index such as C-reactive protein, procalcitonin and Leukocyte count didn’t change significantly as IPA developed. IPA also exacerbated the overall condition of ACLF patients, as indicated by higher CLIF-C ACLFs score (median: 44.9 vs 46.8, p = 0.028) and trends for higher MELD-Na (median: 25.7 vs 28.6, p = 0.064) and CLIF-SOFA score (median: 8 vs 10, p = 0.083). In detail, the numbers of lung failure (0 to 3), cerebral failure (1 to 3), liver failure (16 to 19) and coagulation failure (9 to 11) increased as IPA developed, although they were not statistically different (Table 1).
CLIF-SOFA lung score >1 at IPA diagnosis was associated with higher mortality
Among the 20 probable IPA patients, 11 died within 90 days. Most of the deaths were caused by lung failure associated with IPA (9/11), the other two deaths were caused by intra-cerebral and digestive tract hemorrhage, respectively.
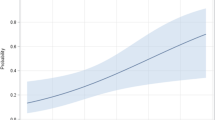
Patients with poor 28-day outcome had a significantly worsen lung condition at the time of IPA diagnosis, as shown by higher CLIF-SOFA lung scores (Fig. 2a). Further analysis showed that lung score was associated with death within 28-days in ACLF patients complicated with IPA (RR: 2.803, 95% CI: 1.543–5.091, p = 0.001). Lung score with cut-off at 1, which was defined with maximally selected log-rank statistic (M = 3.4256, p < 0.001), was adopted as the index to identify patients with high mortality. The 28-day (0% vs 84.6%, p < 0.001) and 90-day (0% vs 69.2%, p < 0.001, Fig. 2b) survival rates calculated from the time of IPA diagnosis were much poorer in patients with lung score >1 (n = 7) than those <2 (n = 13). Consistent results were found with respect to 28-day and 90-day IPA-associated mortality rates (Fig. 2c).
Between patients with CLIF-SOFA lung score >1 and those <2, the median time interval (days) from ACLF diagnosis to IPA diagnosis was comparable [18 (0–44) vs 12 (0–33), p = 0.780]. All (7/7) patients with lung score >2 had fever as IPA diagnosed and for cases with lung score <1 the number was 11/13, p = 0.521. The median time interval (days) from fever to IPA diagnosis was also comparable [6.5 (2–14) vs 4.0 (1–14), p = 0.237] between patients with lung score >1 and those with lung score <2.
Probable IPA patients with lung score >1 had more severe conditions than those <2, as determined by higher CURB-65, PSI16, MELD, MELD-Na, CLIF-C ACLFs and CLIF-SOFA scores. Infection index such as C-reactive protein, procalcitonin and leukocyte count was comparable between these two groups.
Optimization of the voriconazole regimen
Voriconazole is a first-line option for primary treatment of IPA12, however experience of its use in ACLF patients is limited due to concerns of potential liver injuries. In our previous clinical practice, several ACLF patients with a prompt IPA diagnosis were treated with voriconazole using the recommended protocol for Child-Pugh A or B liver cirrhosis. The doses always had to be adjusted due to overt drug-related adverse effects. Thus, we explored to optimize the voriconazole regimen in ACLF patients since plasma voriconazole concentrations monitoring methods became available in our hospital (in October 2014).
The detail of the process was shown in the Supplementary material: Voriconazole monitoring protocol. Briefly, we optimized the loading doses of voriconazole based on ideal trough plasma concentrations (1–5 µg/mL) measured on day 1 and optimized maintenance doses based on plasma concentrations on days 3–5. In total, 10 IPA patients (8 probable and 2 possible) underwent plasma voriconazole concentration monitoring.
The first four patients began with the dose of the standard regimen (loading dose, 0.4 g, po, q12h; maintenance dose, 0.2 g, po, q12h) and underwent dosage adjustment based on the plasma voriconazole levels. The standard regimen usually produced extremely high plasma drug concentrations (Fig. 3a), much further exceeding the efficient therapeutic ranges (1–5 µg/mL). Two of them even complicated with severe adverse events (visual disturbance), and had to stop voriconzole for about half a mouth awaiting for the voriconazole concentrations falling to rational ranges. Finally, after several adjustments based on plasma voriconazole levels, the maintenance doses were set at 0.1 g/d for two (body weight: 65 Kg, 44 Kg), 0.2 g/d for one (body weight: 99 Kg), 75 miligrams for one (body weight: 65 Kg).
Thus, in the subsequent 6 patients (median body weight with range: 64 [62–71] Kg), the loading dose was set at 0.2 g, po, q12h; in one patient, we give a maintenance dose of 0.2 g/d, which was finally reduced to 0.1 g/d because of elevated plasma voriconazole level (6.48 µg/mL); the other 5 patients received maintenance dose of 0.1 g/d de novo, which resulted in stable and optimal trough voriconazole concentrations (1–5 µg/mL, Fig. 3b). No observed adverse events were recorded in these 6 patients.
Finally, the optimal voriconazole regimen was established (loading dose, 0.2 g, po, q12h; maintenance dose, 0.1 g, po, qd), which achieved optimal therapeutic plasma drug concentrations.
Probable IPA patients treated with optimal voriconazole regimen (n = 8) achieved a comparable 90-day survival to ACLF patients without IPA in our cohort (Fig. 4a). Also, an obvious reduction in total bilirubin (median: 23.6 mg/dl vs 20.8 mg/dl, p = 0.017) or resolution of pulmonary lesions on thoracic CT (median:172.33 mm2 vs 35.97 mm2, p = 0.011) was observed in these patients (Fig. 4b,c).
Clinical outcomes in IPA patients treated with optimal voriconazole regimen. (a) IPA Patients treated with optimal voriconazole regimen had similar 90-day survival as patients without IPA. (b) Bilirubin level (median with range) reduced with one weeks of optimal voriconazole regimen treatment. (c) Lesions on pulmonary CT scans (median with interquartile range) resolved obviously with 1–2 weeks of voriconazole treatment.
The median treatment duration of the optimal voriconazole regimen was 54 days (14–110), which was similar to those recommended for the standard one in non-ACLF patients12. However, the cumulative Defined Daily Dose (cDDD) reduced dramatically to 15.25 (4–40).
Discussion
The present study provides several conclusions with clinical merits. Firstly, we found that IPA exacerbated organ failures in ACLF patients, especially lung failures. Secondly, we confirmed the prognostic values of CLIF-SOFA lung scores in ACLF patients complicated with probable IPA. Finally, we introduced an optimal voriconazole regimen, which resulted in rational trough plasma drug concentrations (1–5 μg/mL), no observed adverse events and good anti-fungal responses.
As the progression of IPA, despite of the overall deteriorated condition, lung dysfunction was the most predominant and distinct symptom in IPA patients, and lung failure was the crucial cause of death in these patients. These findings indicated the important role of lung condition at the time of IPA diagnosis in predicting the therapeutic efficiency and outcomes in these patients.
Subsequently, we proved that CLIF-SOFA lung score >1 at IPA diagnosis was able to identify patients with poor prognosis. Patients with lung score >1 at IPA diagnosis had poor responses to anti-fungal treatments and high short-term mortality, which indicated that lung score >1 might represent a delayed diagnosis of IPA, regardless of variable IPA progressions due to different host and pathogenic factors. Thus, early diagnosis of IPA could have a determined role in improve the outcome in ACLF patients. This idea was in accordance with other studies. Wang et al.11 and Gustot et al.6 reported that in the context of HBV-ACLF or severe alcoholic hepatitis, patients with IPA ceased mainly due to respiratory failure within a very short time of IPA diagnosis, regardless of various anti-fungal therapies. Both reports suggested the vital importance of a prompt IPA diagnosis6,11.
However, due to non-specific clinical manifestations and lack of conscious on IPA in ACLF patients, early diagnosis has remained a challenge. Since September 2012, we implemented an IPA screening strategy according to the AspICU study group17. Thus, ACLF patients were screened with thoracic CT driven by clinical symptoms and signs, such as fever refractory to at least 3 days of broad-sputum antibiotic therapy, pleuritic chest pain, pleuritic rub, dyspnoea, haemoptysis. The percentage of patients encountered higher CLIF-SOFA lung scores decreased these years. Except for this symptom-driven screening strategy, further studies on time-triggered or overall condition-triggered screening strategy, including low-radiation doses thoracic CT and plasma GM tests in high-risk ACLF patients were warranted for early diagnosis furthermore18. And CLIF-SOFA lung score <2 had potential as a surrogate marker defining early IPA diagnosis, albeit further validations were warranted in future studies.
We summarized the reports about IPA in patients with advanced liver diseases and found the overall high short-term mortality (>70%, Table 2). We realized that in addition to difficulty of prompt diagnosis, the other challenging issue was the treatment options of IPA in ACLF patients. The first-line treatment is voriconazole, but this drug is potentially hepatotoxic and contraindicated because of hepatic metabolism13. Using therapeutic drug monitoring methods, we established an optimal voriconazole regimen in ACLF patients. Our voriconazole regimen was able to maintain stable and rational therapeutic trough concentrations between 1 and 5 µg/mL19. Meanwhile, patients treated with optimal voriconazole regimen had good clinical outcomes and 90-days survival rate high as 75%, which was also benefitted from early IPA diagnosis as indicated by lower CLIF-SOFA lung score (<2) in all patients prior to our optimal regimen prescribed. Thus, the anti-fungal efficacy of our optimal voriconazole regimen on ACLF patients with higher CLIF-SOFA lung score was unclear and needed prospective observations.
The dosage of oral voriconazole in our optimal regimen for ACLF patients was much lower than that recommended for patients without liver disease or with Child-Pugh A/B liver cirrhosis12,19. Voriconazole undergoes extensive hepatic metabolism by cytochrome P450 system enzymes, including CYP2C19, 2C9 and 3A419. Patients with liver failure may have a diminished capacity to metabolize the drug, leading to its accumulation and hazardous plasma concentrations. Drug interactions in the cytochrome P450 system may also influence the plasma concentration of voriconazole20,21. For example, proton pump inhibitors, especially omeprazole which was commonly used in our ACLF patients, have a potential role in elevating plasma voriconazole levels22. Our optimal voriconazole regimen was derived from Chinese ACLF patients, who had moderate body weights and CYP2C19 polymorphism for slow voriconazole metabolism. Thus, its feasibility in other ethnicities who differ in CYP2C19 polymorphisms and body weight need further validation.
Our study has several limitations that are mainly related to its retrospective nature. Because there were no lung biopsies due to coagulation disorders in liver failure patients and no autopsies for the deceased patients, we did not establish the proven IPA diagnosis. It was a single-centre study and the sample size was small. Our optimal voriconazole needed further validations in future studies, which should be multi-centered, prospective, large sized and ideally multi-ethnicity ones.
Our study showed for the first time that CLIF-SOFA lung score was a valuable prognostic index in ACLF patients complicated with IPA, and our optimal voriconazole regimen was safe and effective in those critical ill patients.
Patients and Methods
Patients with ACLF
A retrospective chart review was conducted to identify ACLF patients with a documented clinical diagnosis of IPA between July 2011 and April 2016. Patients diagnosed with liver failure during hospitalization (Hepatology Unit, Department of Infectious Diseases, Nanfang Hospital) were screened (Supplementary Table 1 showed the ICD-10 code for liver failure). ACLF diagnosis was reassessed according to the Asian Pacific Association for the Study of the Liver (APASL) 2009 consensus recommendations for ACLF23. Patients who were diagnosed with a malignant tumour or who were found pregnant before or during hospitalization were excluded from the study.
Diagnosis of IPA
Radiological evaluations
Among the included ACLF patients, those underwent pulmonary computed tomography (CT; Philips Brilliance 256-slice spiral CT) scans after enrolment and showed signs of lung infection were reassessed by Dr Wu and Dr Yu (Supplementary Table 2 showed the ICD-10 code for lung infection). For patients with suspected IPA features, the numbers, sizes and locations (based on the pulmonary segments) of pulmonary lesions such as nodules or masses shown on CT images prior to and after 1–2 week anti-fungal treatment were measured and recorded.
Mycological criteria
Among the included ACLF patients, sputum culture reports from the clinical laboratory were reviewed to identify patients positive for Aspergillus (A.) spp. In addition, in patients showed suspicious IPA image manifestation, available cryogenic plasmas, which had been stored at −20 °C for 6 months to 4 years, were tested for galactomannan (GM) following the manufacturer’s instructions (EIA, Bio-Rad Laboratories) with a cut-off value of 0.524.
Diagnostic criteria of IPA and non-aspergillus lung infection
The IPA diagnosis was established according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) definitions, considering ACLF as a moderate risk factor for IPA (Supplementary Table 3)17,25. In detail, ACLF patients must meet with the radiologic features of IPA (dense, well-circumscribed lesions with or without a halo sign, aircrescent sign or cavity, masses), and the mycological criteria: direct test (direct microscopy, or culture) indicating the presence of Aspergillus species in appropriate lower-respiratory tract sputum or galactomannan antigen detected in plasma.
Non-aspergillus lung infection was established by the combination of clinical symptoms and new infiltration on pulmonary CT26, with probable or possible IPA excluded.
IPA treatment
Anti-fungal therapies for ACLF patients complicated with IPA have evolved in our Hepatology Unit. In the first few years (2011–2014), our patients were treated with echinocandins (Cancidas, caspofungin acetate, Merck) or standard voriconazole (voriconazole tablet, 50 milligrams/tablet; Huashen, Chendu, PR China) dosage (loading dose as 0.4 g, po, q12h, maintenance dose as 0.2 g, po, q12h, which was adjusted mainly based on overt clinical adverse effects).
In October 2014, plasma voriconazole concentrations monitoring tests became available in our hospital. We then began to explore an optimal voriconazole regimen based on the plasma drug level (Supplementary material: Voriconazole monitoring protocol). Finally, an optimal voriconazole regimen was established, which consisted of a lower loading dose (0.2 g, po, q12h) and a lower maintenance dose (0.1 g, po, qd), and this optimal regimen turned to be the preferred anti-fungal option for the majority of the subsequent IPA cases with underlying ACLF.
Data collection and outcome evaluation
Clinical data including history, physical examination, laboratory measurements, imaging findings and treatment strategies were recorded. Outcomes were evaluated based on the medical records or by direct contact with patient or a kin. Total bilirubin changes were evaluated after around one week of anti-fungal treatment. CT lesions changes were evaluated 1–2 weeks since anti-fungal therapies started. We documented overall 28-day and 90-day survivals and IPA-associated deaths. IPA-associated death was defined as death related to respiratory failure as alternative causes of death excluded, which was restricted in patients with no sustained favourable response to anti-fungal treatment as evaluated with pulmonary CT changes and clinical symptoms and signs27. Informed consent had been obtained from all patients and/or their relatives about usage of their clinical data and/or samples. This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-201105-K1). Methods were carried out in accordance with the approved guidelines.
Statistical analysis
Data were presented as the median (range) for continuous variables. Fisher’s exact test, the Wilcoxon test, or the Mann-Whitney U test was performed as appropriate. The Cox model was used to test the association between lung scores and short-term mortality in IPA patients. Patient survival rates were assessed using the Kaplan-Meier method and compared with the log-rank test. All above mentioned statistical analyses were performed using SPSS software (IBM SPSS, version 17.0). Differentiation of delayed from prompt IPA diagnosis using CLIF-SOFA lung score was determined with maximally selected log-rank statistic (R statistical software, version 3.4.0). All tests were two sided with a significance level of 0.05.
Availability of materials and data
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 144, 1426–1437 (2013).
Li, H. et al. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci Rep. 6, 25487 (2016).
Shi, Y. et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 62, 232–242 (2015).
Bienvenu, A. L. et al. Acute liver failure may lead to lethal pulmonary aspergillosis. J Clin Gastroenterol. 44, 593–594 (2010).
Falcone, M., Massetti, A. P., Russo, A., Vullo, V. & Venditti, M. Invasive aspergillosis in patients with liver disease. Med Mycol. 49, 406–413 (2011).
Gustot, T. et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 60, 267–274 (2014).
Koulenti, D., Garnacho, M. J. & Blot, S. Approach to invasive pulmonary aspergillosis in critically ill patients. Curr Opin Infect Dis. 27, 174–183 (2014).
Segal, B. H. Aspergillosis. N Engl J Med. 360, 1870–1884 (2009).
Chen, J., Yang, Q., Huang, J. & Li, L. Risk factors for invasive pulmonary aspergillosis and hospital mortality in acute-on-chronic liver failure patients: a retrospective-cohort study. Int J Med Sci. 10, 1625–1631 (2013).
Wu, Z., Ling, Z., Shao, F., Sheng, J. & Li, L. Invasive pulmonary aspergillosis in patients with acute-on-chronic liver failure. J Int Med Res. 40, 1958–1965 (2012).
Wang, W. et al. Invasive pulmonary aspergillosis in patients with HBV-related liver failure. Eur J Clin Microbiol Infect Dis. 30, 661–667 (2011).
Patterson, T. F. et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 63, 433–442 (2016).
Dolton, M. J. & McLachlan, A. J. Voriconazole pharmacokinetics and exposure-response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents. 44, 183–193 (2014).
Pfizer Inc. Vfend (voriconazole) package insert. New York, NY (2011).
Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. Journal of Hepatology. 61(5), 1038 (2014).
Richards, G. et al. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J Intensive Care Med. 26(1), 34–40 (2011).
Blot, S. I. et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 186, 56–64 (2012).
Małgorzata, M. et al. Screening With Serum Galactomannan Might Be Associated With Better Outcome Than Symptom-Triggered Galactomannan Testing in Allogeneic HSCT Recipients With Invasive Aspergillosis[J]. Clinical Infectious Diseases. 57(12), 1786–1787 (2013).
Dolton, M. J. et al. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother. 56, 4793–4799 (2012).
Gubbins, P. O. Triazole antifungal agents drug-drug interactions involving hepatic cytochrome P450. Expert Opin Drug Metab Toxicol. 7, 1411–1429 (2011).
Yakiwchuk, E. M., Foisy, M. M. & Hughes, C. A. Complexity of interactions between voriconazole and antiretroviral agents. Ann Pharmacother. 42, 698–703 (2008).
Chayakulkeeree, M., Poovipirom, N., Siengwattana, P. & Maneerattanaporn, M. Effect of proton pump inhibitor on plasma voriconazole concentration in Thai patients. J Med Assoc Thai. 98, 232–237 (2015).
Sarin, S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 3, 269–282 (2009).
Wheat, L. J. et al. Long-term stability at −20 degrees C of Aspergillus galactomannan in serum and bronchoalveolar lavage specimens. J Clin Microbiol. 52, 2108–2111 (2014).
De, P. B. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 46, 1813–1821 (2008).
Fernã, J. et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 314240, https://doi.org/10.1136/gutjnl-2017-314240 (2017).
Raad, I. I. et al. Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int J Antimicrob Agents. 45, 283–288 (2015).
Zhao, H. et al. The clinical characteristics of aspergillosis in patients with liver failure. The journal of Hai Nan Medical University. 18(1), 48–51 (2012).
Zhou, Y., Qian, Z. P., Zhang, Y. Y., Wang, J. F. & Wang, J. W. The clinical characteristics and treatment of aspergillosis in patients with liver failure. The infection journal of Chinese Medical Hospital. 22(4), 705–8 (2012).
Liu, J. Y., Xu, Y. L., Wei, L. R., Wang, H. Z. & Song, L. H. The risk factor and pulmonary radiologic characteristics of IPA in patients with liver failure. The journal of Chinese experiment and clinical infection disease. electric version (2013).
Acknowledgements
We appreciate critical comments from Prof Hai Li (Department of Gastroenterology, Renji Hospital, Shanghai). This work was supported by National Natural Science Foundation of China [81270533 to Jinjun Chen]; National Natural Science Foundation of China [81470038 to Jinjun Chen]; National Science and Technology Major Project [2017ZX10203203 to Jinjun Chen]; National Key Research and Development Program of China [2017YFC0908100 to Jinjun Chen] and The Key Scientific and Technological Program of Guangzhou City [201508020262 to Jinjun Chen].
Author information
Authors and Affiliations
Contributions
Jie Gao, Qing Zhang, Yuankui Wu contributed equally to this work; Jinjun Chen, Jie Gao, Qing Zhang and Yuankui Wu contributed to the conception and design of the study; Qing Zhang and Sijia Liu contributed to testing the plasma voriconazole level; Yuankui Wu and Ruoxi Yu contributed to the re-evaluation and measurement of the lesions on pulmonary CT scanning; Ying Li, Tingting Qi, Congyan Zhu, Qinjun He, Weiqun Wen, Fuyuan Zhou and Yongpeng Chen contributed to data acquisition; Jinjun Chen, Jie Gao contributed to data analysis and interpretation, and writing of article; Jinlin Hou approved the study and this submission. The authors listed above have all contributed to, and approve the submitted version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, J., Zhang, Q., Wu, Y. et al. Improving survival of acute-on-chronic liver failure patients complicated with invasive pulmonary aspergillosis. Sci Rep 8, 876 (2018). https://doi.org/10.1038/s41598-018-19320-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19320-2
This article is cited by
-
Efficacy and safety of voriconazole in the treatment of invasive pulmonary aspergillosis in patients with liver failure: study protocol for a randomized controlled clinical trial
Trials (2023)
-
Spontaneous Fungal Ascites Infection in Patients with Cirrhosis: An Analysis of 10 Cases
Infectious Diseases and Therapy (2021)
-
Invasive Pulmonary Aspergillosis in Acute-on-Chronic Liver Failure Patients: Short-Term Outcomes and Antifungal Options
Infectious Diseases and Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.